Abstract
Telomere shortening with age may lead to genomic instability and an increased risk of cancer. Given the role of the microenvironment in the pathophysiology of the myelodysplastic syndrome (MDS), primarily a disease of older age, we determined telomere length in primary cultured marrow stroma cells using quantitative-fluorescence in situ hybridization (q-FISH) and quantitative-PCR (q-PCR). qFISH showed comparable rates of decrease in telomere length with age in MDS patients and age-matched healthy controls. Telomere length assessment by qPCR showed similar results. These findings suggest a lack of significant differences between MDS patients and healthy controls in terms of telomere stability in marrow stroma in contrast to that observed in hematopoietic cells. In conclusion, this demonstrates that although MDS stroma cells and hematopoietic cells share the same microenvironment, the stromal cells do not share the processes that contribute to accelerated telomere attrition, suggesting that stromal cell proliferative potential is not limiting in MDS.
Keywords: marrow stroma, telomere, stroma, qFITC
INTRODUCTION
Telomeres, the region of repetitive DNA sequences (TTAGGG) at the end of each chromosome provide stability and protect chromosomes from end-to-end fusions, degradation, and recombination. Telomere attrition exposes chromosome ends, activates cell cycle checkpoints and cellular senescence, and, in the absence of proficient cell cycle checkpoints, promotes cycles of bridge-breakage-fusion [14]. The pronounced age-related decline in telomere length may promote genetic instability and increase the risk of malignancy [1]. The incidence of myelodysplastic syndrome (MDS) increases progressively with age, and abnormal shortening of telomeres has been shown in hematopoietic cells from patients with MDS [5, 6, 16–18, 20, 22].
The role of the marrow microenvironment in the pathophysiology of clonal marrow disorders such as MDS remains controversial [7, 23]. While macrophages, for example, are part of the hematopoietic clone, stroma cells are non-clonal [2]. However, several investigators have reported dysfunction of MDS-derived stroma [7], and our own work shows abnormal expression of cytokines and receptors in marrow stroma from patients with MDS [13]. Conversely, hematopoietic cell transplantation from allogeneic donors is generally successful [21], although a recent report support suggests slower kinetics than in other diseases [4].
To further characterize marrow stroma from patients with MDS, we investigated telomere length in marrow fibroblasts as one parameter of integrity of the MDS marrow microenvironment.
MATERIALS AND METHODS
Patients and controls
Fresh bone marrow aspirates were obtained from patients with all subtypes of MDS, including one patient with myelomonocytic leukemia (CMML) (n=38). Patients were 42 to 88 years old, 24 were male and 14 female. In addition, samples were obtained from 13 healthy volunteers, 35–80 years of age. All patients and healthy volunteers had given informed consent as required by the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Cell lines and culture
HS5 is a human marrow stroma cell line that has been extensively characterized [8, 9, 11, 24]. HS5 cells were maintained in complete medium (RPMI 1640 supplemented with 10% heat inactivated fetal bovine serum [FBS], 1% glutamine, and 1% sodium pyruvate) and propagated at 37°C in a 5% C02/air atmosphere. Before experimental manipulation, cells were sedimented at 1200 rpm (300 g) and resuspended in complete medium and aliquoted in 6-well plates at 106 cells/mL.
Culture, isolation and purification of bone marrow stroma cells
Bone marrow mononuclear cells (BMMC) were isolated as described [13]. Approximately 25 × 106 cells were incubated in 75-ml cell culture flasks using non-hematopoietic expansion medium (Miltenyi Biotec) at 37°C in a humidified atmosphere containing 5% CO2. The medium was replaced weekly, and non-attached cells were discarded. When adherent cells reached 70% confluence, cultures were split for further propagation or used for studies. Population doubling times were determined weekly for 7 weeks.
In preparation for studies, adherent cells were washed with phosphate-buffered saline (PBS), trypsinized (0.25%) for 10 minutes, and analyzed by flow cytometry to detect and exclude possible myeloid/lymphoid cell contamination. The antibody specificities used for identification of myeloid/lymphoid cells included CD45, CD11b, CD14 and CD34. To phenotypically characterize adherent (“stroma”) cells, we used antibodies for CD54, CD73, CD90, and CD177, obtained from BD Biosciences (San Jose, CA, USA) and R&D Systems (Minneapolis, MN, USA). After phenotypic characterization, highly purified stroma cells were plated in 6-well plates containing glass-covered slides coated with Poly-(Lys) (Invitrogen, La Jolla, CA). After reaching 75% confluence, those cells were fixed for 30 min in 4% paraformaldehyde at room temperature and washed 3 times in PBS. Slides were air-dried and frozen at −80°C, and subsequently analyzed using fluorescent in situ hybridization (FISH). Genomic DNA was extracted from parallel cell samples using the Qiamp DNA extraction kit (Qiagen,Hilden, Germany) following the manufacturer’s instructions.
Peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were obtained from blood drawn into heparinized syringes by separation over a Ficoll hypaque gradient as described [12]. DNA was extracted as described for stroma cells above.
Quantitative analysis of telomeres using (q-FISH) and (q-PCR)
Dual-color (telomere and centromere) q-FISH was performed as previously described using confocal microscopy and image analysis [15]. A minimum of 50 stroma cells was analyzed per case to obtain an average epithelial/stromal telomere intensity (proportional to telomere length), which was calculated as the epithelial/stromal ratio [15].
Telomere and centromere q-FISH was also performed on aliquots of the HS5 cell line as an internal control, and these values were used for inter-experimental normalization. The intra and inter-assay variability (CV) for the normalized q-FISH was 0.1087 (10%).
Following genomic DNA extraction as described above, telomere length was measured by q-PCR. Briefly, each sample was amplified for telomeric DNA and for 36B4, a single-copy control gene that provided an internal control to normalize the starting amount of DNA. Two control samples were included in each experiment to allow for normalization, and periodic reproducibility experiments were carried out to confirm correct measurements. The intra-assay and inter-assay variabilities (coefficient of variation) for quantitative PCR were 6% and 7%, respectively. Because the T/S ratio is a relative measure of telomere length, the mean of T/S ratio of the cohort was normalized to 1.0 to facilitate comparisons.
Statistical analysis
Linear regression was used to evaluate the relationship between telomere length and age and between telomere length and doubling time of stroma cells in culture. The slopes of the regression lines were compared between MDS patients and healthy volunteers by fitting an interaction term between the slope and group. Comparison of mean telomere lengths between MDS patients and healthy volunteers was performed with the two-sided t test. Telomere lengths were compared between multiple diagnostic subgroups among the MDS patients using analysis of variance.
RESULTS AND DISCUSSION
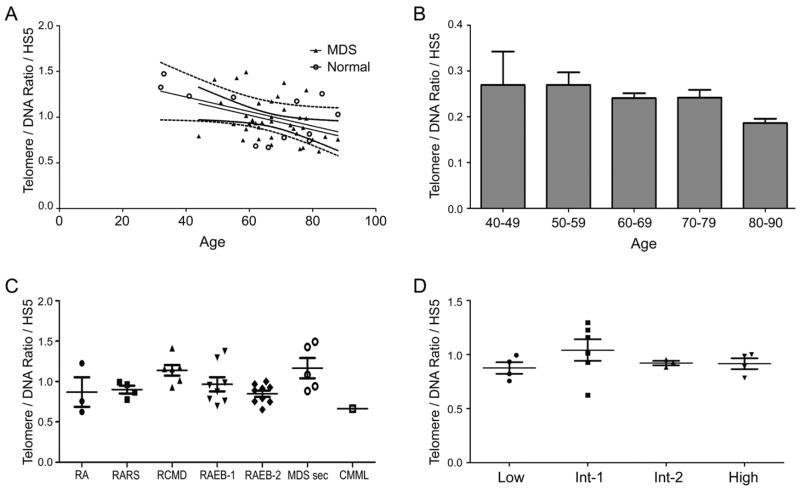
We compared telomere length in stroma cells from 38 patients with MDS to 13 healthy controls in the same age bracket. The centromere and DNA probes served as internal controls for telomere brightness that might have resulted from reduced accessibility of the telomere probe. To control for inter-experiment differences in sample fixation, processing, and staining, data are expressed as the ratio of telomere-to-DNA cell fluorescence divided by the average telomere-to-DNA cell fluorescence of the HS5 cell line that was included in every experiment. Telomere length shortening in MDS patients measured by q-FISH was not statistically significantly different from that in age matched controls (mean MDS=0.96, standard deviation [SD]=0.23; mean control= 1.03,SD=0.28, p=.39, 95% confidence interval for difference in telomere length, - 0.2283 to 0.091). While there was considerable variation in telomere length between individuals with MDS, telomere lengths progressively declined with age in both MDS and control groups (R=−0.36, slope=−0.008) (each increase in age of one year associated with decrease in telomere length of 0.008); and R=-0.55, slope=-0.0079, respectively (p=.99, interaction test; Figure 1A). A second technique, q-PCR, also failed to show a substantial difference in the association between telomere length and age between patients with MDS and controls (data not shown).
Figure 1. A) Telomere shortening in normal and MDS marrow stroma.
Telomere length (by q-FISH) decreased progressively with age for both patients with MDS (broken curves; R= −0.36, slope= −16) and controls (solid curves; R= −0.55, slope= −38, .p=0.15). B) Telomere shortening in patients with MDS. Decrease by 0.08% of total length per year. C) Telomere length by MDS category. There was no correlation between telomere length and MDS category (RA= refractory anemia; RARS= RA with ring sideroblasts; RCMD+ refractory cytopenia with multilineage dysplasia; RAEB = RA with excess blasts [1 =5–9%; 2 = 10–19%]; MDS sec = treatment related MDS; CMML = chronic myelomonocytic leukemia). D) Telomere length and IPSS score. There was no correlation between IPSS category [10]and telomere length.
The doubling times of stroma cells were similarly correlated with telomere length (R=0.36; p=0.02), and as shown in Figure 2, the slopes for population doubling were similar for patients with MDS and healthy individuals (p=0.5), as would be expected in view of comparable telomere length.
Figure 2. Population doubling times of marrow stroma from healthy donors and MDS patients.
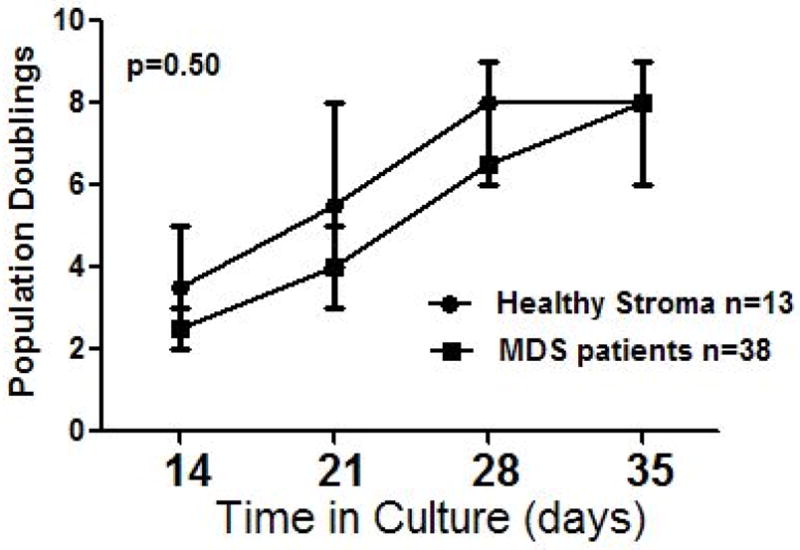
Slopes for patients and healthy controls were similar (p=0.50).
Others have indicated a common pattern of telomere length shortening with age in many different tissues, at a rate of 100 to 150 base pairs/year [3], or approximately 0.08% annually. Our findings are consistent with those previous results (Figure 1B). When IPSS was treated as a continuous linear variable, the correlation with telomere length was not statistically significant (p=0.83), and a trend test on IPSS as categorized in Figure 1D yielded p=−0.78. Further, there was no statistically significant difference in telomere length between the seven diagnostic groups as categorized in Figure 1C (p=0.13), although the number of patients in these groups was small, thereby limiting the power to detect a difference. Results of q-FISH, karyotyping and stratification by IPSS [10] are shown in Table 1.
Table 1.
Clinical Variables of MDS Patients and Telomere Size
| Diagnosis | Chemotherapeutic Treatment | Age | Cytogenetic Markers | IPSS | BM Cellularity | Telomere/DNA/HS5 DNA |
|---|---|---|---|---|---|---|
| RA | Untreated | 69 | Normal | 0.5 | hyper | 1.2266 |
| RA | Untreated | 82 | Normal | 0.5 | normal | 0.6244 |
| RA | Untreated | 88 | Normal | 0.0 | normal | 0.7561 |
| RARS | ATG and etanercept | 59 | Normal | 0.5 | hyper | 0.8689 |
| RARS | Etanercept and 5-azacytidine | 61 | Normal | N/A | hyper | 0.9637 |
| RARS | Lenalidomide | 67 | Normal | N/A | hyper | 0.7773 |
| RARS | Untreated | 76 | Normal | 0.0 | normal | 0.9935 |
| RCMD-RS | Untreated | 60 | Normal | 0.5 | hyper | 0.9290 |
| RCMD | Thalidomide | 63 | t(2:11) | 1.0 | hyper | 1.1520 |
| RCMD | Untreated | 49 | Complex, del(7) | 1.5 | hypo | 1.4119 |
| RCMD | Untreated | 51 | Normal | 0.5 | normal | 1.1578 |
| RCMD | Untreated | 59 | Normal | 0.5 | normal | 1.0133 |
| RCMD | Etanercept | 67 | tri(8),del(7) | 1.0 | normal | 1.1715 |
| RAEB-1 | Filgastrim and epoetin beta | 67 | Normal | 2.0 | hyper | 0.9538 |
| RAEB-1 | Untreated | 78 | Complex | 2.5 | hyper | 0.9852 |
| RAEB-1 | Untreated | 78 | Normal | 1.0 | hyper | 1.2953 |
| RAEB-1 | Untreated | 84 | del7 | 2.5 | hypo | 0.7844 |
| RAEB-1 | Etanercept and 5-azacytidine | 63 | Normal | 0.0 | normal | 0.7584 |
| RAEB-1 | Etanercept and 5-azacytidine | 67 | Complex | 1.5 | normal | 0.6967 |
| RAEB-1 | Etanercept and 5-azacytidine | 71 | Complex, del(7) | 2.5 | normal | 1.3762 |
| RAEB-1 | Etanercept and 5-azacytidine | 74 | Normal | 2.0 | normal | 0.8742 |
| RAEB-2 | 7+3 | 44 | Normal | 0.5 | hyper | 0.7911 |
| RAEB-2 | 7+3 and 5-azacytidine | 61 | Normal | 2.5 | hyper | 0.9675 |
| RAEB-2 | Untreated | 63 | Complex, del(7) | 3.0 | hyper | 0.8900 |
| RAEB-2 | Untreated | 67 | Complex | 3.0 | hyper | 1.0004 |
| RAEB-2 | Untreated | 76 | t(6:9) | 2.5 | hyper | 0.6525 |
| RAEB-2 | Etanercept and 5-azacytidine | 80 | Normal | 1.0 | hyper | 0.7538 |
| RAEB-2 | Etanercept and 5-azacytidine | 73 | del(5q) | 1.0 | hypo | 0.9119 |
| RAEB-2 | Untreated | 55 | t(10:22) | 1.5 | normal | 0.9264 |
| RAEB-2 | 5-azacytidine, thalidomide | 58 | tri(21) | 0.5 | normal | 0.7469 |
| MDS - 5q(-) | Untreated | 62 | del(5q) | 0.0 | normal | 0.9332 |
| MDS-unclassified | Untreated | 75 | Normal | 0.0 | normal | 0.8211 |
| MDS sec | FLAM | 64 | Normal | N/A | hyper | 0.9402 |
| MDS sec | Untreated | 77 | add(11q) | 1.5 | hyper | 0.8824 |
| MDS sec | 7+3 | 56 | Normal | N/A | hypo | 1.4281 |
| MDS sec | Irradiation | 59 | del(13) | 0.5 | normal | 1.4933 |
| MDS sec | Clofarabine | 71 | tri(8),del(7) | N/A | normal | 1.0847 |
| CMML | Untreated | 77 | Normal | N/A | normal | 0.6641 |
Finally, to place our data into the context of previously published results on telomere shortening in cells derived from the hematopoietic clone [5, 6, 17, 18, 22], we carried out a comparison of telomere length in stroma to telomere length in PBMC, which were available in two MDS patients and one healthy donor. The ratio of PBMC to stroma telomere length as determined by qPCR was 0.7 and 0.6 in the two patients, respectively, and 1.2 in the healthy donor. The telomere length in PBMC in the healthy donor was exactly in the range published by R.A. Risques et al. [19], while the lengths were 8 to 10-fold shorter in the two MDS patients.
Thus, these data provide no evidence for accelerated telomere shortening in the marrow stroma of patients with MDS beyond what is expected among healthy volunteers. While the findings do not exclude a role for stroma in the pathophysiology of MDS, they argue strongly against enhanced turn-over or genetic instability in those cells. We have not excluded the possibility that telomere shortening was counter-acted by increased telomerase activity. However, the most likely explanation for the present results is that MDS marrow stroma cells are not clone-derived, and that in spite of sharing the microenvironment with hematopoietic cells, they are not subject to the combination of increased proliferation or oxidative stress that presumably underlies the telomere attrition of MDS hematopoietic cells previously reported [5, 6, 16–18, 20, 22].
In summary, these data show that marrow stroma from patients with MDS undergoes telomere shortening only at a rate that corresponds to patient age [22], and that any abnormality of stroma function in MDS more likely occurs in response to signals derived from the hematopoietic clone.
Acknowledgments
The authors thank Emily Spaulding, for technical assistance and Helen Crawford and Bonnie Larson for help with manuscript preparation.
Supported in part by grants HL082941, HL036444, and AG013280 from the National Institutes of Health (NIH) Bethesda, MD. A. Mario Marcondes. is also supported by a grant from the J.P. McCarthy Fund.
Footnotes
Authors’ contributions. Mario Marcondes designed the experiments, conducted the experiments and wrote the manuscript.
Steve Bair contributed to the analysis.
Peter Rabinovitch provided reagents, made suggestions to the experimental design, assisted in interpreting the results, and revised the manuscript.
Ted Gooley carried out the statistical analysis.
H. Joachim Deeg provided marrow samples, assisted in analyzing the results and provided revisions to the manuscript.
Rosana Risques contributed technical expertise.
References
- 1.Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 2.Awaya N, Rupert K, Bryant E, Torok-Storb B. Failure of adult marrow-derived stem cells to generate marrow stroma after successful hematopoietic stem cell transplantation. Exp Hematol. 2002;30:937–942. doi: 10.1016/s0301-472x(02)00821-4. [DOI] [PubMed] [Google Scholar]
- 3.Baird DM, Kipling D. The extent and significance of telomere loss with age (Review) Ann NY Acad Sci. 2004;1019:265–268. doi: 10.1196/annals.1297.044. [DOI] [PubMed] [Google Scholar]
- 4.Bitan M, Or R, Shapira MY, Resnick IB, Gesundheit B, Ackerstein A, Samuel S, Elad S, Slavin S. Time to engraftment following allogeneic stem cell transplantation is significantly longer in patients with myelodysplastic syndrome than with acute myeloid leukemia. Bone Marrow Transplant. 2008;41:69–78. doi: 10.1038/sj.bmt.1705878. [DOI] [PubMed] [Google Scholar]
- 5.Boultwood J, Fidler C, Kusec R, Rack K, Elliott PJ, Atoyebi O, Chapman R, Oscier DG, Wainscoat JS. Telomere length in myelodysplastic syndromes. Am J Hematol. 1997;56:266–271. doi: 10.1002/(sici)1096-8652(199712)56:4<266::aid-ajh12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Fern L, Pallis M, Ian CG, Seedhouse C, Russell N, Byrne J. Clonal haemopoiesis may occur after conventional chemotherapy and is associated with accelerated telomere shortening and defects in the NQO1 pathway; possible mechanisms leading to an increased risk of t-AML/MDS. Br J Haematol. 2004;126:63–71. doi: 10.1111/j.1365-2141.2004.05006.x. [DOI] [PubMed] [Google Scholar]
- 7.Flores-Figueroa E, Arana-Trejo RM, Gutierrez-Espindola G, Perez-Cabrera A, Mayani H. Mesenchymal stem cells in myelodysplastic syndromes: phenotypic and cytogenetic characterization. Leuk Res. 2005;29:215–224. doi: 10.1016/j.leukres.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Goerner M, Roecklein B, Torok-Storb B, Heimfeld S, Kiem H-P. Expansion and transduction of nonenriched human cord blood cells using HS-5 conditioned medium and FLT3-L. Journal of Hematotherapy and Stem Cell Research. 2000;9:759–765. doi: 10.1089/15258160050196803. [DOI] [PubMed] [Google Scholar]
- 9.Graf L, Iwata M, Torok-Storb B. Gene expression profiling of the functionally distinct human bone marrow stromal cell lines HS-5 and HS-27a (Letter to Editor) Blood. 2002;100:1509–1511. doi: 10.1182/blood-2002-03-0844. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J. International scoring system for evaluating prognosis in myelodysplastic syndromes (erratum appears in Blood 1998 Feb 1;91(3):1100) Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 11.Kerbauy DMB, Lesnikov V, Torok-Storb B, Bryant E, Deeg HJ. Engraftment of distinct clonal MDS-derived hematopoietic precursors in NOD/SCID- β2microglobulin-deficient mice after intramedullary transplantation of hematopoietic and stromal cells (Letter to the Editor) Blood. 2004;104:2202–2203. doi: 10.1182/blood-2004-04-1518. [DOI] [PubMed] [Google Scholar]
- 12.Lesnikov V, Lesnikova M, Deeg HJ. Pro-apoptotic and anti-apoptotic effects of transferrin and transferrin-derived glycans on hematopoietic cells and lymphocytes. Exp Hematol. 2001;29:477–489. doi: 10.1016/s0301-472x(00)00687-1. [DOI] [PubMed] [Google Scholar]
- 13.Marcondes AM, Mhyre AJ, Stirewalt DL, Kim S-H, Dinarello CA, Deeg HJ. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. PNAS. 2008;105:2865–2870. doi: 10.1073/pnas.0712391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maser RS, DePinho RA. Connecting chromosomes, crisis, and cancer (Review) Science. 2002;297:565–569. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan JN, Finley JC, Risques RA, Shen WT, Gollahon KA, Moskovitz AH, Gryaznov S, Harley CB, Rabinovitch PS. Telomere length assessment in tissue sections by quantitative FISH: image analysis algorithms. Cytometry Part A: The Journal of the International Society for Analytical Cytology. 2004;58:120–131. doi: 10.1002/cyto.a.20006. [DOI] [PubMed] [Google Scholar]
- 16.Ohyashiki JH, Iwama H, Yahata N, Ando K, Hayashi S, Shay JW, Ohyashiki K. Telomere stability is frequently impaired in high-risk groups of patients with myelodysplastic syndromes. Clin Cancer Res. 1999;5:1155–1160. [PubMed] [Google Scholar]
- 17.Ohyashiki JH, Ohyashiki K, Fujimura T, Kawakubo K, Shimamoto T, Iwabuchi A, Toyama K. Telomere shortening associated with disease evolution patterns in myelodysplastic syndromes. Cancer Res. 1994;54:3557–3560. [PubMed] [Google Scholar]
- 18.Ohyashiki K, Iwama H, Yahata N, Tauchi T, Kawakubo K, Shimamoto T, Ohyashiki JH. Telomere dynamics in myelodysplastic syndromes and acute leukemic transformation (Review) Leuk Lymphoma. 2001;42:291–299. doi: 10.3109/10428190109064585. [DOI] [PubMed] [Google Scholar]
- 19.Risques RA, Vaughan TL, Li X, Odze RD, Blount PL, Ayub K, Gallaher JL, Reid BJ, Rabinovitch PS. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiology, Biomarkers & Prevention. 2007;16:2649–2655. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- 20.Sashida G, Ohyashiki JH, Nakajima A, Sumi M, Kawakubo K, Tauchi T, Ohyashiki K. Telomere dynamics in myelodysplastic syndrome determined by telomere measurement of marrow metaphases. Clin Cancer Res. 2003;9:1489–1496. [PubMed] [Google Scholar]
- 21.Scott BL, Deeg HJ. Hematopoietic cell transplantation for acquired nonmalignant diseases and myelodysplastic syndrome. In: Hoffman R, et al., editors. Hematology: Basic Principles and Practice. Churchill Livingstone, Inc; in press. [Google Scholar]
- 22.Sieglova Z, Zilovcova S, Cermak J, Rihova H, Brezinova D, Dvorakova R, Markova M, Maaloufova J, Sajdova J, Brezinova J, Zemanova Z, Michalova K. Dynamics of telomere erosion and its association with genome instability in myelodysplastic syndromes (MDS) and acute myelogenous leukemia arising from MDS: a marker of disease prognosis? Leuk Res. 2004;28:1013–1021. doi: 10.1016/j.leukres.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Soenen-Cornu V, Tourino C, Bonnet ML, Guillier M, Flamant S, Kotb R, Bernheim A, Bourhis JH, Preudhomme C, Fenaux P, Turhan AG. Mesenchymal cells generated from patients with myelodysplastic syndromes are devoid of chromosomal clonal markers and support short- and long-term hematopoiesis in vitro. Oncogene. 2005;24:2441–2448. doi: 10.1038/sj.onc.1208405. [DOI] [PubMed] [Google Scholar]
- 24.Stirewalt DL, Mhyre AJ, Marcondes M, Pogosova-Agadjanyan E, Abbasi N, Radich JP, Deeg HJ. Tumour necrosis factor-induced gene expression in human marrow stroma: clues to the pathophysiology of MDS? Br J Haematol. 2008;140:444–453. doi: 10.1111/j.1365-2141.2007.06923.x. [DOI] [PubMed] [Google Scholar]