Abstract
The reactivity of two DNA-targeted platinum–acridine conjugates with cysteine sulfur was studied. The conjugate containing an amidine-NH donor group cis to the chloride leaving group showed considerably reduced reactivity with N-acetylcysteine compared to the prototypical derivative containing a thiourea-S linkage. The opposite scenario has been observed previously in reactions with nucleobase nitrogen. Possible consequences of the unique target-selective tuning of the substitution chemistry for the pharmacodynamic properties and biological activity of these agents are discussed.
Introduction
The high reactivity of the anticancer drug cisplatin (cis-[PtCl2(NH3)2]) with the intracellular sulfur-containing nucleophile glutathione (γ-l-glutamyl-l-cysteinylglycine, GSHa) causes adverse effects, such as severe systemic toxicities and tumor resistance.1, 2 One major goal in platinum drug development, therefore, is to design complex geometries that react less avidly with cysteine sulfur. This has been achieved, for instance, in picoplatin (cis-[PtCl2(NH3)(2-methylpyridine)]), by replacing one ammine ligand in cisplatin with a sterically hindered pyridine derivative,3, 4 and in carboplatin (cis-[Pt(CBDCA)(NH3)2], CBDCA = cyclobutane-1,1-dicarboxylate), by introducing a bidentate leaving group in place of the chlorido ligands. While carboplatin is remarkably inert in reactions with GSH,5 the stability of the dicarboxylate chelate compromises the metal's ability to bind to its pharmacological target, DNA.6 This dilemma is manifest in the fact that the second-generation drug, while less toxic than cisplatin, has to be administered at significantly higher doses to achieve the same therapeutic effect as the parent drug.7
Here we report a unique case of a chemical modification to a DNA-targeted platinum agent that reduces the reactivity of the metal with cysteine sulfur while it was previously found to accelerate the reaction with nucleobase nitrogen. In a recent study,8 we investigated the biological effect of replacing a thiourea (sp2-S) with an amidine (sp2-NH) donor cis to a chloride leaving group. The new platinum–acridine agent reported, [PtCl(en)(L)](NO3)2 (en = ethane-1,2-diamine; L = N-(2-(acridin-9-ylamino)ethyl)-N-methylpropionamidine, acridinium cation) (compound 1, Figure 1) proved to be a significantly more efficient DNA binder and more cytotoxic agent than the prototypical agent, PT-ACRAMTU9, 10 (ACRAMTU = 1-(2-(acridin-9-ylamino)ethyl)-1,3-dimethylthiourea, acridinium cation) (compound 2, Figure 1). Moreover, this work has lead to the first member of this class of compounds endowed with antitumor activity in aggressive non-small cell lung cancer in a mouse xenograft model.8 To test the effect of this simple structural modification on the metal's reactivity with protein sulfur, we incubated both analogues with N-acetylcysteine (N-AcCys), a modified amino acid mimicking GSH, and monitored the progress of the reactions by 1H NMR spectroscopy. In addition, we analyzed the mixtures after completion of the reactions by in-line high-performance liquid chromatography electrospray mass spectrometry (LC–MS).
Figure 1.

Structures of compound 1 and PT-ACRAMTU (2) with structural differences highlighted.
Results and Discussion
In arrayed one-dimensional 1H NMR experiments, the reaction of complex 1 with one equivalent of N-AcCys (25 °C, 10 mM phosphate buffer, D2O, pH* 6.8) was found to proceed considerably slower than the reaction of PT-ACRAMTU (2) under the same conditions. The relative rates at which the amino acid reacted with 1 and 2 were deduced from the change in integral intensities of 1H NMR signals assigned to the platinum complexes (see Supporting Information for spectral data and detailed procedures). Under the conditions chosen, the reaction between 1 and N-AcCys follows a second-order rate law, typical of a mechanism that is dominated by direct substitution of the chlorido ligand by cysteine sulfur and is not limited by the rate of aquation.2 In contrast, the more rapid reaction of PT-ACRAMTU (2) with N-AcCys followed neither second-order nor first-order kinetics, suggesting that the reaction of this derivative proceeds via a more complicated mechanism (Figure 2). In both cases, the 1H NMR spectra of the mixtures indicated formation of multiple products. Attempts to assign the species formed by 1 and 2 using two-dimensional [1H-15N] NMR spectroscopy, a technique applied previously in nucleotide binding studies of [15N]-en labeled conjugates,8, 11 were unsuccessful due to the complexity of the reaction mixtures.
Figure 2.
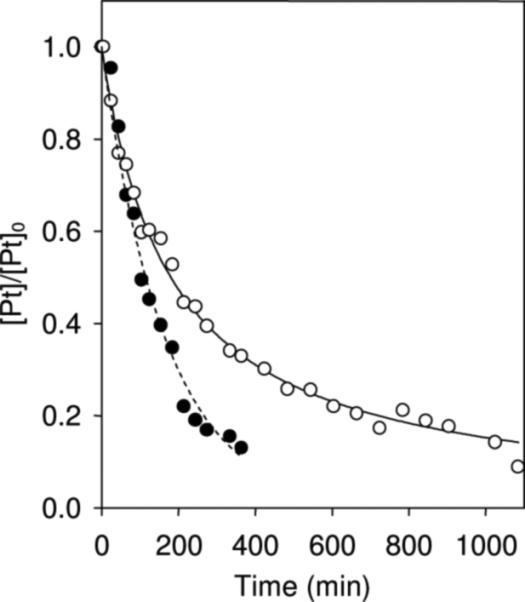
Progress of the reactions of compound 1 (open circles) and compound 2 (filled circles) with one equivalent of N-acetylcysteine (N-AcCys) at 25 °C monitored by 1H NMR spectroscopy. The data shown is the mean of two experiments. The solid line (for 1) represents a curve fit assuming second-order conditions, [Pt]/[Pt]0 = (1 + k [Pt]0 t)−1; (t1/2 = 180 min). The dashed trend line (for 2) has no physical significance.
To overcome this difficulty, LC–MS was used to analyze the product distribution in mixtures of platinum complex incubated with one equivalent of sulfur nucleophile (37 °C, 6 h, 10 mM phosphate buffer, pH 7.2). The two most abundant adducts formed by compound 1 were unambiguously identified as the mononuclear species [Pt(en)(L)(N-AcCys*)]+ ([I]+, 14%) and the dinuclear complex [{Pt(en)(L)}2(μ-N-AcCys*)]4+ ([II]4+, 72%) in electrospray mass spectra recorded in positive-ion mode (Figure 3). (The asterisk indicates the dianionic form of metal-bound N-AcCys at neutral pH.) These findings suggest that adduct I, formed by substitution of chloride by cysteine sulfur, rapidly reacts with unreacted complex 1 to form the dinuclear species, II (Scheme 1). The tendency of cysteine sulfur to readily induce bridged complexes with platinum(II) drugs has been amply demonstrated.2 The platinum–amidine linkage and the en chelate in complex 1 appear to be resistant to nucleophilic attack by cysteine based on the presence of only a minor amount of free acridine L (III, 3%) and the absence of ring-opened adducts. In contrast, the LC–MS data acquired for PT-ACRAMTU (2) reveal a more complicated reaction pattern. In analogy to complex 1, the chloride substitution pathway leads to the dinuclear adduct, [{Pt(en)(ACRAMTU)}2(μ-N-AcCys*)]4+ (II′, 32%), most likely via intermediate I′, which could not be detected in this case (Figure 3). In addition, significant amounts of ACRAMTU (III′, 33%) and the bisintercalator complex, [Pt(en)(ACRAMTU)2]4+ (IV, 26%) (Scheme 1), were observed in the HPLC profiles (Supporting Information). Formation of the latter complex can be explained with chloride substitution in compound 2 by free ACRAMTU in the reaction mixture. The release of ACRAMTU by N-AcCys was unexpected and shows that thiourea, while a typical nonleaving group in reactions of compound 2 with DNA nitrogen,12, 13 becomes substitution-labile in the presence of cysteine sulfur.
Figure 3.
Positive-ion electrospray mass spectra of adducts I (A) and II (B) showing peaks for the molecular ions, [I]+ (m/z 725.2) and [II-2H]2+ (m/z 643.7, z = 2), and fragment ions resulting from in-source collisionally activated dissociation (CAD). The insets illustrate the observed fragmentation patterns. The discrepancy between the calculated and observed m/z values for some of the platinum containing ions is due to distorted Pt isotope patterns.
Scheme 1.
Reactivity of Compounds 1 and 2 with N-Acetylcysteine (N-AcCys)
The reactivity of the Pt–Sthiourea bond and its cis labilizing effect on chloride are potential contributors to the increased cysteine reactivity of PT-ACRAMTU (2) compared to analogue 1. The rationale for incorporating a thiourea-based nonleaving group in the original design was to enhance the reactivity of the metal with DNA nucleobases by exploiting the cis activating effect of sulfur. In their pioneering studies of the inorganic kinetic cis-effect, Tobe and coworkers demonstrated for the complexes [PtCl(en)(dmso-S)]+ and [PtCl(en)(dms)]+ (dmso = dimethylsulfoxide, dms = dimethylsulfide) that the sulfur donors greatly accelerate chloride substitution by an incoming nucleophile relative to analogous complexes with [N3Cl] donor sets.14, 15 In the same studies, the authors demonstrated that the relative rate enhancement (kS/kN) is large for strong incoming nucleophiles, such as sulfur ligands, but negligible for weak ligands like water. The comparative kinetic study of amidine-modified complex 1 and thiourea-based PT-ACRAMTU (2) shows that replacement of the sulfur with an imino donor group reduces, as expected, the metal's reactivity with cysteine sulfur but enhances its binding with DNA nitrogen. These findings strongly suggest that, unlike the reaction with cysteine, the reaction with DNA is not controlled by the electronic cis-effect but, as concluded previously, most likely by steric factors and/or H-bonding that favor complex aquation and facilitate nucleophilic attack of nucleobase nitrogen on the metal center.8
In addition to the unique monofunctional–intercalative adducts formed by PT-ACRAMTU and its second-generation analogues, one critical feature that sets these hybrid agents apart from cisplatin-type cross linkers is their inherent DNA-targeted character. While intercalation plays an important role in the mechanism of action of these agents, rapid platination of DNA nucleobases appears to be crucial for potent inhibition of cancer cell proliferation. Characteristically, derivatives that react with DNA sluggishly or not at all have proven only marginally cytotoxic.16, 17 Since the Pt–Scysteine bond is resistant to nucleophilic attack by DNA nitrogen,2 the GSH metabolites formed by compounds 1 and 2 (analogous to I/I′ and II/II′, Scheme 1), even if they reach the nucleus, should be relatively nontoxic because of their inability to induce cytotoxic DNA adducts. Likewise, ACRAMTU (III′) and the bisintercalator IV (a reversible DNA binder18, 19), which are potential metabolites formed in the reaction between 2 and GSH, are considerably less cytotoxic than the hybrid agents.9, 19
Conclusions
In summary, we report the first case of a simple structural modification within a DNA-targeted platinum antitumor agent that increases the target affinity of the metal while concomitantly reducing unwanted reactivity with protein sulfur. Based on these findings, the amidine analogue, 1, should have a major pharmacological advantage over PT-ACRAMTU (2), which may contribute to the superior potency of the new analogue in vitro and in vivo. To test this hypothesis, future studies will delineate relationships between the cytotoxicity and intracellular distribution of 1, PT-ACRAMTU, and related complexes in cancer cells characterized by elevated cytosolic GSH levels. Another important issue to be addressed is the relatively high toxicity of our new hybrid agent in treated animals. The results of a necropsy performed on the test animals treated with at sublethal doses of the hybrid agent (unreported data) revealed mild to yellow discoloration of the kidneys. We speculate that the adverse effects on the kidneys are the result of platinum binding to sulfur of glutathione (GSH). Cisplatin–GSH adducts are metabolized to strong nephrotoxins that cause damage to the renal proximal tubules.2 Thus, additional structural modifications in compound 2 may be needed to reduce this toxicity to levels as low as those observed for picoplatin and carboplatin.
Experimental Section
Materials
The platinum-acridine complexes 1 and 2 were synthesized according to published procedures.8, 9 For the preparation of biological buffers, biochemical grade chemicals (Fisher/Acros) were used. HPLC grade solvent were used in all chromatographic separations. All other chemicals and reagents were purchased from common vendors and used without further purification. Stock solutions of the platinum compounds were prepared immediately before use.
NMR Spectroscopy
NMR spectra in arrayed experiments were collected at room temperature on a Bruker 500 DRX spectrometer equipped with a triple-resonance broadband inverse probe and a variable temperature unit. Reactions were performed in 5-mm NMR tubes containing 2 mM complex 1 or 2 and 2 mM N-acetylcysteine (10 mM phosphate buffer, D2O, pH* 6.8). The 1-D 1H kinetics experiments were carried out as a standard arrayed 2-D experiments using a variable-delay list. Incremented 1-D spectra were processed exactly the same, and suitable signals were integrated. Data were processed with XWINNMR 3.6 (Bruker, Ettlingen, Germany). The concentrations of platinum complex at each time point were deduced from relative peak intensities, averaged over multiple signals to account for differences in proton relaxation.
Incubations and Chromatographic Separations
Reactions of 1 and 2 with N-acetylcysteine were performed at a 1:1 drug-to-amino acid ratio in 10 mM sodium phosphate buffer (pH 7.1). Incubations were performed at 37 °C for 6 h. The mixtures were separated by reverse-phase HPLC using the LC module of an Agilent Technologies 1100 LC/MSD Trap system equipped with a multi-wavelength diode-array detector and an autosampler. A 4.6 × 150 mm reverse-phase Agilent ZORBAX SB-C18 (5 μm) analytical column was used in all of the assays, which was maintained at 25 °C during separations. A monitoring wavelength of 413 nm was used to detect acridine-containing fragments. The following eluent systems were used for the separations: solvent A, degassed water/0.1% formic acid, and solvent B, methanol/0.1% formic acid. The gradient used in the separation was 98% → 30% solvent A over 30 min at a flow rate of 0.5 mL/min. Peak integration was done using the LC/MSD Trap Control 4.0 data analysis software.
Mass Spectrometry
Mass spectra were recorded on an Agilent 1100LC/MSD ion trap mass spectrometer. After separation by in-line HPLC, adducts were directly infused into the atmospheric-pressure electrospray source. Ion evaporation was assisted by a flow of N2 drying gas (350 °C) at a pressure of 40 psi and a flow rate of 10 L/min. Positive-ion mass spectra were recorded with a capillary voltage of 2800 V and a mass-to-charge scan range of 150 to 2200 m/z.
Supplementary Material
Acknowledgment
This work was, in part, supported by a grant from the National Institutes of Health (Grant CA101880).
Footnotes
Abbreviations: N-AcCys, N-acetylcysteine; ACRAMTU, 1-(2-(acridin-9-ylamino)ethyl)-1,3-dimethylthiourea; GSH, glutathione; pH*, uncorrected pH meter reading in D2O.
Supporting Information Available: Plots of arrayed NMR spectra, HPLC profiles of the reaction mixtures, electrospray mass spectra and tabulated mass data for all identified products. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am. J. Med. Sci. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Guo Z. The role of sulfur in platinum anticancer chemotherapy. Anticancer Agents Med. Chem. 2007;7:19–34. doi: 10.2174/187152007779314062. [DOI] [PubMed] [Google Scholar]
- 3.Holford J, Sharp SY, Murrer BA, Abrams M, Kelland LR. In vitro circumvention of cisplatin resistance by the novel sterically hindered platinum complex AMD473. Br. J. Cancer. 1998;77:366–373. doi: 10.1038/bjc.1998.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beale P, Judson I, O'Donnell A, Trigo J, Rees C, Raynaud F, Turner A, Simmons L, Etterley L. A Phase I clinical and pharmacological study of cis-diamminedichloro(2-methylpyridine)platinum(II) (AMD473). Br. J. Cancer. 2003;88:1128–1134. doi: 10.1038/sj.bjc.6600854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnham KJ, Djuran MI, Murdoch PS, Ranford JD, Sadler PJ. Ring-Opened Adducts of the Anticancer Drug Carboplatin with Sulfur Amino Acids. Inorg. Chem. 1996;35:1065–1072. doi: 10.1021/ic950973d. [DOI] [PubMed] [Google Scholar]
- 6.Hah SS, Stivers KM, de Vere White RW, Henderson PT. Kinetics of carboplatin-DNA binding in genomic DNA and bladder cancer cells as determined by accelerator mass spectrometry. Chem. Res. Toxicol. 2006;19:622–626. doi: 10.1021/tx060058c. [DOI] [PubMed] [Google Scholar]
- 7.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 8.Ma Z, Choudhury JR, Wright MW, Day CS, Saluta G, Kucera GL, Bierbach U. A Non-Cross-Linking Platinum-Acridine Agent with Potent Activity in Non-Small-Cell Lung Cancer. J. Med. Chem. 2008;51:7574–7580. doi: 10.1021/jm800900g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins ET, Baruah H, Kramarczyk J, Saluta G, Day CS, Kucera GL, Bierbach U. Design, synthesis, and biological activity of a novel non-cisplatin-type platinum-acridine pharmacophore. J. Med. Chem. 2001;44:4492–4496. doi: 10.1021/jm010293m. [DOI] [PubMed] [Google Scholar]
- 10.Guddneppanavar R, Bierbach U. Adenine-N3 in the DNA minor groove - an emerging target for platinum containing anticancer pharmacophores. Anticancer Agents Med. Chem. 2007;7:125–138. doi: 10.2174/187152007779313991. [DOI] [PubMed] [Google Scholar]
- 11.Guddneppanavar R, Wright MW, Tomsey AK, Bierbach U. Guanine binding of a cytotoxic platinum-acridin-9-ylthiourea conjugate monitored by 1-D 1H and 2-D [1H-15N] NMR spectroscopy: Hydrolysis is not the rate-determining step. J. Inorg. Biochem. 2006;100:972–979. doi: 10.1016/j.jinorgbio.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Budiman ME, Alexander RW, Bierbach U. Unique base-step recognition by a platinum-acridinylthiourea conjugate leads to a DNA damage profile complementary to that of the anticancer drug cisplatin. Biochemistry. 2004;43:8560–8567. doi: 10.1021/bi049415d. [DOI] [PubMed] [Google Scholar]
- 13.Baruah H, Day CS, Wright MW, Bierbach U. Metal-intercalator-mediated self-association and one-dimensional aggregation in the structure of the excised major DNA adduct of a platinum-acridine agent. J. Am. Chem. Soc. 2004;126:4492–4493. doi: 10.1021/ja038592j. [DOI] [PubMed] [Google Scholar]
- 14.Bonivento M, Canovese L, Cattalini L, Marangoni G, Michelson G, Tobe ML. Cis effect of dimethylsulfide and leaving group effect in reactions of the cationic complex chloro(dimethylsulfide)(1,2-diaminoethane)platinum(II) chloride. Inorg. Chem. 1981;20:3728–3730. [Google Scholar]
- 15.Bonivento M, Cattalini L, Marangoni G, Michelson G, Schwab AP, Tobe ML. The cis effect of dimethyl sulfoxide in the reactions of the cationic complex chloro(dimethyl sulfoxide)(1,2-diaminoethane)platinum(II) chloride. Inorg. Chem. 1980;19:1743–1746. [Google Scholar]
- 16.Ackley MC, Barry CG, Mounce AM, Farmer MC, Springer BE, Day CS, Wright MW, Berners-Price SJ, Hess SM, Bierbach U. Structure-activity relationships in platinum-acridinylthiourea conjugates: effect of the thiourea nonleaving group on drug stability, nucleobase affinity, and in vitro cytotoxicity. J. Biol. Inorg. Chem. 2004;9:453–461. doi: 10.1007/s00775-004-0541-4. [DOI] [PubMed] [Google Scholar]
- 17.Guddneppanavar R, Choudhury JR, Kheradi AR, Steen BD, Saluta G, Kucera GL, Day CS, Bierbach U. Effect of the diamine nonleaving group in platinum-acridinylthiourea conjugates on DNA damage and cytotoxicity. J. Med. Chem. 2007;50:2259–2263. doi: 10.1021/jm0614376. [DOI] [PubMed] [Google Scholar]
- 18.Choudhury JR, Bierbach U. Characterization of the bisintercalative DNA binding mode of a bifunctional platinum-acridine agent. Nucleic Acids Res. 2005;33:5622–5632. doi: 10.1093/nar/gki869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choudhury JR, Guddneppanavar R, Saluta G, Kucera GL, Bierbach U. Tuning the DNA conformational perturbations induced by cytotoxic platinum-acridine bisintercalators: effect of metal cis/trans isomerism and DNA threading groups. J. Med. Chem. 2008;51:3069–3072. doi: 10.1021/jm8003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




