Abstract
It is now recognized that geometric structures of scaffolds at several size levels have profound influences on cell adhesion, viability, proliferation and differentiation. This study aims to develop an integrated process to fabricate scaffolds with controllable geometric structures at nano-, micro- and macro-scales. A phase separation method is used to prepare interconnected poly(l-lactide) (PLLA) nanofibrous (NF) scaffolds. The pore size of the NF scaffold at the scale of several hundred micrometers is controlled by the size of porogen, paraffin spheres. At millimeter scale and above, the overall shape of the scaffold is defined by a wax mold produced using a three-dimensional printer. The printer utilizes a stereo lithographic file generated from computed tomographic files retrieved from the National Library of Medicine's Visual Human Project. NF PLLA scaffolds with a human digit shape are successfully prepared using this process. Osteoblast cell line MC3T3-E1 cells are then seeded and cultured in the prepared scaffolds. Cell proliferation, differentiation and biomineralization are characterized to demonstrate the suitability of the scaffolds for the digit bone tissue engineering application.
Introduction
The geometric structures of scaffolds at several size levels influence cell adhesion, viability, proliferation and differentiation [1]. At macro-scale level (mm and above), it is desirable for scaffold to assume the shape of the defective part of the tissue/organ to help the neo tissue organize into the needed three-dimensional (3D) structure [2-5]. At the micro-scale level, structural parameters such as pore size (usually ranging from 50 to 1000 μm), pore shape and porosity have to be controlled so that living cells can grow throughout the entire scaffold, and nutrients and metabolic wastes can be readily transported into or out of the scaffold [6-9]. At an even smaller scale (nm), surface topography influences cellular behaviors such as adhesion and differentiation [10]. To meet these criteria, a unique phase-separation process was developed to fabricate interconnected polylactide scaffolds with nanofibrous (NF) matrix [11-14]. The superior properties compared to scaffolds without NF features (also called solid-walled scaffolds), have been proven in a series of studies [11, 15-18]. Significantly larger amounts of serum proteins can be adsorbed onto the NF scaffold, facilitating cellular adhesion and growth [4, 19], making NF scaffolds likely advantageous for a variety of tissue engineering applications. Particularly for applications in bone tissue engineering, the results suggest that NF scaffolds can better promote osteoblast differentiation and biomineralization. Runt-related transcription factor 2 (Runx2) protein and bone sialoprotein (BSP) mRNA are expressed at higher levels in osteoblastic cells cultured on NF scaffolds, compared to cells cultured on solid-walled scaffolds. It was also found that biomineralization was enhanced substantially on NF scaffolds as confirmed by von Kossa staining and transmission electron microscopy [15]. The enhanced differentiation of osteoblastic cells on NF matrix was found to be associated with the RhoA/ROCK signaling pathway [20].
Although the importance of controlling scaffold geometries is now recognized, few existing technologies are capable of fabricating NF scaffolds with accurate anatomical shape, tunable inner pore size and controllable interpore connectivity. The electrospinning technique is probably the most intensively studied fabrication technique for polymer nanofibers [21-25]. Great efforts have been made to fabricate nanofibers using various polymers and to understand how to control the diameter of fibers. However, it is difficult to use the electrospinning technique to create inner micro-pores and macroscopic shapes. That is partially why electrospun fibers are often used only in the form of mats, limiting their wide applications in tissue engineering.
In this study, we combined the unique phase separation based nanofiber preparation technique [11-13] with a prototyping technique. Our aim was to fabricate the NF scaffolds with a controlled overall shape, inner pore size and pore connectivity. The overall shape of the scaffold was ultimately controlled by computed tomographic (CT) files, taken from the patient's defective part, so that the scaffold shape matches exactly the specific anatomic shape, such as a proximal phalanx in this study. A 3D printer was used to print a mold for scaffold preparation based on a stereo lithography (STL) file generated from the CT files. The inner pore size of the scaffold was controlled by the size of porogen, paraffin particles that were made with the emulsion method. The size of the paraffin spheres were adjusted by varying the emulsion process conditions (i.e. stirring speed and surfactant concentration). The interconnectivity of the inner pores of scaffolds was tailored by heating paraffin spheres for different periods of time. A unique phase separation process was used to fabricate the interconnected nanofibers, which constitute the pore walls and provide the surface for cell adhesion and growth. Murine osteoblast cell line MC3T3-E1 subclone 26 (MC-26) cells were then seeded and cultured in the prepared scaffolds. Cell proliferation, differentiation and biomineralization were characterized to evaluate the performance of the scaffolds for bone regeneration.
Materials and Methods
Scaffold fabrication
PLLA (inherent viscosity 1.6) was purchased from Alkermes (Cambridge, MA). Printing materials, wax and polysulphonamide (PSA), for 3D mold fabrication were bought from Solidscape Inc. (Merrimack, NH). All solvents, including dioxane, methanol, ethanol, hexane and cyclohexane, were purchased from Fisher Scientific (Pittsburgh, PA).
The CT data was part of a proximal phalanx that was retrieved from the National Library of Medicine's Visual Human Project. CT cross sectional image files were converted into a stereo lithography (STL) file using the software Mimics 8.11 (Materialise USA, Ann Arbor, MI). Based on the STL file, a wax mold was printed in a layer-by-layer fashion using a Modelmaker II (Solidscape Inc.). Paraffin spheres prepared in our lab were poured into the mold and heated at 37 degrees Celsius for 15 min to bond neighboring spheres together. PLLA was dissolved in a mixture of dioxane and methanol (volume ratio 4:1) to prepare a 9% (w/v) solution. The solution was cast into the mold filled with paraffin spheres. The mold loaded with PLLA solution and paraffin spheres was kept at -20 degrees Celsius to allow the PLLA solution to phase separate for approximately 2 hrs. The solvent mixture was then extracted by ethanol at -20 degrees Celsius and water at 4 degrees Celsius sequentially for 2 hrs each. Paraffin spheres and the wax mold were dissolved away with cyclohexane. Thus obtained was a porous PLLA scaffold in the shape of the part of phalanx with inner pore size determined by the size of paraffin spheres.
Mechanical property measurement
To characterize the mechanical properties of the scaffolds, PLLA NF scaffolds with a regular disk shape were prepared using a Teflon vial as a mold. The scaffold preparation procedure was the same as that described in the above paragraph except using a different mold. The diameter of scaffold disk was 7.2 mm and the thickness was 2 mm. A compressive mechanical test was carried out using a universal testing machine (MTS Synergie 200, MTS Systems, MN). The crosshead speed was 0.5 mm/min. The porosity and mechanical properties were calculated as previously reported [11, 14].
Cell Culture and osteoblast differentiation
MC3T3-E1 subclone 26 (MC-26) cells were cultured in ascorbic acid (AA)-free α modified essential medium (α-MEM) supplemented with 10% FBS, 1% penicillin/streptomycin in a humidified incubator at 37 degrees Celsius with 5% CO2. For cell seeding and culture, the scaffolds were sterilized using ethylene oxide and soaked in 70% ethanol solution for 0.5 hrs under reduced air pressure to allow the ethanol solution to penetrate the scaffold. Afterwards, the ethanol solution was replaced with PBS three times for 30 min each on an orbital shaker (Model 3520, Lab-Line Instruments, Melrose Park, IL) at 75 rpm. Scaffolds were washed with the medium twice for 2 hrs each, transferred to custom-built Teflon trays, and then seeded with 2×106 MC3T3-E1 cells per scaffold. After 48 hrs of incubation, the cell-scaffold constructs were transferred into 6-well tissue culture plates containing 3 mL of medium per well, supplemented with 50 μg/mL ascorbic acid and 10 mM β-glycerol phosphate, and cultured for specified times. The medium was changed every other day.
For proliferation studies, the harvested cell-scaffold constructs were washed with PBS for 5 min, homogenized with a Tissue-Tearor (BioSpec Products, Inc., Bartlesville, OK), and the DNA content was determined with a fluorescence assay kit from Sigma (St. Louis, MO) [19].
For gene expression studies, real-time PCR was used to detect the amounts of mRNAs encoding bone sialoprotein (BSP) and osteocalcin (OCN). The total RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA) with RNase-Free DNase set (Qiagen) according to the manufacturer's protocol after cell-scaffold constructs were mechanically homogenized with a Tissue-Tearor. The cDNA was made using a PCR machine (Applied Biosystems, Foster City, CA) with TaqMan (Applied Biosystems) reverse transcription reagents and 10 min incubation at 25 degrees Celsius, 30 min reverse transcription at 48 degrees Celsius, and 5 min inactivation at 95 degrees Celsius. Real-time PCR was set up using TaqMan Universal PCR Master mix and specific primer sequence for OCN (5′-CCGGGAGCAGTGTGAGCTTA-3′ and 5′-TAGATGCGTTTGTAGGCGGTC-3′) and BSP (5′-CAGAGGAGGCAAGCGTCACT-3′ and 5′-CTGTCTGGGTGCCAACACTG-3′) with 2 min incubation at 50 degrees Celsius, a 10 min Taq Activation at 95 degrees Celsius, and 40 cycles of denaturation for 15 s at 95 degrees Celsius followed by an extension for 1 min at 72 degrees Celsius on an ABI Prism 7500 Real-Time PCR System (Applied Biosystems) [4]. Target genes were normalized against GAPDH.
For histological analysis, samples were fixed in 10% neutral buffered formalin solution (Sigma), dried through an ethanol gradient, and embedded in paraffin. Embedded samples were cut into 5 μm sections and stained with hematoxylin and eosin or 5% silver nitrate for von Kossa staining.
Data are reported as mean ± S.D. based on triplicate cell cultures. To test the significance of observed differences between the study groups, an unpaired Student's t-test was applied. A value of p < 0.05 was considered to be statistically significant.
Results and Discussion
Scaffold fabrication
Figure 1 shows an image of a female left hand bones generated by converting CT images using software Mimics 8.11. Purple color denotes the defective part to be repaired. From left to right, Figure 2 shows a mold right after printing (with green supporting material PSA still inside), a mold after rinsing away the supporting material, a mold filled with paraffin spheres, and a finished PLLA scaffold. The mold was printed using a 3D printer in a layer-by-layer fashion. The thickness of each layer could reach as small as ten micrometers. In the current study, the thickness of each layer was set to 50 μm to balance the need of resolution and speed. A few seconds was needed to cool down the molten printing materials after each layer was printed. Due to the use of a supporting material, this method in principle can be employed to manufacture any complicated 3D structures as long as PSA and wax form a bicontinuous structure.
Figure 1.
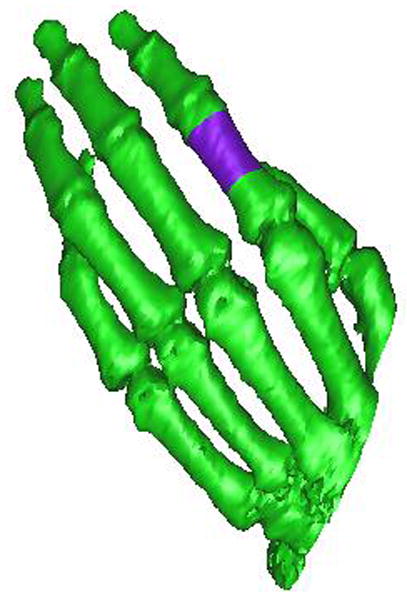
An image of a female left hand bones (purple color indicates the defective part).
Figure 2.
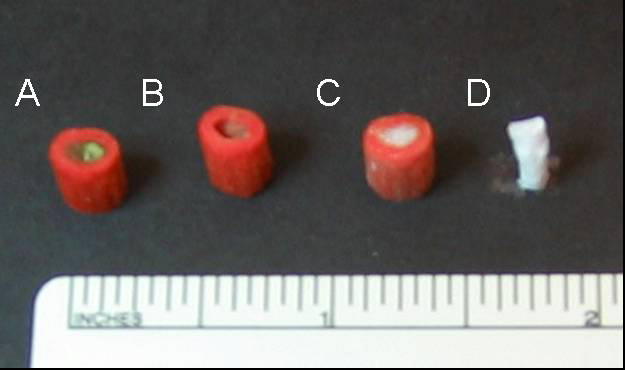
Fabrication of porous NF scaffolds with an anatomic shape: (A) an image of the mold right after printing, (B) the mold after rinsing away supporting material PSA, (C) mold filled with white paraffin spheres, and (D) the final PLLA NF scaffold.
After fabrication of the wax mold, micro-sized paraffin spheres were poured into the mold. The thermal treatment step afterwards is critical to controlling the interconnectivity of inner pores of scaffolds. In this study, a temperature of 37 degrees Celsius and a heating time of 50 min were used to bond paraffin spheres together. Heating the mold packed with paraffin spheres longer or at a higher temperature can lead to a higher interconnectivity as long as that there still is continuous space allowing polymer solution to be filled in.
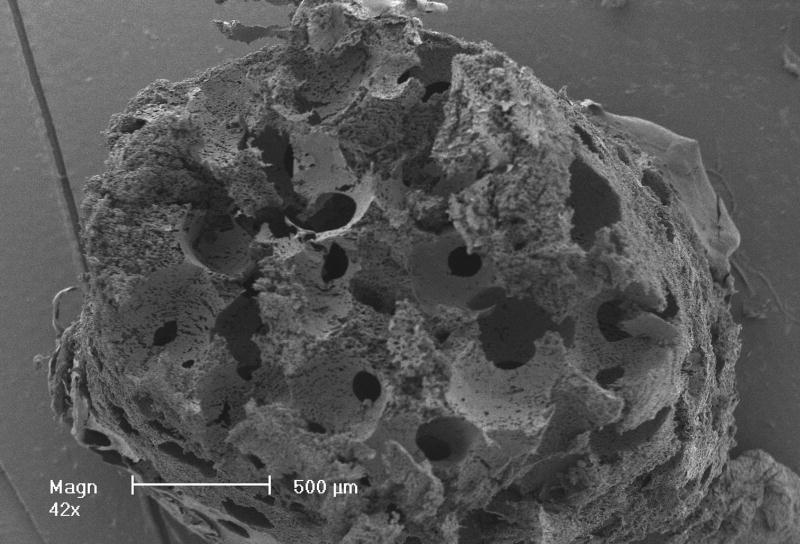
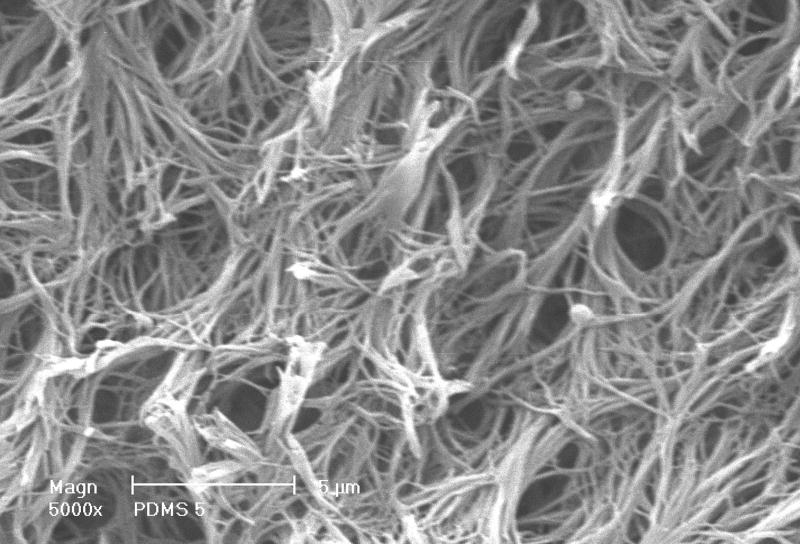
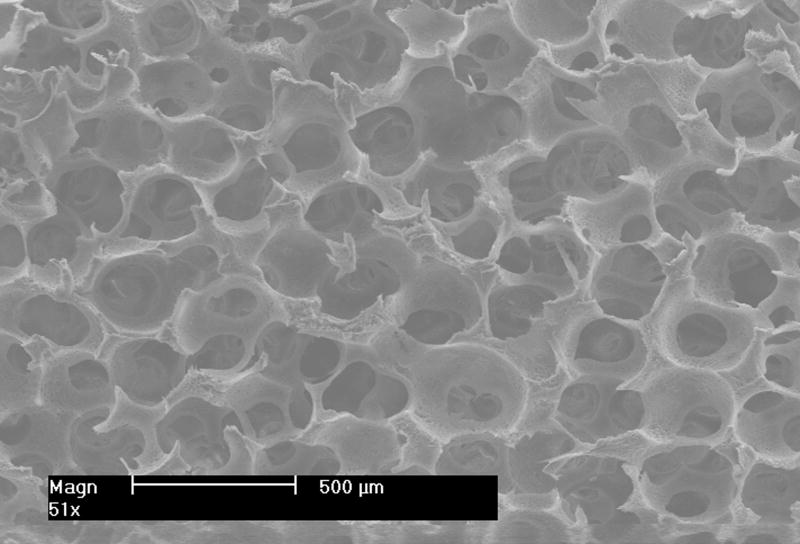
After thermal bonding of paraffin spheres, a PLLA solution was cast into the mold to fill the empty space around spheres. To make the NF structure mimicking natural collagen fibers, a special solvent (a mixture of dioxane and methanol with the volume ratio of 4:1) was used to prepare the PLLA solution. The PLLA solution in the paraffin sphere-loaded mold was then induced to phase separate at -20 degrees Celsius for at least 2 hrs. The solvent was extracted using ethanol and water sequentially for 2 hrs each. The last step was to dissolve the wax mold and the paraffin spheres using cyclohexane. Figure 3 shows a porous scaffold thus made. In this case, paraffin spheres with a diameter between 250 and 420 μm were used. A cross sectional picture of the scaffold shows good interconnectivity among pores, which is desirable for cells to grow throughout the scaffold. A higher magnification picture (Figure 4) reveals the formation of interconnected nanofibers with diameter between 50 nm and 300 nm. The interconnecting characteristics of these nanofibers make the scaffold mechanically strong enough to maintain the 3D structure. It is worthwhile to point out that the type of solvent in polymer solution and the phase-separation temperature were two key parameters for the formation of nanofibers. Our experimental results suggest that the temperature needs to be lower than 15 degrees Celsius to produce nanofibers. Above this critical temperature, platelets instead of NF structures would form.
Figure 3.
SEM images of outer surface (left) and cross-section (right) of a scaffold at low magnification. Scale bar: 500 μm.
Figure 4.
SEM image of a scaffold showing the NF matrix structure observed at high magnification. Scale bar: 5 μm.
Pore size and mechanical properties
Recent studies suggest that the inner pore size and porosity of scaffolds affect cell adhesion, growth, migration and differentiation [26, 27]. Pore size directly affects the mass transport rate, which in turn affects cell growth and function [1, 28, 29]. For example in bone regeneration, smaller pores generally favor hypoxic conditions and tend to induce osteochondral formation before osteogenesis. In contrast, larger pores tend to allow for better vascularization [27].
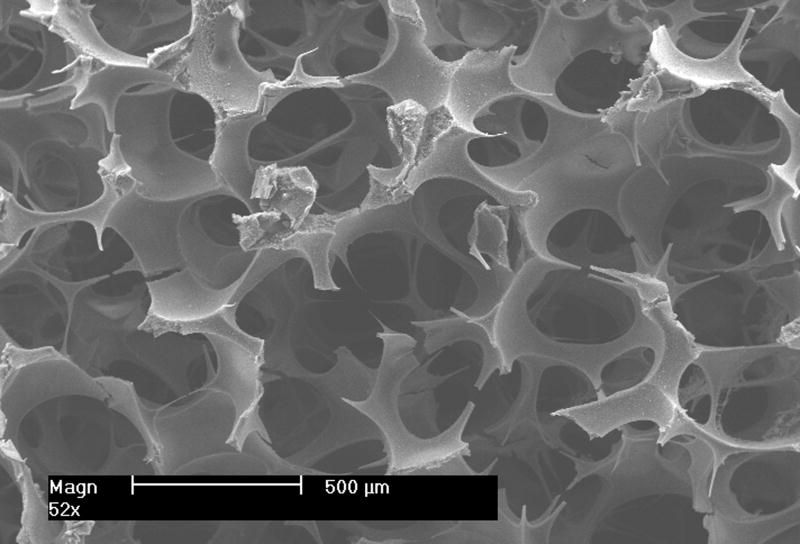
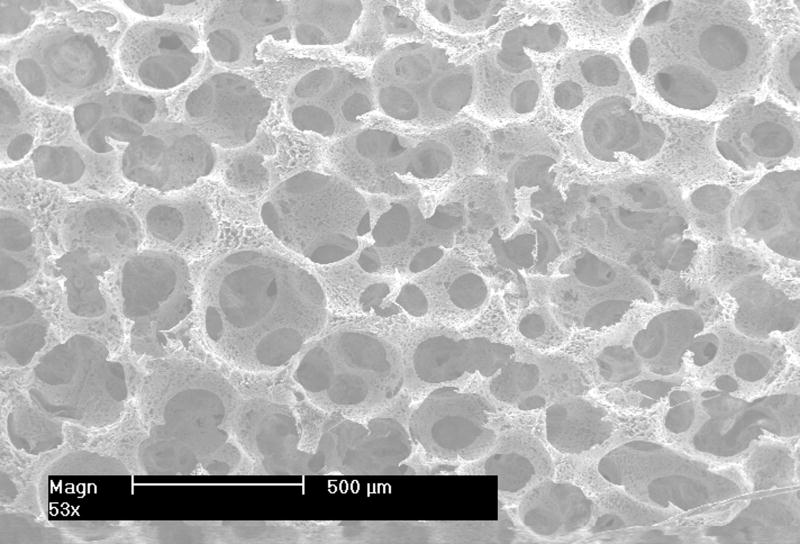
Appropriate mechanical properties are essential for load bearing scaffolds. Even for non-load bearing scaffolds mechanical properties are important since they affect the readiness of handling and storage of scaffolds. Commonly used methods for generation of pores in scaffolds include using sugar or salt particles as leachable porogens [30-35], foaming the biomaterials using gas or blowing agent [30, 31, 36-39]. However, it is often difficult to fabricate scaffolds with open-pores and well-controlled pore size using such methods. In this study, the emulsion method and a sieve screening process were used to prepare the porogen, paraffin spheres with desired sizes. The sphere size controls the pore size of the scaffold (as shown in Figure 5), whereas the pore interconnectivity is tailored by adjusting thermal treatment conditions.
Figure 5.
SEM images of PLLA NF scaffolds with different pore sizes (from left to right 420-500 μm, 250-420 μm and 150-250 μm respectively). Scale bar: 500 μm.
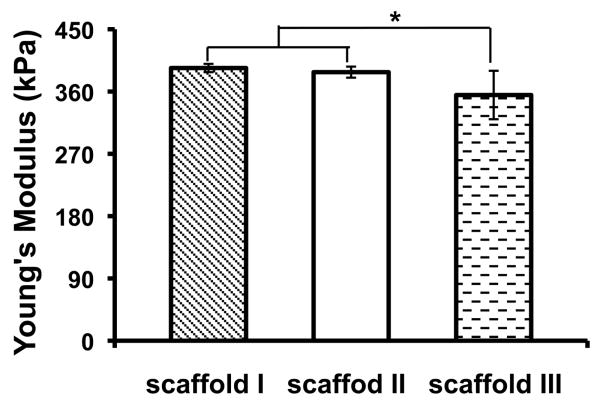
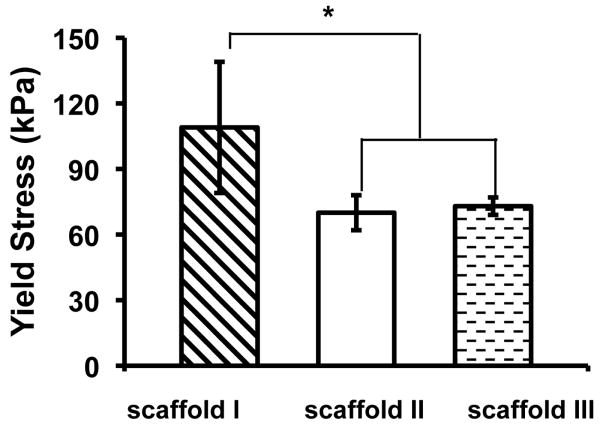
The mechanical properties of nanofibrous PLLA scaffolds with three different pore sizes were evaluated (I: pore size = 420-500 μm, porosity = 86%; II: pore size = 250-420 μm, porosity = 89%; III: pore size = 150-250 μm, porosity = 91%). The porosity was slightly higher for scaffolds made from small particles, which might be caused by the differences in paraffin sphere packing or polymer solution filling during the fabrication. The Young's modulus and yield stress of these PLLA scaffolds are shown in Figure 6. The Young's modulus of scaffolds I and II were not statistically different while both were statistically slightly higher than that of scaffold III. The yield stress of scaffold I was statistically higher than those of scaffolds II and III, whereas the yield stresses of scaffolds II and III were not statistically different. The slightly better mechanical properties of the scaffolds with larger pore sizes could have resulted from their slightly lower porosities, which would be consistent with our previous studies [11]. The mechanical properties of these scaffolds are comparable to those previously used for bone tissue engineering [15, 16, 18, 19]. These experimental results have demonstrated that PLLA NF scaffolds can be made with desirable anatomic shape, various pore sizes, and suitable mechanical properties for tissue engineering applications.
Figure 6.
Mechanical properties of scaffolds with different pore structures.
Cell culture and osteogenic differentiation
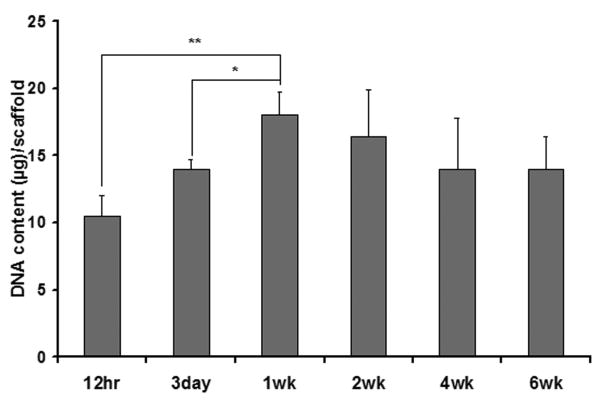
The NF scaffolds with a pore size of 250-420 μm were used for in vitro cell culture and differentiation studies. The DNA content of the osteoblast–scaffold constructs was measured to quantify osteoblast adhesion onto and proliferation in the scaffolds. A substantial number of cells adhered to the scaffolds as shown by the DNA amount 12 hr after cell seeding (Figure 7). Osteoblast cells grew quickly within the first week with the DNA amount almost doubled by the end of the first week. Cell number became steady from then on as the DNA amount did not change significantly in the following five weeks.
Figure 7.
DNA contents of cell-scaffold constructs during a 6-week culture (* p<0.05).
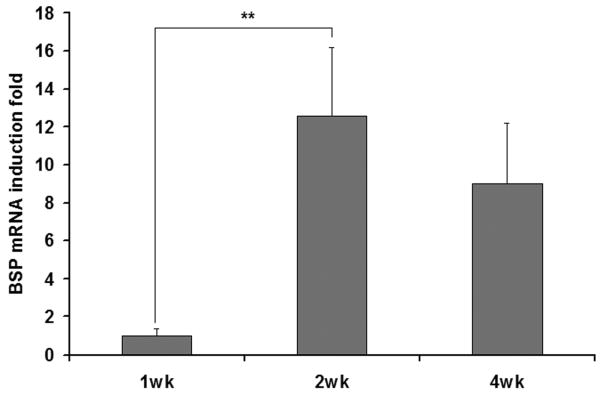
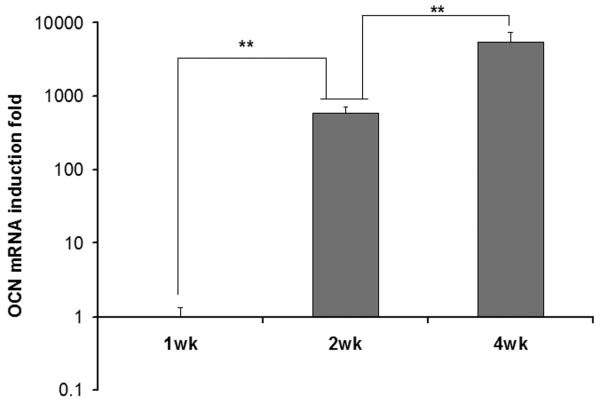
Real-time PCR was used to detect the mRNAs encoding BSP and OCN, two osteoblastic differentiation makers. The values were normalized against GAPDH. The expression of these two genes was at a negligible level in cells at the end of the first week of culture (Figure 8), indicating weak differentiation at the early stage of cultivation. The mRNA encoding BSP increased with culture time and reached the maximum at the end of the second week. The mRNA encoding OCN kept increasing throughout the 4 weeks of culture. These results indicate that the cell proliferation occurred mainly during the first week of culture, while the differentiation proceeded primarily after the first week and continued during the remaining culture period.
Figure 8.
Real-time RT-PCR analysis of BSP gene and OCN gene expression during the cell culture in the NF scaffolds. (** p<0.01).
The histological analysis shows the cell distribution and mineralization within the scaffold after 6 wk of culture (Figure 9). Cells grew well into the scaffolds, but the cell density is higher in the outer regions of the scaffolds. The cell-scaffold constructs were highly mineralized in the densely cellularized regions. A relatively higher cellular density at surface regions (compared to that at the central region) indicates the potential need for improved cell seeding conditions and culture environment (such as using a bioreactor). Optimizing inter-pore connectivity, pore size, and porous network design may also result in more uniform cell and neo tissue distribution.
Figure 9.
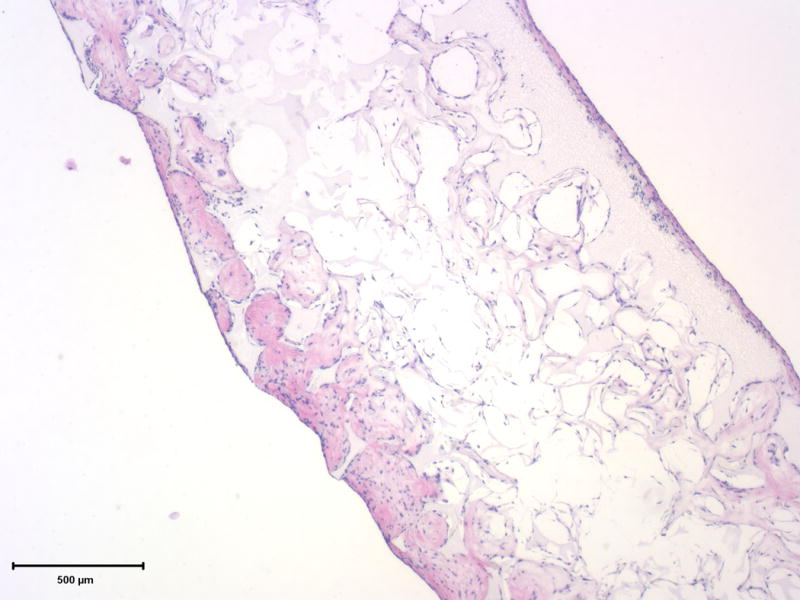
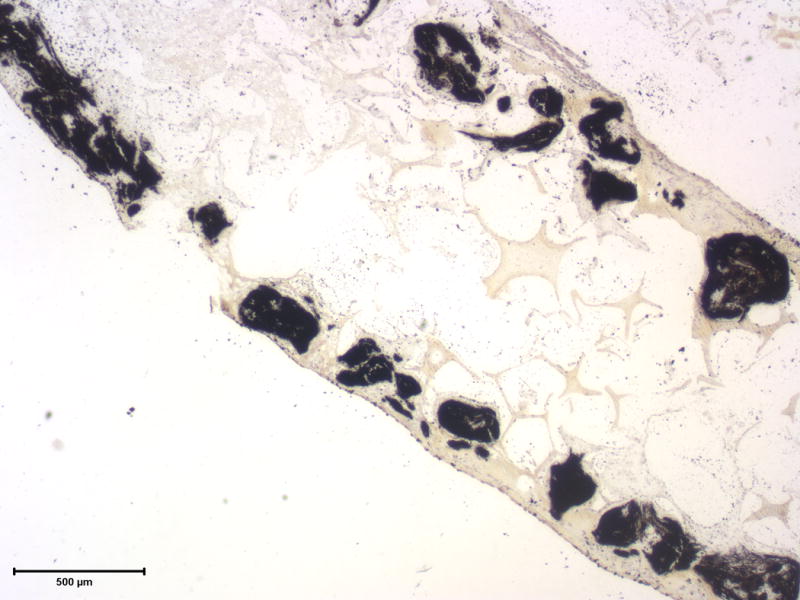
Histological analysis of cell-scaffold constructs after 6 weeks of culture. The H&E staining shows cells throughout the scaffold and embedded within extracelluar matrix (left), while the von Kossa staining shows highly mineralized cell-scaffold constructs (right). Scale bar: 500 μm.
Conclusions
A phase separation technique and a porogen-leaching technique have been integrated with a 3D printing technology to prepare porous NF scaffolds with tunable pore size, interpore connectivity, and patient-specific anatomic shape. In vitro cell culture results indicate that osteoblast cells grow and differentiate well inside the NF scaffolds. At the early stage of cultivation, cells proliferate at a fast pace without significant differentiation. The cell number then stabilizes and cell differentiation becomes evident, leading to mineralized bone formation. Taken together, this porous NF scaffold with medical image-generated anatomical shape, tailored pore size and interconnectivity, shows the promise to regenerate patient specific bone tissue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60(2):184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nature Biotechnology. 2002;20(11):1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- 3.Quadrani P, Pasini A, Mattioli-Belmonte M, Zannoni C, Tampieri A, Landi E, et al. High-resolution 3D scaffold model for engineered tissue fabrication using a rapid prototyping technique. Medical & Biological Engineering & Computing. 2005;43(2):196–199. doi: 10.1007/BF02345954. [DOI] [PubMed] [Google Scholar]
- 4.Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27(21):3973–3979. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 5.Naing MW, Chua CK, Leong KF, Wang Y. Fabrication of customised scaffolds using computer-aided design and rapid prototyping techniques. Rapid Prototyping Journal. 2005;11(4):249–259. [Google Scholar]
- 6.Starly B, Lau W, Bradbury T, Sun W. Internal architecture design and freeform fabrication of tissue replacement structures. Computer-Aided Design. 2006;38(2):115–124. [Google Scholar]
- 7.Yunoki S, Ikoma T, Monkawa A, Ohta K, Kikuchi M, Sotome S, et al. Control of pore structure and mechanical property in hydroxyapatite/collagen composite using unidirectional ice growth. Materials Letters. 2006;60(8):999–1002. [Google Scholar]
- 8.Boschetti F, Raimondi MT, Mighavacca F, Dubini G. Prediction of the micro-fluid dynamic environment imposed to three-dimensional engineered cell systems in bioreactors. Journal of Biomechanics. 2006;39(3):418–425. doi: 10.1016/j.jbiomech.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Siewe M, Madilhally SV. Effect of spatial architecture on cellular colonization. Biotechnology and Bioengineering. 2006;93(1):64–75. doi: 10.1002/bit.20703. [DOI] [PubMed] [Google Scholar]
- 10.Wei G, Ma PX. Nanostructured Biomaterials for Regeneration. Advanced Functional Materials. 2008;18:3568–3582. doi: 10.1002/adfm.200800662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen VJ, Ma PX. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25(11):2065–2073. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 12.Ma PX, Zhang RY. Synthetic nano-scale fibrous extracellular matrix. Journal of Biomedical Materials Research. 1999;46(1):60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Zhang RY, Ma PX. Synthetic nano-fibrillar extracellular matrices with predesigned macroporous architectures. Journal of Biomedical Materials Research. 2000;52(2):430–438. doi: 10.1002/1097-4636(200011)52:2<430::aid-jbm25>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Wei GB, Ma PX. Macroporous and nanofibrous polymer scaffolds and polymer/bonelike apatite composite scaffolds generated by sugar spheres. Journal of Biomedical Materials Research Part A. 2006;78A(2):306–315. doi: 10.1002/jbm.a.30704. [DOI] [PubMed] [Google Scholar]
- 15.Woo KM, Jun JH, Chen VJ, Seo JY, Baek JH, Ryoo HM, et al. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28(2):335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Woo KM, Chen VJ, Jung HM, Kim TI, Shin HI, Baek JH, et al. Comparative evaluation of nano-fibrous scaffolding for bone regeneration in critical size calvarial defects. Tissue Engineering: Part A. 2009 doi: 10.1089/ten.tea.2008.0433. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith LA, Liu X, Hu J, Wang P, Ma PX. Enhancing Osteogenic Differentiation of Embryonic Stem Cells by Nanofibers. Tissue Engineering: Part A. 2009 doi: 10.1089/ten.tea.2008.0227. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith LA, Liu X, Hu J, Ma PX. The Influence of Three Dimensional Nanofibrous Scaffolds on the Osteogenic Differentiation of Embryonic Stem Cells. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.01.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. Journal of Biomedical Materials Research Part A. 2003;67A(2):531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 20.Hu J, Liu X, Ma PX. Induction of osteoblast differentiation phenotype on poly(l-lactic acid) nanofibrous matrix. Biomaterials. 2008;29(28):3815–3821. doi: 10.1016/j.biomaterials.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boland ED, Coleman BD, Barnes CP, Simpson DG, Wnek GE, Bowlin GL. Electrospinning polydioxanone for biomedical applications. Acta Biomaterialia. 2005;1(1):115–123. doi: 10.1016/j.actbio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Wutticharoenmongkol P, Sanchavanakit N, Pavasant P, Supaphol P. Preparation and characterization of novel bone scaffolds based on electrospun polycaprolactone fibers filled with nanoparticles. Macromolecular Bioscience. 2006;6(1):70–77. doi: 10.1002/mabi.200500150. [DOI] [PubMed] [Google Scholar]
- 23.Tuzlakoglu K, Bolgen N, Salgado AJ, Gomes ME, Piskin E, Reis RL. Nano- and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. Journal of Materials Science-Materials in Medicine. 2005;16(12):1099–1104. doi: 10.1007/s10856-005-4713-8. [DOI] [PubMed] [Google Scholar]
- 24.Venugopal J, Ramakrishna S. Applications of polymer nanofibers in biomedicine and biotechnology. Applied Biochemistry and Biotechnology. 2005;125(3):147–157. doi: 10.1385/abab:125:3:147. [DOI] [PubMed] [Google Scholar]
- 25.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26(15):2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 26.Mastrogiacomo M, Scaglione S, Martinetti R, Dolcini L, Beltrame F, Cancedda R, et al. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials. 2006;27(17):3230–3237. doi: 10.1016/j.biomaterials.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 27.Karageorgiou V, Kaplan D. Porosity of 3D biornaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, Ma PX. Synthetic nano-fibrillar extracellular matrices with predesigned macroporous architectures. J Biomed Mater Res. 2000;52(2):430–438. doi: 10.1002/1097-4636(200011)52:2<430::aid-jbm25>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.Ma PX, Choi JW. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001;7(1):23–33. doi: 10.1089/107632701300003269. [DOI] [PubMed] [Google Scholar]
- 30.Yoon JJ, Kim JH, Park TG. Dexamethasone-releasing biodegradable polymer scaffolds fabricated by a gas-foaming/salt-leaching method. Biomaterials. 2003;24(13):2323–2329. doi: 10.1016/s0142-9612(03)00024-3. [DOI] [PubMed] [Google Scholar]
- 31.Yoon JJ, Song SH, Lee DS, Park TG. Immobilization of cell adhesive RGD peptide onto the surface of highly porous biodegradable polymer scaffolds fabricated by a gas foaming/salt leaching method. Biomaterials. 2004;25(25):5613–5620. doi: 10.1016/j.biomaterials.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Huang H, Oizumi S, Kojima N, Niino T, Sakai Y. Avidin-biotin binding-based cell seeding and perfusion culture of liver-derived cells in a porous scaffold with a three-dimensional interconnected flow-channel network. Biomaterials. 2007;28(26):3815–3823. doi: 10.1016/j.biomaterials.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Taqvi S, Roy K. Influence of scaffold physical properties and stromal cell coculture on hematopoietic differentiation of mouse embryonic stem cells. Biomaterials. 2006;27(36):6024–6031. doi: 10.1016/j.biomaterials.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 34.Lee YH, Lee JH, An IG, Kim C, Lee DS, Lee YK, et al. Electrospun dual-porosity structure and biodegradation morphology of Montmorillonite reinforced PLLA nanocomposite scaffolds. Biomaterials. 2005;26(16):3165–3172. doi: 10.1016/j.biomaterials.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Pattison MA, Wurster S, Webster TJ, Haberstroh KM. Three-dimensional, nanostructured PLGA scaffolds for bladder tissue replacement applications. Biomaterials. 2005;26(15):2491–2500. doi: 10.1016/j.biomaterials.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Wang XX, Li W, Kumar V. A method for solvent-free fabrication of porous polymer using solid-state foaming and ultrasound for tissue engineering applications. Biomaterials. 2006;27(9):1924–1929. doi: 10.1016/j.biomaterials.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Neves NM, Kouyumdzhiev A, Reis RL. The morphology, mechanical properties and ageing behavior of porous injection molded starch-based blends for tissue engineering scaffolding. Materials Science & Engineering C-Biomimetic and Supramolecular Systems. 2005;25(2):195–200. [Google Scholar]
- 38.Barry JJA, Gidda HS, Scotchford CA, Howdle SM. Porous methacrylate scaffolds: supercritical fluid fabrication and in vitro chondrocyte responses. Biomaterials. 2004;25(17):3559–3568. doi: 10.1016/j.biomaterials.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Mathieu LM, Mueller TL, Bourban PE, Pioletti DP, Muller R, Manson JAE. Architecture and properties of anisotropic polymer composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(6):905–916. doi: 10.1016/j.biomaterials.2005.07.015. [DOI] [PubMed] [Google Scholar]