Abstract
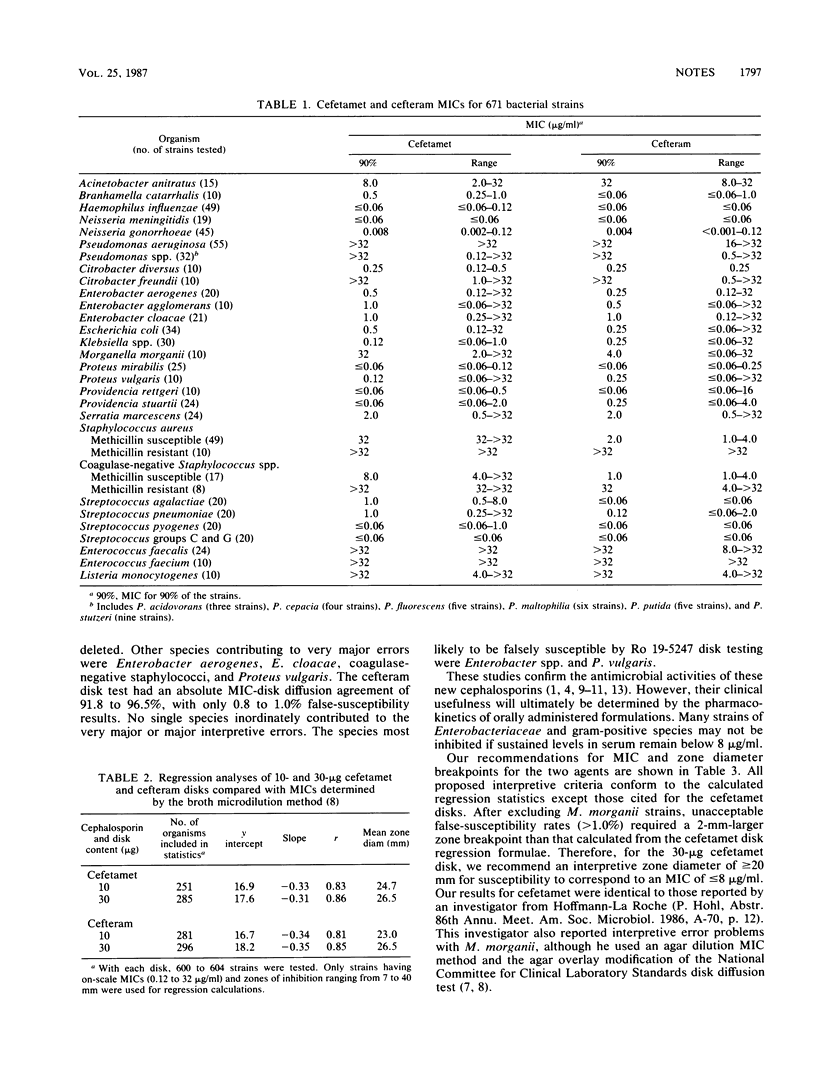
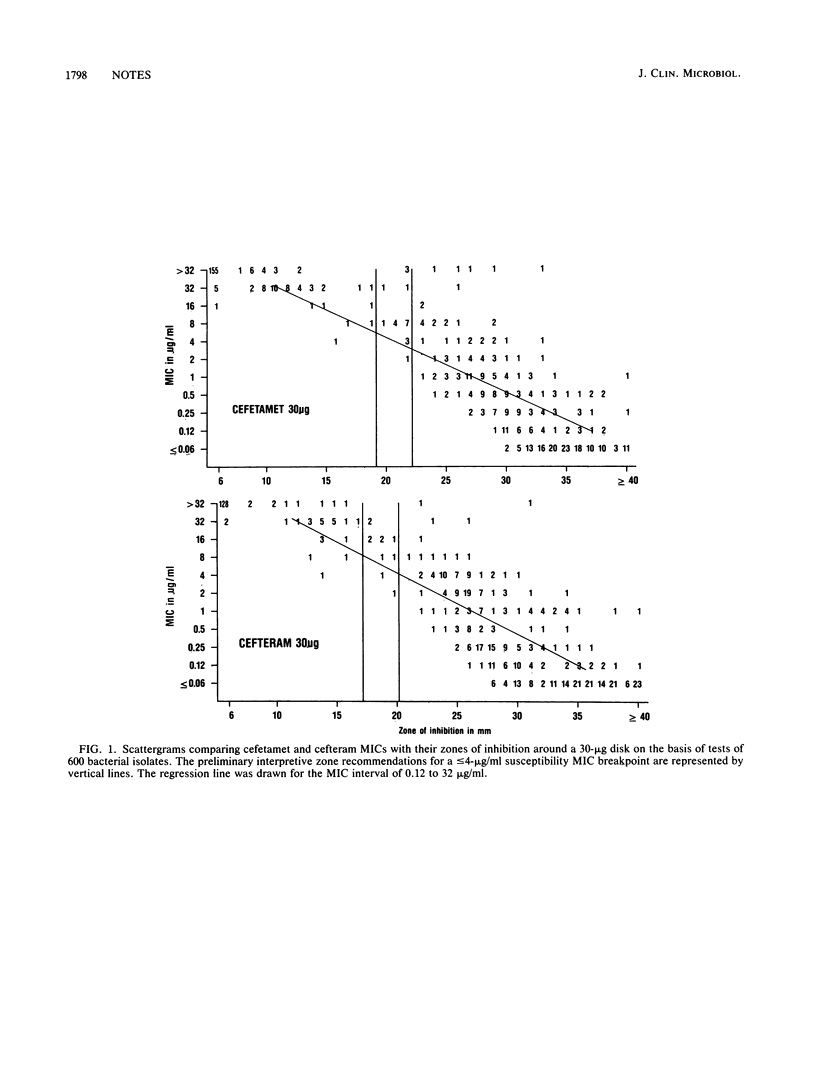
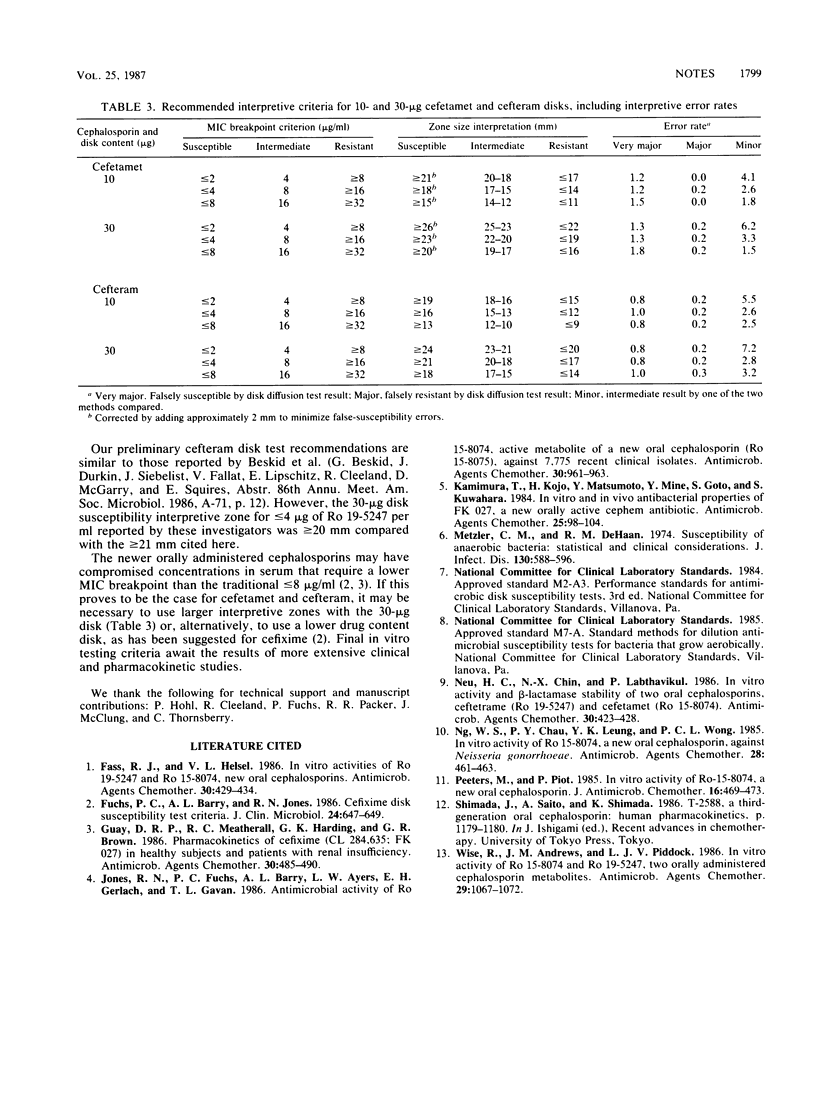
Preliminary interpretive zone criteria were calculated for cefetamet (Ro 15-8074) and cefteram (Ro 19-5247) by using 10- and 30-micrograms disks and three possible MIC susceptibility breakpoints. Absolute interpretive agreement between MICs and zone size criteria ranged from 91.8 to 97.2%. Very major errors (false susceptibility) were less than or equal to 1.2% for both cephalosporin disk tests. Morganella morganii strains appeared to produce the highest rates of very major interpretive errors with cefetamet disks.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fass R. J., Helsel V. L. In vitro activities of Ro 19-5247 and Ro 15-8074, new oral cephalosporins. Antimicrob Agents Chemother. 1986 Sep;30(3):429–434. doi: 10.1128/aac.30.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Jones R. N. Cefixime disk susceptibility test criteria. J Clin Microbiol. 1986 Oct;24(4):647–649. doi: 10.1128/jcm.24.4.647-649.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay D. R., Meatherall R. C., Harding G. K., Brown G. R. Pharmacokinetics of cefixime (CL 284,635; FK 027) in healthy subjects and patients with renal insufficiency. Antimicrob Agents Chemother. 1986 Sep;30(3):485–490. doi: 10.1128/aac.30.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Barry A. L., Ayers L. W., Gerlach E. H., Gavan T. L. Antimicrobial activity of Ro 15-8074, active metabolite of a new oral cephalosporin (Ro 15-8075), against 7,775 recent clinical isolates. Antimicrob Agents Chemother. 1986 Dec;30(6):961–963. doi: 10.1128/aac.30.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura T., Kojo H., Matsumoto Y., Mine Y., Goto S., Kuwahara S. In vitro and in vivo antibacterial properties of FK 027, a new orally active cephem antibiotic. Antimicrob Agents Chemother. 1984 Jan;25(1):98–104. doi: 10.1128/aac.25.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler C. M., DeHaan R. M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974 Dec;130(6):588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chin N. X., Labthavikul P. In vitro activity and beta-lactamase stability of two oral cephalosporins, ceftetrame (Ro 19-5247) and cefetamet (Ro 15-8074). Antimicrob Agents Chemother. 1986 Sep;30(3):423–428. doi: 10.1128/aac.30.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. S., Chau P. Y., Leung Y. K., Wong P. C. In vitro activity of Ro 15-8074, a new oral cephalosporin, against Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1985 Sep;28(3):461–463. doi: 10.1128/aac.28.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M., Piot P. In-vitro activity of Ro-15-8074, a new oral cephalosporin. J Antimicrob Chemother. 1985 Oct;16(4):469–473. doi: 10.1093/jac/16.4.469. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Piddock L. J. In vitro activity of Ro 15-8074 and Ro 19-5247, two orally administered cephalosporin metabolites. Antimicrob Agents Chemother. 1986 Jun;29(6):1067–1072. doi: 10.1128/aac.29.6.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]



