Abstract
Rearing rats in isolation has been shown to produce behavioral and neurochemical alterations similar to those observed in psychoses such as schizophrenia. Also, a dysregulation in both the endocannabinoid and dopaminergic systems has been implicated in schizophrenia. The aim of this study was to determine if there are differences in CB1 receptor and fatty acid amide hydrolase (FAAH) protein expression, as well as D2 dopamine receptor expression in different brain regions in rats reared in different environmental conditions. Twenty-one day old male Sprague-Dawley rats were either reared in individual cages (isolated rats) or in group cages of 6 per cage (group housed rats) for 8 weeks. Quantitative fluorescence immunohistochemistry was performed on brain slices using antibodies specific to the CB1 or D2 receptor, or the enzyme FAAH. Raising rats in isolation led to a significant decrease in CB1 receptor expression in the caudate putamen and the amygdala, a significant increase in FAAH expression in the caudate putamen and the nucleus accumbens core and shell, and no significant change in D2 receptor expression in any region studied. These results indicate that the endocannabinoid system is altered in an animal model of aspects of psychosis. This implies that rearing rats under different housing conditions may provide new insight into the role of the endocannabinoid system in the development of psychoses.
Keywords: Cannabinoid, social isolation, dopamine, fatty acid amide hydrolase, schizophrenia
Introduction
Rearing rats in isolation for eight weeks following weaning has long term effects that are thought to arise from the lack of sensory information and input from social contact that is important for the normal development of brain structure and neurotransmitter function (Muchimapura et al., 2002). Isolation rearing produces a number of post-pubertal behavioral and neurochemical changes, some of which are similar to those observed in psychoses such as schizophrenia (Geyer et al., 1993; Muchimapura et al., 2003; Cilia et al., 2005). The most notable behavioral change induced by isolation rearing is the disruption in prepulse inhibition (PPI) which is indicative of deficits in sensorimotor gating. PPI is also disrupted in medicated but still symptomatic schizophrenia patients (Braff et al., 1992; Bolino et al., 1994) and in non-medicated schizotypal patients (Cadenhead et al., 1993). As opposed to social isolation following weaning, isolation of adult rats does not result in disruptions in PPI and predictive validity of the social isolation model has been shown as disruptions in PPI induced by isolation rearing can be reversed with antipsychotics (Geyer et al., 1993; Varty and Higgins, 1995). Thus, the social isolation paradigm has been suggested to be an animal model of aspects of psychosis (Geyer et al., 1993; Lapiz et al., 2003; Muchimapura et al., 2003; Roberts and Greene, 2003; Harte et al., 2004).
A conserved endocannabinoid system has been identified in mammals and includes at least two G-protein coupled receptors (For review see (Petrocellis et al., 2004). The CB1 receptor subtype is widely expressed, with particularly high levels in the hippocampus, cerebellum and basal ganglia, and mediates most CNS effects of cannabinoids (Herkenham et al., 1990). The high densities found in association with limbic cortices suggests an important role of CB1 receptors in motivational and cognitive information processing (Howlett et al., 2004). A number of endogenous cannabinoid ligands (endocannabinoids) have been identified, such as the arachidonic acid derivatives anandamide (AEA) and 2-arachidonylglycerol (2-AG). The highest concentrations of AEA can be found in the hippocampus, cortex, thalamus and cerebellum (Porter and Felder, 2001). Fatty acid amide hydrolase (FAAH) is one of the enzymes involved in endocannabinoid catabolism (Cravatt et al., 1996). The importance of FAAH in AEA metabolism is demonstrated by the observation that genetic deletion of this enzyme in mice results in 10 to 15 fold elevations in brain AEA (Cravatt et al., 2001). FAAH activity in the rat brain is highest in the cerebellum, hippocampus and neocortex (Egertova et al., 1998).
Several lines of evidence support an association between an altered endocannabinoid system and the pathogenesis of psychoses such as schizophrenia. Cannabis use can exacerbate psychosis or precipitate schizophrenia in vulnerable individuals (Hambrecht and Hafner, 2000; van Os et al., 2002; Hall et al., 2004; Henquet et al., 2005) and synthetic CB1 receptor agonists such as CP 55940 (Mansbach et al., 1996; Martin et al., 2003) and WIN 55212-2 (Schneider and Koch, 2002) have been reported to disrupt sensorimotor gating in rats. However, other studies have not shown cannabinoid–induced disruption of sensorimotor gating (Stanley-Cary et al., 2002; Bortolato et al., 2005). Also, increases in CB1 receptor density in frontal cortex subregions have been identified in post-mortem schizophrenic brains (Dean et al., 2001; Zavitsanou et al., 2004), and elevated levels of AEA were detected in cerebrospinal fluid (Leweke et al., 1999; Giuffrida et al., 2004; Leweke et al., 2007) and blood (De Marchi et al., 2003) of anti-psychotic naïve schizophrenics.
Previous studies involving the dopaminergic system have suggested that D2 receptors are altered in the striatum (Guisado et al., 1980) and nucleus accumbens (Hall, 1998) of socially isolated rats compared with group housed rats. Perhaps linking D2 receptors and the endocannabinoid system is the observation that D2 receptors and CB1 receptors heterodimerize (Kearn et al., 2005) creating a distinct signal transduction repertoire. Moreover, it has been clearly demonstrated that exogenous and endogenous cannabinoids influence dopamine neurotransmission (for review see (Gardner, 2005).
We have previously demonstrated that socially isolated rats are more susceptible to disruptions in sensorimotor gating produced by Δ9-tetrahydrocannabinol (Δ9-THC), the major psychoactive constituent of Cannabis sativa (Malone and Taylor, 2006). The primary aim of this study was to investigate the relative expression of CB1 receptor and FAAH protein in a number of brain regions in group housed versus socially isolated rats. Due to the reported involvement of changes in the dopaminergic system in isolated vs group housed rats, and given the ability of the endocannabinoid system to modulate the dopaminergic system and vice versa, the relative expression of dopamine D2 receptors was also investigated in the same rats.
Experimental Procedures
Animals and Housing
Male Sprague-Dawley rats (bred at the animal facilities of the Victorian College of Pharmacy, Monash University, Parkville) were obtained at weaning (21 days postnatal) and randomly housed either individually (isolated rats) or in groups of 6 (group housed rats) for 8 weeks in opaque, high top plastic cages lined with animal bedding. The cage size for isolated rats was 360 cm × 230 cm × 190 cm (length × width × height) and 540 cm x 360 cm × 190 cm for group housed rats. Both group housed and isolated rats were kept on a reversed lighting 12 h light/dark cycle (lights on at 1900, off at 0700) and maintained at a temperature of 22°C. The reverse lighting conditions were used for previous experiments where behavioral studies were performed (Malone and Taylor, 2006). All rats were housed in the same holding room so that socially isolated rats maintained visual, auditory and olfactory contact with other animals but did not have any physical contact. Animals were handled once a week for cleaning purposes. Food and drinking water was available ad libitum.
All experimental procedures were approved by the Victorian College of Pharmacy, Monash University Animal Ethics Committee. All rats received humane care in accordance to the guidelines set out by the National Health and Medical Research Council, as well as to the legal requirements in Australia.
Immunohistochemistry
To identify the relative expression of CB1 receptors, D2 receptors and FAAH in group housed and isolated rats, 7 animals from each group were heavily anaesthetised with pentobarbitone (0.3 mL of 325 mg/mL; > 90.0 mg/rat) and transcardially perfused with 200 mL phosphate buffered saline, pH 7.4 (PBS), followed by 200 mL of a fixative composed of 4% v/v formaldehyde in PBS. Rats were decapitated using a guillotine and whole brains were removed and postfixed in 4% v/v formaldehyde and 10% w/v sucrose in PBS at 4°C overnight. The following day brains were transferred to a solution containing 4% v/v formaldehyde and 30% w/v sucrose in PBS at 4°C for 48 h. Brains were then frozen using solid carbon dioxide (dry ice, −70°C) and stored in a −80°C freezer. Coronal rat brain sections were examined to find those specimens that most closely matched the bregma level identified in the Rat Brain Atlas of Paxinos and Watson (1998). 40 μm thick sections containing either the prefrontal cortex (at approximately 4.7 mm rostral to bregma), the nucleus accumbens core and shell, the cingulate cortex, the caudate putamen (at approximately 1.6 mm rostral to bregma), the hippocampus (containing specific hippocampal regions CA1, CA2 and CA3), the amygdala (at approximately 3.3 mm caudal to bregma), the substantia nigra (at approximately 4.8 mm caudal to bregma) or the brainstem (at approximately 12.72 mm caudal to bregma) were cut on a cryostat (CM 1850, Leica, Gladesville, NSW, Australia) and placed in PBS until processed for immunohistochemistry.
All immunostaining procedures were performed on free-floating sections. For the double labelling of: 1. CB1 receptors and D2 receptors, and 2. CB1 receptors and FAAH, rat brain sections were rinsed 4 times in PBS for 5 min each and blocked in PBS containing 5% v/v normal donkey serum (Chemicon International, Temecula, CA, USA) and 0.1% v/v Triton X-100 for 1 to 2 h at room temperature. To allow for concurrent evaluation of two antigens, sections were incubated overnight at 4°C in a cocktail of primary antibodies to which 2.5% v/v normal donkey serum and 0.1% v/v Triton X-100 in PBS were added. Either a combination of: 1. Goat anti-CB1-CT raised against residues 401–473 of the rat CB1 receptor (1:2500 dilution) and rabbit anti-D2 antibody raised against residues 216–311 of the human D2 receptor (1:1500 dilution) or 2. Goat anti-CB1-CT (1:2500 dilution) and rabbit anti-FAAH raised against the last 102 amino acid residues of rat FAAH (1:5000 dilution) was employed. The goat anti-CB1 receptor has been characterized previously and is specific in adsorption controls using the antigenic peptide sequence (Harkany et al., 2003).
The affinity purified rabbit anti-D2 antibody was raised in the laboratory of authors CK and KM at the University of Washington against previously identified epitopes of the D2 receptor (McVittie et al., 1991; Boundy et al., 1993b; Boundy et al., 1993a; Chazot et al., 1993). This antibody has been shown to be specific by positive immunolabeling in human embryonic kidney (HEK) cells transiently transfected with the pcDNA-FLAG-D2L plasmid (Kearn et al., 2005). In addition, Western blots and adsorption controls have been performed in rat brain membranes and brain slices to further establish the specificity of the D2 antiserum (Pickel et al., 2006).
The rabbit anti-FAAH antibody was generated against a glutathione-S-transferase fusion protein by inserting the coding sequence for the terminal 102 amino acids of rat FAAH into a pGEX-3X vector by standard methods. The antibody was affinity purified and recognized recombinant FAAH expressed in HEK293 cells, but exhibited no detectable immunoreactivity to wild-type cells and recognized a single band by Western immunoblot (data not shown). Additionally, Helliwell et al. (2004) have demonstrated the utility of this antibody for immunocytochemistry (Harkany et al., 2003; Helliwell et al., 2004). All three antibodies were generated in the laboratory of authors CK and KM at the University of Washington.
Sections were extensively rinsed in PBS containing 0.05% v/v Tween 20 (four washes of 15 min each) and then incubated with a mixture of Alexa Fluor® 680 conjugated donkey anti-goat IgG (H+L) (1:5000 dilution; Molecular Probes, Eugene, OR, USA) and IRDye® 800CW conjugated donkey anti-rabbit IgG (H+L) (1:1500 dilution; Rockland Immunochemicals, Gilbertsville, PA, USA) which was diluted in PBS containing 2.5% v/v normal donkey serum and 0.1% v/v Triton X-100 for 2 h at room temperature. Following incubation, sections were then washed four times for 15 min each with PBS containing 0.05% v/v Tween 20 and were finally rinsed in PBS and water, respectively (four times of 5 min each), and mounted onto fluorescence-free glass slides and air dried. The fluorescent immunocomplexes were then detected using a LI-COR Odyssey Imaging® System (21 μm resolution, 1 mm offset at the highest quality setting). Channel sensitivity was optimized for each set of stained sections then maintained for that group of samples. Typical sensitivity settings ranged from 1.5 to 3.0.
The relative location of the brain tissue slice and identification of brain regions were determined with the aid of a Rat Brain Atlas (Paxinos and Watson, 1998). Integrated intensities of CB1 receptor, D2 receptor and FAAH immunoreactivity were determined with the associated Odyssey software. Integrated intensity values measured from the external capsule of each slice were used to account for background staining of brain regions of interest from all slices except for slices containing the prefrontal cortex and brainstem. Results are expressed as integrated intensity counts per mm2 and are presented as mean ± standard error of the mean (SEM; n = 7 per group). Differences were determined by an analysis of variance for the integrated intensity of each protein investigated (CB1 receptor, D2 receptor and FAAH) with housing condition as the between subjects factor. Differences were considered significant when P values were less than 0.05. Statistical analyses were performed with GraphPad Prism version 4.0.
Results
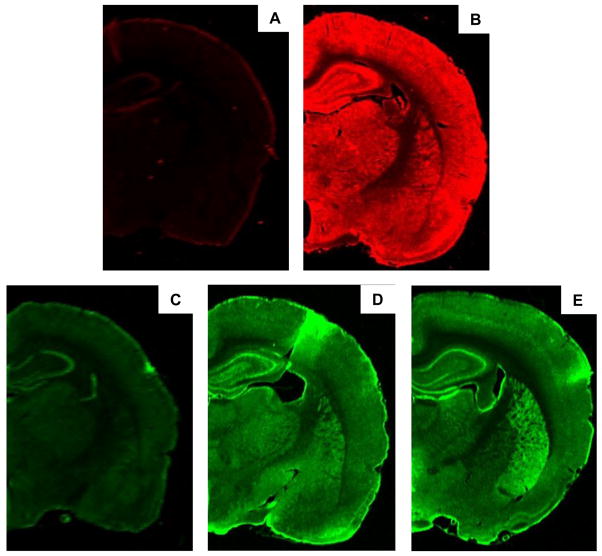
The binding of the Alexa Fluor® 680 conjugated donkey anti-goat IgG and IRDye® 800CW conjugated donkey anti-rabbit IgG was specific for the primary antibodies used as incubation of these secondary antibodies with no primary antibodies failed to produce any significant fluorescence (Figure 1A and C). In group housed rats, CB1, D2 and FAAH expression was observed in all brain regions investigated with the exception of the brainstem.
Figure 1.
Representative images showing brain slices approximately 3.3 mm caudal to bregma from group housed rats incubated with A) Alexa Fluor® 680 conjugated donkey anti-goat IgG B) Anti-CB1-CT raised in goat and Alexa Fluor® 680 anti-goat antibody, C) IRDye® 800CW conjugated donkey anti-rabbit IgG, D) anti-FAAH raised in rabbit and IRDye® 800CW conjugated donkey anti-rabbit IgG and E) anti-D2 raised in rabbit and IRDye® 800CW conjugated donkey anti-rabbit IgG.
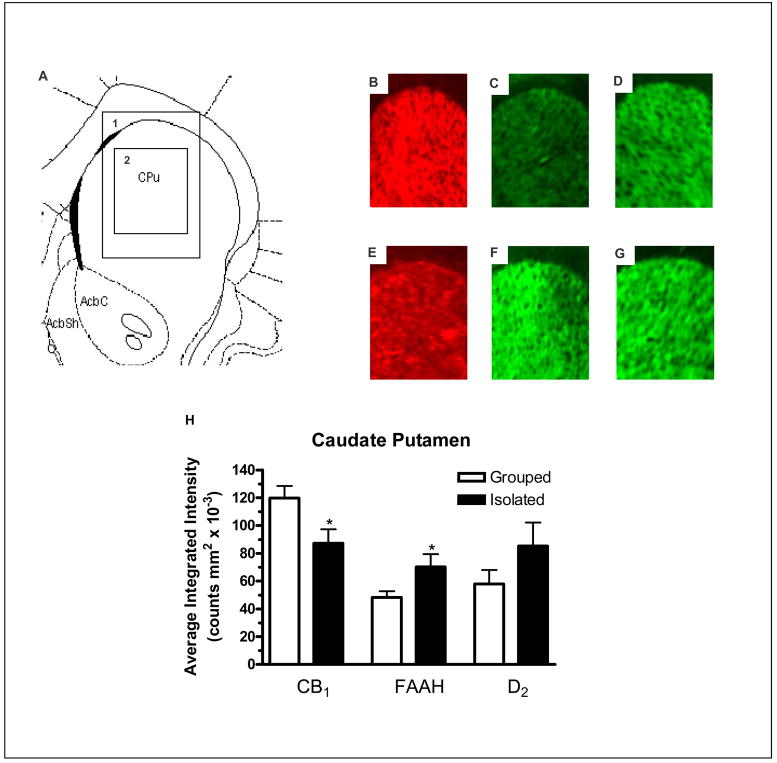
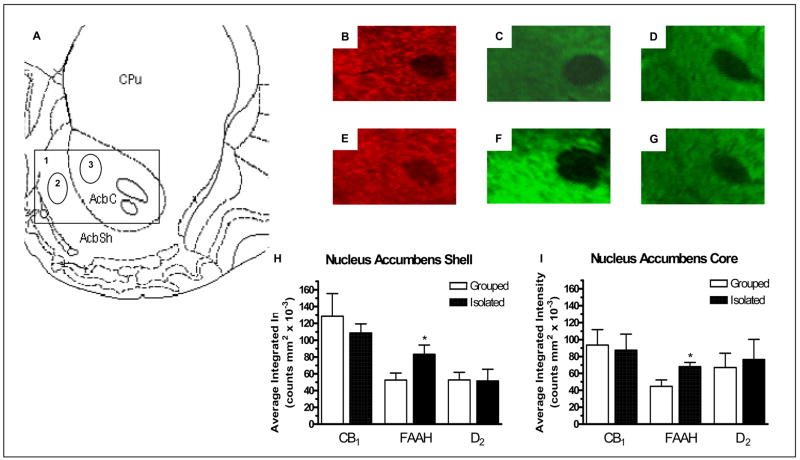
In the caudate putamen there was a significant reduction in CB1 receptor expression (F1,13 = 5.883, P < 0.05; Figure 2B and E), a significant increase in expression of FAAH protein (F1,13 = 4.813, P < 0.05; Figure 2C and F) and no significant effect on D2 receptor expression (Figure 2D and G) in socially isolated rats when compared with group housed rats (Figure 2H). In both the nucleus accumbens core (F1,13 = 6.195, P < 0.05) and shell (F1,13 = 5.198, P < 0.05) there was a significant increase in FAAH expression (Figure 3C and F) and no change in CB1 (Figure 3B and E) or D2 (Figure 3D and G) receptor expression in socially isolated rats when compared with group housed rats (Figure 3H and I).
Figure 2.
A) Modified image from Paxinos and Watson (1998) 1.6 mm rostral to bregma showing: 1) The area depicted in images B–G and 2) The area measured for intensity of staining for the caudate putamen. Image B, C and D are representative images from group housed rats showing: B) CB1 expression, C) FAAH expression and D) D2 expression. Image E, F and G are representative images from socially-isolated rats showing: E) CB1 expression, F) FAAH expression and G) D2 expression and H) Effect of social isolation on CB1, FAAH and D2 relative fluorescence density of staining in the caudate putamen compared with group housed rats. * denotes P < 0.05 when compared with group housed rats (n = 7).
Figure 3.
A) Modified image from Paxinos and Watson (1998) 1.6 mm rostral to bregma showing 1) The area depicted in images B–G, 2) The area measured for intensity of staining for the nucleus accumbens shell and 3) The area measured for intensity of staining for the nucleus accumbens core. Image B, C and D are representative images from group housed ratsshowing: B) CB1 expression, C) FAAH expression and D) D2 expression. Image E, F and G are representative images from socially-isolated rats showing: E) CB1 expression, F) FAAH expression and G) D2 expression. Image H and I show the effect of social isolation on CB1, FAAH and D2 relative fluorescence density of staining in the nucleus accumbens shell and core respectively, compared with group housed rats. * denotes P < 0.05 when compared with group housed rats (n = 7).
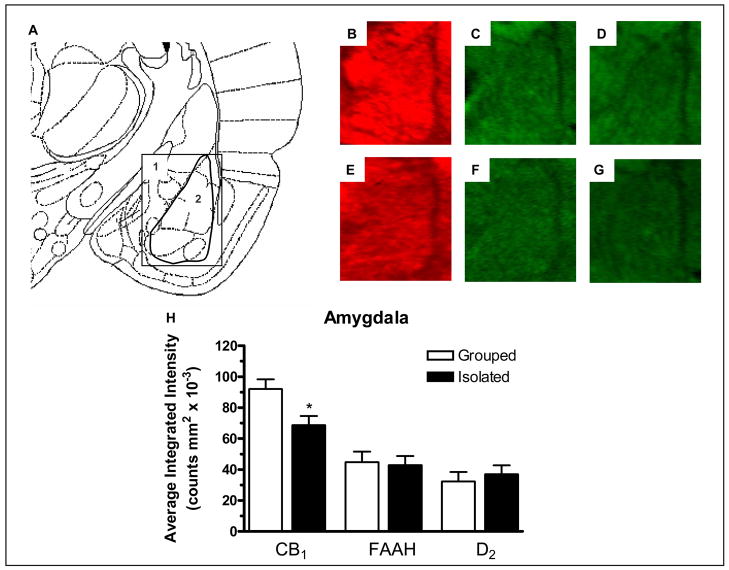
There was a significant decrease in CB1 receptor expression in the amygdala (F1,13 = 7.211, P < 0.05) of socially isolated rats when compared with group housed rats (Figure 4B and E). No significant difference in FAAH (Figure 4C and F) or D2 receptor (Figure 4D and G) expression was detected in the amygdala of socially isolated rats compared with group housed rats (Figure 4H).
Figure 4.
A) Modified image from Paxinos and Watson (1998) 3.3 mm caudal to bregma showing 1) The area depicted in images B–G, 2) The area measured for intensity of staining for the amygdala. Image B, C and D are representative images from group housed rats showing: B) CB1 expression, C) FAAH expression and D) D2 expression. Image E, F and G are representative images from socially-isolated rats showing: E) CB1 expression, F) FAAH expression and G) D2 expression and H) Effect of social isolation on CB1, FAAH and D2 relative fluorescence density of staining in the amygdala compared with group housed rats. * denotes P < 0.05 when compared with group housed rats (n = 7).
The expression of CB1 receptors, D2 receptors or FAAH in the other brain regions evaluated, the prefrontal cortex, hippocampus (or specific hippocampal regions CA1, CA2 and CA3), Cg1, Cg2 and substantia nigra, were not significantly different in socially isolated rats when compared with group housed rats (data not shown).
Discussion
The present study investigated the effect of social isolation on CB1 and D2 receptor expression as well as FAAH protein expression in specific brain regions implicated in sensorimotor gating and psychoses. The immunohistochemistry approach was chosen because of the ability to evaluate the concurrent expression of CB1 and D2 receptors, as well as the metabolic enzyme FAAH in specific brain regions using thoroughly characterized antibodies. Relative expression of these proteins in brain regions from socially isolated compared with group housed rats was able to be performed as brain slices from the differently housed rats were processed simultaneously.
Schizophrenia is thought to be at least in part a developmental disorder (Weinberger, 1995). Isolation rearing of weaned rats for eight weeks produces behavioral and neurochemical changes similar to those seen in schizophrenia and has therefore been suggested as an animal model of aspects of psychosis (Geyer et al., 2001; Lapiz et al., 2003; Leng et al., 2004). However, the reductions found in CB1 receptor expression in several brain regions, and the no change in CB1 receptor expression in the prefrontal cortex and the cingulate cortex are in contrast to previous human studies. Using [3H]CP-55940 binding, a lack of change in the caudate putamen and an increase in CB1 receptor expression was reported in the prefrontal cortex of schizophrenic patients when compared with control subjects (Dean et al., 2001). Another study reported an increase in CB1 receptors in the cingulate cortex of humans with schizophrenia (Zavitsanou et al., 2004). This may reflect the fact that it is not possible to model all aspects of psychosis in animals due to the subjective nature of the disease. Also, it has long been known that there are major differences in cortical areas between primates and rats (Kolb, 1984). Such differences between primate and rat cortices include afferent neurons from the nucleus medialis dorsalis to regions such as the cingulate cortex and prefrontal cortex and cytoarchitectural differences in cortical regions (Kolb, 1984).
Previous studies have found increased levels of endocannabinoids in blood and CSF of patients with schizophrenia (Leweke et al., 1999; De Marchi et al., 2003; Giuffrida et al., 2004; Leweke et al., 2007). A possible mechanism leading to the reduced expression of the CB1 receptors in socially isolated rats is receptor down-regulation in response to an increase in endocannabinoid levels (e.g., AEA). Supporting this hypothesis is the reciprocal increase in FAAH and decrease CB1 receptor expression observed in the caudate putamen of socially isolated rats. However, a decrease in CB1 receptor expression was not always accompanied by an increase in FAAH expression. For example, in the nucleus accumbens core and shell, an increase in FAAH expression and no change in CB1 receptor expression was observed in socially isolated rats compared with group housed rats, while in the amygdala, a decrease in CB1 receptor expression was observed in isolated rats compared with group housed rats, with no change in FAAH expression. While it is clear that FAAH is the primary enzyme responsible for degrading AEA, other enzymes degrade 2-AG in vivo (Cravatt et al., 2001), FAAH degrades other fatty acid ethanolamides, including lipids with no activity at cannabinoid receptors such as oleamide (Cravatt et al., 1995). Also, FAAH is expressed in some regions of the brain that are largely devoid of CB1 receptors such as the thalamus (Thomas et al., 1997; Tsou et al., 1998). For these reasons, obligate reciprocal changes may not be expected.
The results of this study show that socially isolated rats had reduced levels of CB1 receptor expression in brain regions such as the caudate putamen and the amygdala compared with group housed rats. However, we have previously shown that socially isolated rats are more sensitive to the sensorimotor gating disruptive effects of the CB1 receptor agonist Δ9-THC (Malone and Taylor, 2006). It may be expected that if an increased effect occurred due to CB1 receptor activation in isolated rats, then one may expect this to be the result of an increase in CB1 receptor expression in socially isolated rats compared with group housed rats. Whilst multiple explanations may account for this apparent paradox, two prominent findings are evident. Breivogel et al. (1997) have demonstrated that the efficiency of cannabinoid receptors, in terms of capability to activate GTP-binding proteins, is higher in the hypothalamus (an area of relatively low CB1 receptor expression) as compared with other regions (Breivogel et al., 1997). It is possible that areas of lower CB1 receptor expression in isolated rats compared with group housed rats have higher capability to activate G proteins, thus are more efficient in terms of activating intracellular second messenger systems. This may account for the increased sensitivity of Δ9-THC in disrupting sensorimotor gating in isolated rats compared with group housed rats (Malone and Taylor, 2006). Alternatively, it has been hypothesised that since Δ9-THC is a partial agonist at CB1 receptors, some effects of Δ9-THC may be mediated by inhibiting endocannabinoid signalling (particularly 2-AG) (Straiker and Mackie, 2005), suggesting that the mechanism of action of Δ9-THC is more complex than merely acting as an agonist at CB1 receptors.
Rats reared in social isolation showed a significant increase in FAAH expression in the caudate putamen and nucleus accumbens (core and shell). It has been previously reported that CSF levels of AEA are markedly elevated in schizophrenics (Giuffrida et al., 2004). In addition, De Marchi et al. (2003) found that AEA levels and FAAH mRNA transcripts were higher in blood samples taken from patients during an acute schizophrenic episode compared to the same patients in clinical remission (De Marchi et al., 2003). It is possible that this increase in FAAH expression is due to an increase in endocannabinoid activity and thus a compensatory increase in FAAH in these regions has ensued to metabolise endocannabinoids. However, whether endocannabinoid levels are altered in rats as a result of social isolation remains an open question. Interestingly, we (Malone et al., 2004) and others (Ballmaier et al., 2007) have shown that PPI gating deficits induced by drugs such as apomorphine can be reversed by pretreatment with the CB1 receptor antagonist SR 141716 (rimonabant), implying that the endocannabinoid system has a role in modulating sensorimotor gating processes.
Rats reared in social isolation had no significant difference in D2 receptor immunoreactivity when compared with group housed rats in any region studied. Previous results investigating D2 receptor expression in socially isolated rats have been conflicting, with an increase (Djouma et al., 2006), a decrease (Jones et al., 1992) or no effect (Del Arco et al., 2004) on D2 receptor expression observed in various brain regions compared to group housed rats. This may be due in part to the use of different strains of rat in social isolation experiments including Lister hooded (Jones et al. 1992), Fawn hooded (Djouma et al. 2006), Wistar (Guisado et al. 1980) and Sprague-Dawley (Del Arco et al. 2004). Similarly, differences in the affinity state of the D2 receptor have been shown using [35S]GTPγS binding in different strains of rats, thus making a comparison between radioligand binding and immunohistochemistry studies problematic (Swerdlow et al., 2006). In the same strain of rats (Sprague Dawley) used in the present study, it has been reported that social isolation did not alter [3H]-raclopride binding to D2 receptors in the dorsal or ventral striatum (Del Arco et al., 2004). These results are in agreement with the findings of the current study that social isolation did not alter D2 receptor expression in these brain regions.
Conclusions
The present study provides the first evidence that social isolation alters CB1 receptor and FAAH expression, hence suggesting that isolation rearing affects the endocannabinoid system. The lack of changes in D2 receptor expression is in agreement with some previous studies, supporting the notion that this aspect of the dopaminergic system is unaffected in socially isolated rats. As isolation rearing is thought to represent an animal model of aspects of psychosis, these results imply there is a dysregulation in the endocannabinoid system of schizophrenics. This alteration of the endocannabinoid system of rats reared in different housing conditions (i.e. isolated vs. group housed rats), provides new insight into the possible role of the endocannabinoid system in schizophrenic individuals.
Acknowledgments
The authors wish to thank Jim Rabba for his assistance with some of the experiments and Dr Helen Irving and Dr Virginia Pickel for their critical reviews of the manuscript. These studies were supported in part by grants DA11322 and DA015916 to KM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballmaier M, Bortolato M, Rizzetti C, Zoli M, Gessa G, Heinz A, Spano P. Cannabinoid Receptor Antagonists Counteract Sensorimotor Gating Deficits in the Phencyclidine Model of Psychosis. Neuropsychopharmacology. 2007;32:2098–2107. doi: 10.1038/sj.npp.1301344. [DOI] [PubMed] [Google Scholar]
- Bolino F, Di Michele V, Di Cicco L, Manna V, Daneluzzo E, Casacchia M. Sensorimotor gating and habituation evoked by electro-cutaneous stimulation in schizophrenia. Biol Psychiatry. 1994;36:670–679. doi: 10.1016/0006-3223(94)91176-2. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Aru GN, Frau R, Orru M, Luckey GC, Boi G, Gessa GL. The CB receptor agonist WIN 55,212–2 fails to elicit disruption of prepulse inhibition of the startle in Sprague-Dawley rats. Psychopharmacology. 2005;177:264–271. doi: 10.1007/s00213-004-1941-4. [DOI] [PubMed] [Google Scholar]
- Boundy VA, Luedtke RR, Molinoff PB. Development of polyclonal anti-D2 dopamine receptor antibodies to fusion proteins: inhibition of D2 receptor-G protein interaction. J Neurochem. 1993a;60:2181–2191. doi: 10.1111/j.1471-4159.1993.tb03504.x. [DOI] [PubMed] [Google Scholar]
- Boundy VA, Luedtke RR, Artymyshyn RP, Filtz TM, Molinoff PB. Development of polyclonal anti-D2 dopamine receptor antibodies using sequence-specific peptides. Mol Pharmacol. 1993b;43:666–676. [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Sim LJ, Childers SR. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J Pharmacol Exp Ther. 1997;282:1632–1642. [PubMed] [Google Scholar]
- Cadenhead KS, Geyer MA, Braff DL. Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am J Psychiatry. 1993;150:1862–1867. doi: 10.1176/ajp.150.12.1862. [DOI] [PubMed] [Google Scholar]
- Chazot PL, Doherty AJ, Strange PG. Antisera specific for D2 dopamine receptors. Biochem J. 1993;289 (Pt 3):789–794. doi: 10.1042/bj2890789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia J, Hatcher PD, Reavill C, Jones DNC. Long-term evaluation of isolation-rearing induced prepulse inhibition deficits in rats: an update. Psychopharmacology. 2005;180:57–62. doi: 10.1007/s00213-004-2139-5. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA. Chemical characterization of a family of brain lipids that induce sleep. Science. 1995;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. PNAS. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003;2:5. doi: 10.1186/1476-511X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103:9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Zhu S, Terasmaa A, Mohammed AH, Fuxe K. Hyperactivity to novelty induced by social isolation is not correlated with changes in D2 receptor function and binding in striatum. Psychopharmacology. 2004;171:148–155. doi: 10.1007/s00213-003-1578-8. [DOI] [PubMed] [Google Scholar]
- Djouma E, Card K, Lodge DJ, Lawrence AJ. The CRF receptor antagonist, antalarmin, reverses isolation-induced up-regulation of dopamine D2 receptors in the amygdala and nucleus accumbens of fawn-hooded rats. Eur J Neurosci. 2006;23:3319–3327. doi: 10.1111/j.1460-9568.2006.04864.x. [DOI] [PubMed] [Google Scholar]
- Egertova M, Giang DK, Cravatt BF, Elphick MR. A New Perspective on Cannabinoid Signalling - Complementary Localization of Fatty Acid Amide Hydrolase and the CB1 Receptor in Rat Brain. Proc Royal Soc Lond. 1998;265:2081–2085. doi: 10.1098/rspb.1998.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: Emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry. 1993;34:361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, Klosterkotter J, Piomelli D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- Guisado E, Fernandez-Tome P, Garzon J, Del Rio J. Increased dopamine receptor binding in the striatum of rats after long-term isolation. Eur J Pharmacol. 1980;65:463–464. doi: 10.1016/0014-2999(80)90359-3. [DOI] [PubMed] [Google Scholar]
- Hall FS. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav. 1998;59:859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L, Teesson M. Cannabis use and psychotic disorders: an update. Drug Alcohol Rev. 2004;23:433–443. doi: 10.1080/09595230412331324554. [DOI] [PubMed] [Google Scholar]
- Hambrecht M, Hafner H. Cannabis, vulnerability, and the onset of schizophrenia: an epidemiological perspective. Aust N Z J Psychiatry. 2000;34:468–475. doi: 10.1080/j.1440-1614.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- Harkany T, Hartig W, Berghuis P, Dobszay MB, Zilberter Y, Edwards RH, Mackie K, Ernfors P. Complementary distribution of type 1 cannabinoid receptors and vesicular glutamate transporter 3 in basal forebrain suggests input-specific retrograde signalling by cholinergic neurons. Eur J Neurosci. 2003;18:1979–1992. doi: 10.1046/j.1460-9568.2003.02898.x. [DOI] [PubMed] [Google Scholar]
- Harte MK, Powell SB, Reynolds LM, Swerdlow NR, Geyer MA, Reynolds GP. Reduced n-acetylaspartate in the temporal cortex of rats reared in isolation. Biol Psychiatry. 2004;56:296–299. doi: 10.1016/j.biopsych.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Helliwell RJ, Chamley LW, Blake-Palmer K, Mitchell MD, Wu J, Kearn CS, Glass M. Characterization of the endocannabinoid system in early human pregnancy. J Clin Endocrinol Metab. 2004;89:5168–5174. doi: 10.1210/jc.2004-0388. [DOI] [PubMed] [Google Scholar]
- Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47:345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav. 1992;43:17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent Stimulation of Cannabinoid CB1 and Dopamine D2 Receptors Enhances Heterodimer Formation: A Mechanism for Receptor Cross-Talk? Mol Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res. 1984;320:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol. 2003;33:13–29. doi: 10.1023/a:1021171129766. [DOI] [PubMed] [Google Scholar]
- Leng A, Feldon J, Ferger B. Long-term social isolation and medial prefrontal cortex: dopaminergic and cholinergic neurotransmission. Pharmacol Biochem Behav. 2004;77:371–379. doi: 10.1016/j.pbb.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated endogenous cannabinoids in schizophrenia. NeuroReport. 1999;10:1665–1669. doi: 10.1097/00001756-199906030-00008. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Giuffrida A, Koethe D, Schreiber D, Nolden BM, Kranaster L, Neatby MA, Schneider M, Gerth CW, Hellmich M, Klosterkotter J, Piomelli D. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: Impact of cannabis use. Schizophr Res. 2007;94:29–36. doi: 10.1016/j.schres.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. The effect of Δ9-tetrahydrocannabinol on sensorimotor gating in socially isolated rats. Behav Brain Res. 2006;166:101–109. doi: 10.1016/j.bbr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Malone DT, Long LE, Taylor DA. The effect of SR 141716 and apomorphine on sensorimotor gating in Swiss mice. Pharmacol Biochem Behav. 2004;77:839–845. doi: 10.1016/j.pbb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Rovetti CC, Winston EN, Lowe JA., 3rd Effects of the cannabinoid CB1 receptor antagonist SR141716A on the behavior of pigeons and rats. Psychopharmacology. 1996;124:315–322. doi: 10.1007/BF02247436. [DOI] [PubMed] [Google Scholar]
- Martin R, Secchi R, Sung E, Lemaire M, Bonhaus D, Hedley L, Lowe D. Effects of cannabinoid receptor ligands on psychosis-relevant behavior models in the rat. Psychopharmacology. 2003;165:128–135. doi: 10.1007/s00213-002-1240-x. [DOI] [PubMed] [Google Scholar]
- McVittie LD, Ariano MA, Sibley DR. Characterization of anti-peptide antibodies for the localization of D2 dopamine receptors in rat striatum. Proc Natl Acad Sci U S A. 1991;88:1441–1445. doi: 10.1073/pnas.88.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchimapura S, Mason R, Marsden CA. Effect of isolation rearing on pre- and post-synaptic serotonergic function in the rat dorsal hippocampus. Synapse. 2003;47:209–217. doi: 10.1002/syn.10167. [DOI] [PubMed] [Google Scholar]
- Muchimapura S, Fulford AJ, Mason R, Marsden CA. Isolation rearing in the rat disrupts the hippocampal response to stress. Neuroscience. 2002;112:697–705. doi: 10.1016/s0306-4522(02)00107-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic co-ordinates. 4. New York: Academic press; 1998. [Google Scholar]
- Petrocellis LD, Cascio MG, Marzo VD. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kearn CS, Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J Comp Neurol. 2006;495:299–313. doi: 10.1002/cne.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AC, Felder CC. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol Therapeutics. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Roberts L, Greene JRT. Post-weaning social isolation of rats leads to a diminution of LTP in the CA1 to subiculum pathway. Brain Res. 2003;991:271–273. doi: 10.1016/j.brainres.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. The cannabinoid agonist WIN 55,212–2 reduces sensorimotor gating and recognition memory in rats. Behav Pharmacol. 2002;13:29–37. doi: 10.1097/00008877-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Stanley-Cary CC, Harris C, Martin-Iverson MT. Differing effects of the cannabinoid agonist, CP 55,940, in an alcohol or Tween 80 solvent, on prepulse inhibition of the acoustic startle reflex in the rat. Behav Pharmacol. 2002;13(1):15–28. doi: 10.1097/00008877-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Krupin AS, Bongiovanni MJ, Shoemaker JM, Goins JC, Hammer RP., Jr Heritable differences in the dopaminergic regulation of behavior in rats: relationship to D2-like receptor G-protein function. Neuropsychopharmacology. 2006;31:721–729. doi: 10.1038/sj.npp.1300877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EA, Cravatt BF, Danielson PE, Gilula NB, Sutcliffe JG. Fatty acid amide hydrolase, the degradative enzyme for anandamide and oleamide, has selective distribution in neurons within the rat central nervous system. J Neurosci Res. 1997;50:1047–1052. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1047::AID-JNR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Tsou K, Nogueron MI, Muthian S, Sanudo-Pena MC, Hillard CJ, Deutsch DG, Walker JM. Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci Lett. 1998;254:137–140. doi: 10.1016/s0304-3940(98)00700-9. [DOI] [PubMed] [Google Scholar]
- van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156:319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- Varty GB, Higgins GA. Examination of drug-induced and isolation-induced disruptions of prepulse inhibition as models to screen antipsychotic drugs. Psychopharmacologia. 1995;122:15–26. doi: 10.1007/BF02246437. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. From neuropathology to neurodevelopment. The Lancet. 1995;346:552–557. doi: 10.1016/s0140-6736(95)91386-6. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:355–360. doi: 10.1016/j.pnpbp.2003.11.005. [DOI] [PubMed] [Google Scholar]