Abstract
Language and communication deficits are key diagnostic criteria for autism. However, not all aspects of language are equally affected. Here we present evidence of enhanced performance of a critical aspect of language—word processing—in children with autism. The results have implications for explanatory theories of autism and language, and for the development of therapeutic approaches.
Keywords: Autism, Language, Memory, Sex difference
Introduction
Although deficits of language and communication are defining characteristics of autism, not all aspects of language are equally affected. Whereas impairments are consistently observed in “pragmatics” (how to use language appropriately in social and real-world contexts), “lexical” abilities involving individual words are generally spared (for review, see Walenski et al. 2006). Here we further examine lexical processing, and test a specific hypothesis which predicts that lexical abilities may not only be spared, but could even be enhanced, in autism.
Multiple lines of evidence from healthy and impaired populations, including both adults and children, have linked lexical knowledge to declarative memory and its underlying temporal lobe structures (Friederici 2002; Indefrey and Levelt 2004; Levelt et al. 1999; Levelt 2001; Ullman 2001, 2004; Ullman et al. 1997). In autism, it has been predicted that aspects of declarative memory, in particular lexical and semantic memory (semantic memory is declarative memory for facts), may not only be spared, but perhaps even enhanced (Walenski et al. 2006). This prediction follows from the hypothesized procedural deficit in autism (Mostofsky et al. 2000; Ullman 2004; Walenski et al. 2006), together with independent evidence suggesting that a dysfunction of procedural memory can lead to the enhancement of declarative memory in variety of circumstances (the “see-saw effect”) (Ullman 2004; Walenski et al. 2006).
Previous evidence from autism suggests a sparing of lexical and semantic memory. (Note that although episodic memory, that is declarative memory for personally experienced events, appears to be problematic in autism, this may be explained by the particular dependence of episodic memory on frontal lobe structures, which are implicated in the disorder (Ben Shalom 2003).) Moreover, some evidence suggests that lexical/semantic memory may serve as compensatory mechanisms for aspects of procedural (and perhaps even episodic) memory (Ben Shalom 2003; Mostofsky et al. 2000; Ullman 2004; Walenski et al. 2006), consistent with a relative sparing of lexical and semantic memory. However, to our knowledge no one has specifically tested for, or reported, enhancements in lexical/semantic memory in autism.
To examine the prediction of enhanced lexical and/or semantic processing, we tested subjects with autism on a picture-naming task, in which subjects name pictures of objects. Performance at picture naming depends crucially on lexical/semantic memory, which encodes the arbitrary associations between a word's phonological representation (specifying the sounds to be produced) and its meaning—the concept activated by presentation of the picture (Indefrey and Levelt 2004; Levelt et al. 1999; Levelt 2001).
Few prior studies of lexical processing in autism have examined expressive language (i.e., language production), and only two that we are aware of have reported performance at picture naming tasks. In one of these studies, performance in children with autism was highly correlated with IQ scores, suggesting no particular deficit at the task (Kjelgaard and Tager-Flusberg 2001). However, typically developing control children were not included, so it is not clear whether the performance by the children with autism would be normal, deficient, or possibly even superior, relative to typically developing subjects with similar IQ scores. A second study reported normal performance in three of five adults with autism at the rapid automated naming subtest of the CELF-R (Müller et al. 1999).
While these and other studies suggest that lexical processing may not be specifically impaired in autism, it is possible that enhancements of lexical processing could have been obscured by the inclusion of easier items, such as higher frequency words, thereby leading to ceiling effects. To address this issue, in the current study we examined lexical processing with a picture-naming task that included both higher and lower frequency picture-names.
Methods
Subjects
Subjects were native English-speaking high-functioning boys diagnosed with autism (n = 21) and typically developing control boys (n = 26) and control girls (n = 27). Diagnosis of autism was made according to the autism diagnostic interview-revised (ADI-R) and the autism diagnostic observation schedule-generic (ADOS-G) (Lord et al. 2000, 1994). We focused on boys with autism because autism has a much higher prevalence in boys (Lord and Spence 2006), and because this allowed us to distinguish autism-related and sex-related differences. All subjects had full scale IQ scores greater than 80 (range 81−139; means: autism 106.52; control-boys 116.69; control-girls 115.78; one-way ANOVA F2,71 = 5.25, p = 0.008). Subjects did not differ on age (range 8−14 years; means: autism 10.00; control-boys 10.00; control-girls 9.63; one-way ANOVA F2,71 = 0.51, p = 0.60) or education (range 2−8 years; means: autism 4.89; control-boys 4.54; control-girls 4.37; one-way ANOVA F2,71 = 0.62, p = 0.54). The majority of subjects were right-handed (autism: n = 18 of 21; control-boys: n = 23 of 26; control-girls: n = 23 of 27). Children with identifiable causes of autism (e.g., Fragile X syndrome) were excluded. Control subjects were free of any developmental or psychiatric disorders (Reich et al. 1997).
Materials and Procedure
In the picture-naming task subjects named 96 pictures of objects (including animals, tools, fruits, vegetables, and buildings). Picture names varied in their frequency of occurrence in English (range: 0−6.30), calculated as the natural logarithm of the sum of the raw frequencies from two English-language counts (Church 1988; Francis and Kucera 1982; Ullman 1999). Note that the picture names in our task were all of relatively low-frequency, from the lower half of the frequency range of nouns in English (the full range was 0−11.30 in our counts). Pictures were shown on a computer screen, and remained on-screen for 15 s, or until the subject finished responding, with a 3-s inter-stimulus interval (ISI) between items.
Analysis
First responses to each item were analyzed using multilevel (hierarchical) regression models, with crossed random effects of subject and item. Dependent variables were correct/incorrect (accuracy) and ln-transformed response times (milliseconds) of correct responses. A logit-link function (for binary outcome data) was used for accuracy analyses. Two sets of analyses were carried out. First, we examined group differences in performance (accuracy, response time), with word frequency held constant (covaried out). Second, we examined group differences in the effect of frequency on performance; that is, we treated frequency as a continuous independent variable. Group differences of means (i.e., when holding word frequency constant), and regression-line intercepts and slopes (i.e., when examining effects of frequency) are reported as t-statistics between the appropriate parameters estimated by each model. Two additional potentially confounding subject- and item-related variables (full-scale IQ and item-order) were included as covariates in all analyses. All response-time analyses additionally included a covariate indicating whether the object-name began with a vowel (a word's initial sound can affect computerized response-time measurement) (Kessler et al. 2002). Reported means, intercepts and slopes are adjusted for these covariates.
Nine additional item-related or subject-related variables were examined, but, unlike the included covariates, these did not significantly (ps > 0.1) predict either accuracy or response time (either independently or when included in the regression models), and therefore were not ultimately included as covariates in any analysis: object-name wordlength (syllables); whether or not the name has an initial fricative sound; the manipulability of the depicted object (rated from 1 to 7); subject age, years of education; and handedness (right versus non-right); and whether or not a subject with autism was taking psychiatric medication, had co-morbid obsessive compulsive disorder (OCD), or co-morbid attention deficit hyperactivity disorder (ADHD).
Results and Discussion
Holding frequency constant (covarying it out), the groups did not differ in their mean accuracy (probability of correct response: autism 0.90; control-boys 0.87; control-girls 0.88; autism versus control-boys: t68.6 = 1.08, p = 0.28; autism versus control-girls: t68.3 = 0.73, p = 0.47; control-boys versus control-girls: t68.0 = 0.42, p = 0.68) or response time (ln-transformed milliseconds: autism 7.31; control-boys 7.36; control-girls 7.32; autism versus control-boys: t69.3 = 0.84, p = 0.40; autism versus control-girls: t69.5 = 0.16, p = 0.87; control-boys versus control-girls: t69.3 = 0.77, p = 0.44), consistent with previous findings of spared lexical knowledge in autism (see above).
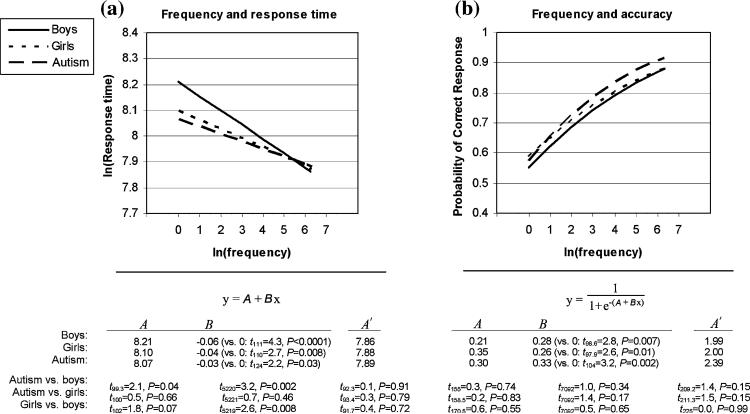
When frequency was examined as a continuous independent variable, all three groups were, as expected, faster and more accurate at higher than lower frequency words (regression-line slopes differed from 0; Fig. 1). No group differences in response time were found for higher-frequency words. However, the groups differed significantly on the lower-frequency words (Fig. 1a). On these items the boys with autism responded faster than control boys, but not faster than control girls, who were themselves faster than the control boys.
Fig. 1.
Regression line equations and group differences of slopes and high- and low-frequency words for (a) response time; (b) accuracy. Regression lines are shown across the range of picture-name frequencies tested, from ln(frequency) = 0 (lowest-frequency; zero-intercept) to ln(frequency) = 6.30 (highest-frequency). In the equations, A corresponds to the zero-intercept and B to the slope of the regression line. Group differences at the high-frequency endpoint (A′) were examined by resetting the intercept to ln(frequency) = 6.30. Significance was assessed with t-statistics, between groups for A, A′ and B, and between B and zero. All p-values are reported two-tailed, with α = 0.05; degrees of freedom are calculated using the Satterthwaite approximation
These frequency-modulated response-time differences cannot be explained by a speed-accuracy tradeoff, as there were no frequency-modulated accuracy differences (Fig. 1b). They are also not explained by a number of potentially confounding item- and subject-related variables such as word-length, object manipulability, item-order, age, education, and IQ, among others (see above). Similarly, evidence suggests that neither the visual processing of the pictures, nor the speed with which articulatory (motor) gestures can be programmed or executed, are able to explain word frequency effects in picture naming tasks (Levelt et al. 1999), and thus these factors are unlikely to explain the pattern observed here.
In contrast, prior evidence suggests that the influence of word frequency on naming speed derives from a stage of lexical processing involving access to the phonological form of the word (Levelt et al. 1999), a stage which furthermore has been localized to temporal lobe regions (Levelt 2001). Thus, the observed pattern of frequency effects—both with respect to the finding that all three groups were faster and more accurate at naming higher than lower frequency words, and with respect to the finding of group differences in response times to lower frequency words—seem likely to derive from accessing lexical representations.
The speeded performance observed for the boys with autism relative to the control boys is thus consistent with the prediction of an enhancement in autism of aspects of declarative memory, and lexical/semantic memory in particular. Moreover, the lack of a difference between the boys with autism and the control girls, and the advantage of control girls as compared to control boys, are consistent with this prediction, since prior evidence suggests a female advantage, relative to males, at lexical and declarative memory (Ullman 2004; Ullman et al. 2007).
It is important to emphasize, however, that the precise source of the enhancement in autism is not yet clear (and may or may not be the same as the cause of improved lexical/declarative memory in typically developing girls). Although it may indeed be explained by the “seesaw” effect, namely that declarative memory is enhanced as a consequence of procedural memory abnormalities (see above), no direct evidence for such an effect has been presented here. Alternatively or additionally, enhanced lexical/semantic memory in autism may reflect the involvement of mechanisms that directly affect aspects of declarative memory. For example, brain-derived neurotrophic factor (BDNF) may play a role. Individuals with autism have been shown to have increased levels of BDNF (for review, see Tsai 2005), which has independently been found to modulate aspects of declarative memory functionality as well as hippocampal activation and grey matter volumes (Egan et al. 2003; Hariri et al. 2003; Pezawas et al. 2004). Moreover, evidence suggests that the female advantage at lexical/declarative memory may depend, at least in part, on BDNF-modulated effects of estrogen (Murphy et al. 1998; Scharfman and MacLusky 2005; Simpkins et al. 1997; Ullman et al. 2007; Woolley 1999). Thus it is plausible that BDNF may (also) play a role in enhanced lexical/semantic memory in autism. However, further research is needed, not only to test the replicability of the findings reported here, but also to identify the exact source of these effects.
To our knowledge, this is the first report of better-than-normal performance at lexical/semantic processing in autism. It demonstrates that even though language deficits are a diagnostic criterion for autism, at least one aspect of language may actually be enhanced. The findings extend to language the view that the “disorder” of autism may constitute not a cluster of deficits, but rather a set of relative strengths and weaknesses across various domains (Frith and Happé 1994). The finding that lexical/semantic memory is a neurocognitive strength in the disorder may also help to explain the high coincidence of savantism and autism, since savants (with talents in both language and non-language domains) also exhibit exceptional memory (Treffert and Christensen 2005). Finally, an enhancement of lexical/semantic memory, and perhaps other aspects of declarative memory as well, may provide important compensatory mechanisms in autism for impaired language and other cognitive functions, which could lead to new therapeutic interventions (Ben Shalom 2003; Ullman 2004; Walenski et al. 2006).
Acknowledgments
Support was provided by the National Institutes of Health, the National Alliance for Autism Research, and the Mabel Flory Trust.
Contributor Information
Matthew Walenski, Brain and Language Laboratory, Department of Neuroscience, Georgetown University, P.O. Box 571464, Washington, DC 20057−1464, USA.
Stewart H. Mostofsky, Kennedy Krieger Institute, Baltimore, MD, USA Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Psychiatry, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Jennifer C. Gidley-Larson, Kennedy Krieger Institute, Baltimore, MD, USA
Michael T. Ullman, Brain and Language Laboratory, Department of Neuroscience, Georgetown University, P.O. Box 571464, Washington, DC 20057−1464, USA
References
- Ben Shalom D. Memory in autism: Review and synthesis. Cortex. 2003;39(4−5):1129–1138. doi: 10.1016/s0010-9452(08)70881-5. [DOI] [PubMed] [Google Scholar]
- Church K. A stochastic parts program and noun phrase parser for unrestricted text.. Paper presented at the Second Conference on Applied Natural Language Processing; Austin, TX. 1988. [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Francis N, Kucera H. Frequency analysis of English usage: Lexicon and grammar. Houghton Mifflin; Boston, MA: 1982. [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends in Cognitive Sciences. 2002;6(2):78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Frith U, Happé F. Autism: Beyond “theory of mind”. Cognition. 1994;50:115–132. doi: 10.1016/0010-0277(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. Journal of Neuroscience. 2003;23(17):6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1−2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Kessler B, Treiman R, Mullennix J. Phonetic biases in voice key response time measurements. Journal of Memory and Language. 2002;47:145–171. [Google Scholar]
- Kjelgaard MM, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic sub-groups. Language and Cognitive Processes. 2001;16(2):287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJ, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral and Brain Sciences. 1999;22(1):1–38. doi: 10.1017/s0140525x99001776. discussion 38−75. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. Spoken word production: A theory of lexical access. Proceedings of the National Academy of Science. 2001;98(23):13464–13471. doi: 10.1073/pnas.231459498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Spence SJ. Autism spectrum disorders: Phenotype and diagnosis. In: Moldin SO, Rubenstein JLR, editors. Understanding autism: From basic neuroscience to treatment. CRC Press; Boca Raton, FL: 2006. pp. 1–23. [Google Scholar]
- Mostofsky SH, Goldberg MC, Landa RJ, Denckla MB. Evidence for a deficit in procedural learning in children and adolescents with autism: Implications for cerebellar contribution. Journal of the International Neuropsychological Society. 2000;6(7):752–759. doi: 10.1017/s1355617700677020. [DOI] [PubMed] [Google Scholar]
- Müller RA, Behen ME, Rothermel RD, Chugani DC, Muzik O, Mangner TJ, et al. Brain mapping of language and auditory perception in high-functioning autistic adults: A PET study. Journal of Autism and Developmental Disorders. 1999;29(1):19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proceedings of the National Academy of Sciences, USA. 1998;95:11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. Journal of Neuroscience. 2004;24(45):10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. Diagnostic interview for children and adolescents-IV (DICA-IV) Multi-Health Systems; Toronto: 1997. [Google Scholar]
- Scharfman HE, MacLusky NJ. Similarities between actions of estrogen and BDNF in the hippocampus: Coincidence or clue? Trends in Neuroscience. 2005;28(2):79–85. doi: 10.1016/j.tins.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer's disease. American Journal of Medicine. 1997;103(3A):19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- Treffert DA, Christensen DD. Inside the mind of a savant. Scientific American. 2005;293(6):108–113. doi: 10.1038/scientificamerican1205-108. [DOI] [PubMed] [Google Scholar]
- Tsai S-J. Is autism caused by early hyperactivity of brain-derived neurotrophic factor? Medical Hypotheses. 2005;65(1):79–82. doi: 10.1016/j.mehy.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Ullman MT. Acceptability ratings of regular and irregular past tense forms: Evidence for a dual-system model of language from word frequency and phonological neighbourhood effects. Language and Cognitive Processes. 1999;14(1):47–67. [Google Scholar]
- Ullman MT. A neurocognitive perspective on language: The declarative/procedural model. Nature Reviews Neuroscience. 2001;2:717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Ullman MT. Contributions of memory circuits to language: The declarative/procedural model. Cognition. 2004;92(1−2):231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Corkin S, Coppola M, Hickok G, Growdon JH, Koroshetz WJ, et al. A neural dissociation within language: Evidence that the mental dictionary is part of declarative memory, and that grammatical rules are processed by the procedural system. Journal of Cognitive Neuroscience. 1997;9(2):266–276. doi: 10.1162/jocn.1997.9.2.266. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Miranda RA, Travers ML. Sex differences in the neurocognition of language. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman J, Young E, editors. Sex on the brain: From genes to behavior. Oxford University Press; NY, NY: 2007. [Google Scholar]
- Walenski M, Tager-Flusberg H, Ullman MT. Language in autism. In: Moldin SO, Rubenstein JLR, editors. Understanding autism: From basic neuroscience to treatment. Taylor and Francis Books; Boca Raton, FL: 2006. pp. 175–203. [Google Scholar]
- Woolley CS. Effects of estrogen in the CNS. Current Opinion in Neurobiology. 1999;9(3):349–354. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]