Abstract
The widely used non-steroidal anti-inflammatory drugs (NSAIDs) function mainly through inhibition of cyclooxygenases 1 and 2 (Cox-1 and Cox-2). Unlike Cox-1, Cox-2 is considered an inducible and pro-inflammatory enzyme. We previously reported that Cox-2 is upregulated in activated human B lymphocytes and using Cox-2 selective inhibitors that Cox-2 is required for optimal antibody synthesis. It is not known whether commonly used non-prescription and non-Cox-2 selective drugs also influence antibody synthesis. Herein, we tested a variety of Cox-1/Cox-2 non-selective NSAIDs, namely ibuprofen, tylenol, aspirin and naproxen and report that they blunt IgM and IgG synthesis in stimulated human peripheral blood mononuclear cells (PBMC). Ibuprofen had its most profound effects in inhibiting human PBMCs and purified B lymphocyte IgM and IgG synthesis when administered in the first few days after activation. As shown by viability assays, ibuprofen did not kill B cells. The implications of this research are that the use of widely available NSAIDs after infection or vaccination may lower host defense. This may be especially true for the elderly who respond poorly to vaccines and heavily use NSAIDs.
Keywords: human B lymphocytes, Cox-2, NSAIDs, antibody
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are regularly used by more than 60 million Americans [1] and are effective in relieving pain, reducing fever and inhibiting inflammation. Most NSAIDs target the activity of the prostaglandin G/H synthases, commonly known as cyclooxygenases. Cyclooxygenases (Cox-1 and Cox-2) catalyze the conversion of arachidonic acid to prostaglandin H2 (PGH2) which is metabolized by tissue-specific isomerases to other prostanoids (PGD2, PGE2, PGF2α, PGI2) and thromboxanes. Classically, Cox-1 has been described as the isoform which is constitutively expressed in most tissues and cells and plays a role in homeostasis, while Cox-2 generates PGs that act as mediators of fever, inflammation and promoters of carcinogenesis [2, 3].
There are over 50 different NSAIDs available [4] and they can be divided into different groups based on their chemical structure, pharmacokinetics and selectivity towards Cox-1 or Cox-2 [5, 6]. NSAIDs can cause liver damage [7], renal failure [8], aseptic meningitis [9] and can interfere with bone fracture healing [10]. Among the side-effects caused by NSAIDs, gastrointestinal toxicity is one of the most common [11] and results mostly from inhibition of Cox-1 in the gastric mucosa. This problem was thought to be circumvented by developing highly selective Cox-2 inhibitors (celecoxib, rofecoxib, valdecoxib), however, their use was associated with an increased incidence of myocardial infarction and stroke [12, 13]. Besides these life-threatening side-effects, there is increased evidence that NSAIDs have immunomodulatory effects by interfering with human monocyte and T lymphocyte activation, proliferation and cytokine synthesis [14–16].
The effects, if any, of Cox-1/Cox-2 nonselective over-the-counter NSAIDs on human B lymphocytes are relatively unknown. Our laboratory recently reported that inhibition of Cox-2 activity in normal human B lymphocytes by highly selective Cox-2 inhibitors (e.g. SC-58125 and NS-398) resulted in a significant decrease in antibody synthesis [17, 18]. We also showed that Cox-2 knock out mice made less antibody than normal mice [17]. Therefore, we hypothesized that widely used Cox-1/Cox-2 non-selective NSAIDs would have a negative effect on normal B cell function. Herein, we have investigated, (1) the effect of aspirin, ibuprofen, naproxen and tylenol on antibody synthesis in human peripheral blood mononuclear cells; (2) the time-frame and the concentrations of ibuprofen required to blunt antibody synthesis and (3) the effect of ibuprofen on B cell lymphocytes. Overall, our findings reveal that over-the-counter NSAIDs have potent negative effects on human B lymphocytes and on antibody production.
Material and methods
Reagents
Aspirin (acetylsalicylic acid), ibuprofen (α-methyl-4-(isobuthyl) phenylacetic acid), indomethacin (1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-3-indoleacetic acid), S-ibuprofen (S-(+)-4-isobutyl-α-methyl-phenylacetic acid), tylenol (acetaminophen), naproxen (S)-(+)-6-Methoxy-α-methyl-2-naphthaleneacetic acid) and 3- (4, 5- dimethylthiazole- 2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were obtained from Sigma (St Louis, MO). SC-58125 was obtained from Cayman (Ann Arbor, MI). Stock solutions of NSAIDs and Cox-2 selective inhibitor were prepared in DMSO and diluted in culture media prior to treatment. For PBMC and B cell stimulation, rabbit antihuman F(ab′)2 anti-IgM Ab (Jackson ImmunoResearch Laboratories, West Grove, PA) and CpG oligodeoxynucleotide 2395 (5′-TCGTCGTTTTCGGCGCGCGCCG-3′) (Coley Pharmaceutical Group, Wellesley, MA) were used. Human IgM and IgG quantitation kit was purchased from Bethyl Laboratories (Montgomery, TX). 7-AAD reagent was obtained from BD Biosciences (San Jose, CA). The following antibodies were used: CD27, CD38 (eBioscience San Diego, CA), IgD, CD19 and CD20 (BD Biosciences, San Jose, CA).
Human peripheral blood B cell (PBMC) isolation and culture conditions
One unit of blood was obtained from healthy donors (who were not taking any NSAIDs) as approved by the University of Rochester Institutional Review Board and Office for Human Subjects Protection. Peripheral blood mononuclear cells were obtained by density-gradient centrifugation of buffy coat using Ficoll-Paque Plus. PBMCs were washed in PBS and used for in vitro assays or further purified to obtain B cells, as follows. PBMCs were incubated with CD19 magnetic beads (Dynal Inc, Brown Deer, WI). CD19 positive cells were captured with a magnet, washed and detached using CD19 Detachabead (Dynal Inc, Brown Deer, WI). An aliquot was used to assess the purity of isolated B cells (which was > 95% as determined by flow cytometry based on CD19 staining).
PBMCs and purified B cells were cultured in RPMI1640 media 1640 (Invitrogen Life Technologies) supplemented with 10% FBS, 50 µM β-mercaptoethanol (Eastman Kodak, Rochester, NY), 10 mM Hepes (U.S. Biochemical, Cleveland, OH), 2 mM L-glutamine (Invitrogen Life Technologies, Carlsbad, CA), 50 µg/ml gentamicin (Invitrogen Life Technologies, Carlsbad, CA) and 5 µM arachidonic acid (Nu-Check-Prep, Elysian, MN). PBMC and B cells were stimulated with anti-IgM (2 µg/ml) plus CpG 2395 (1 µg/ml) for variable time-points as described in figure legends.
IgM and IgG enzyme-linked immunosorbent assay (ELISA)
PBMCs and purified human B cells (5 × 105 cells/ml) were cultured in triplicate in 96-well plates for 7 days, unless otherwise specified. Cells were stimulated in the presence of NSAIDs. Control cells (no drug) received only the vehicle (DMSO). Supernatants were collected and used for IgM and IgG detection using the human-specific ELISA kit (Bethyl Laboratories) as recommended by the manufacturer.
Measurement of PGE2 synthesis
PBMCs (1 × 106 cells/ml) were stimulated with anti-IgM plus CpG 2395 and exposed to varying concentrations of ibuprofen for 2 days. Control cells (no drug) received only the vehicle (DMSO). PGE2 synthesis was determined using an enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI), as recommended by the manufacturer.
Mouse studies
Cox-2 deficient (B6. 129P2-Ptgs 2tm1Smi) mice and their wild-type controls were purchased from Taconic Farms. Mice were maintained in pathogen-free conditions as approved by University of Rochester Division of Laboratory Animal Medicine. Spleens of Cox-2 knock-out and wild-type controls were isolated and red cells removed by density-gradient centrifugation using Ficoll-Paque Plus. Splenocytes were washed in 1XPBS. An aliquot was used for cell surface staining using antibodies against: IgD, CD19, CD27 and CD38. For in vitro studies, splenocytes were resuspended in same media used for human experiments and stimulated with 10 µg/ml LPS (Sigma, St Louis, MO) for 6 days in the presence of ibuprofen (50 µM). At the end of culture period, supernatants were collected. Antibody synthesis was determined using mouse-specific IgM and IgG ELISA kits (Bethyl Laboratories), as recommended by the manufacturer.
IgM and IgG-specific ELISPOT assay
Stimulated human PBMC (anti-IgM+CpG2395) were cultured for 6 days in the presence or absence of ibuprofen (50 µM). Goat anti-human IgM and IgG antibodies (Biosource, Carslbad, CA) were used to coat ELISPOT plates (Millipore, Billerica, MA), as recommended by the manufacturer. ELISPOT plates were blocked in RPMI1640 + 5% FBS for one hour at 37°C and washed in 1XPBS. PBMC (106 or 105 cells/well) were transferred to ELISPOT plates and incubated for 16h at 37°C in 5 % CO2. Plates were washed with 1XPBS and incubated with alkaline phosphatase-conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories) or IgM antibody (Biosource) for 2 hours at room temperature. Plates were washed and developed using Vector AP substrate kit III (Vector Laboratories, Burlingame, CA). ELISPOT plates were counted using a CTL plate reader and immunospot software (Cellular Technologies, Shaker Heights, OH).
Flow cytometry
Human PBMCs or highly purified B lymphocytes were cultured for up to 7 days in the presence of mitogens and ibuprofen (50 µM). Cells were surface stained for CD19 or CD20 (BD, Biosciences, San Jose, CA) for 20 minutes at 4°C, washed in staining buffer (PBS with 0.3% BSA) and then pelleted by centrifugation. For 7-AAD staining, cells were resuspended in 7-AAD solution (BD Biosciences, San Jose, CA) as recommended by the manufacturer. Samples were analyzed on a FACScalibur (BD Bioscience, San Jose, CA) flow cytometer using FlowJo software (Tree Star).
Mitochondrial dehydrogenase activity (MTT) assay
PBMCs and normal B cells (4 × 105 cells/ml) were stimulated with anti-IgM plus CpG 2395 and cultured in triplicate in 96-well plate for up to 7 days. Cells were exposed to ibuprofen. Control cells (no drug) received only the vehicle (DMSO). A solution of 5 mg/ml MTT was added for the last 4h of incubation. Plates were centrifuged, media removed and the precipitates dissolved in DMSO. Absorbance was read at 510 nm on a Benchmark microplate reader (Bio-Rad, Hercules, CA).
Statistical analysis
At least 3 independent experiments were performed for each figure panel. Error bars represent the SD of the mean (n=3). One-way ANOVA test, followed by Tukey’s post hoc test were used for statistical analysis and p values of *, p<0.05; **, p<0.001; ***, p<0.0001 are considered significant.
Results
NSAIDs inhibit human antibody production in vitro
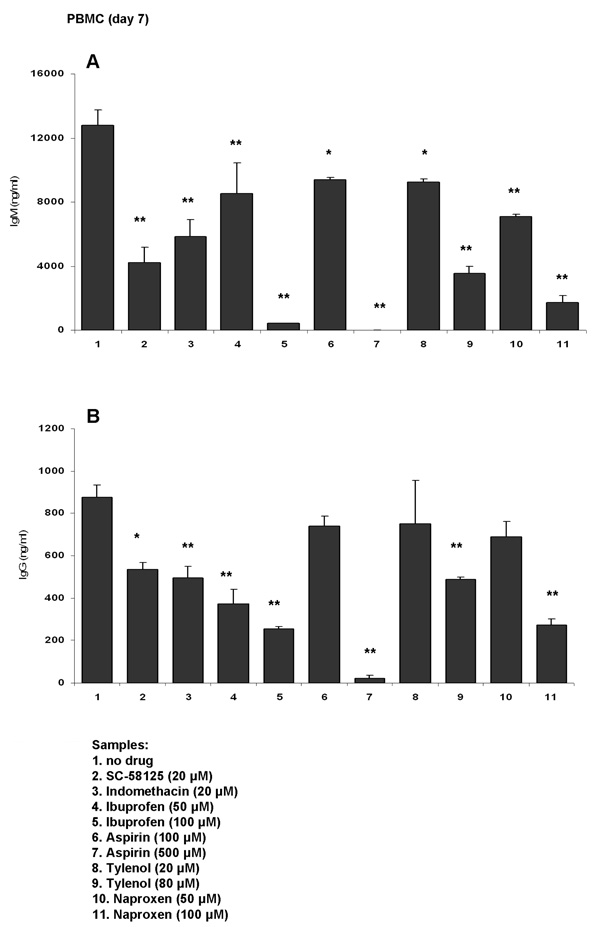
We previously reported that Cox-2 selective inhibitors blunt antibody production in vitro [17, 18]. In our current studies we tested if inhibition of antibody production was drug-specific or whether other widely used over-the counter NSAIDs would also interfere with antibody synthesis. Human PBMCs were stimulated with anti-IgM (2 µg/ml) + CpG 2395 (1 µg/ml) for 7 days and cells were exposed to a panel of NSAIDs: ibuprofen (50 and 100 µM), aspirin (100 µM and 500 µM), tylenol (20 and 80 µM) and naproxen (50 and 100 µM). Indomethacin (20 µM) and SC-58125 (highly selective Cox-2 inhibitor; 20 µM) were also included. Drugs were added to the culture everyday except for SC-58125 which was added every other day. The doses of the NSAIDs used was determined based on drug plasma concentrations attained in humans [19–25]. Figure 1 shows that anti-IgM plus CpG 2395 stimulated PBMC produced higher levels of IgM compared to IgG (~12,000 ng/ml IgM and 800 ng/ml IgG, respectively). As we previously reported, indomethacin and SC-58125 significantly blunted IgM and IgG synthesis. Aspirin, tylenol and naproxen showed similar abilities to inhibit antibody production when used at pharmacological doses. Ibuprofen also reduced IgM (by up to 97%) and IgG (by up to 70%) production.
Figure 1. Non-steroidal anti-inflammatory drugs (NSAIDs) blunt antibody synthesis in human PBMC in vitro.
Human PBMCs were stimulated with anti-IgM (2 µg/ml) plus CpG 2395 (1 µg/ml) for 7 days. Cells were exposed to NSAIDs or to a selective Cox-2 inhibitor, as follows: SC-58125 (Cox-2 selective inhibitor; 20 µM), indomethacin (20 µM), ibuprofen (50 µM or 100 µM), aspirin (100 µM or 500 µM), tylenol (20 µM or 80 µM) or naproxen (50 µM or 100 µM). Drugs were added everyday, except for SC-58125, which was added every other day. The control samples (no drug) received only the vehicle (DMSO). At the end of culture period, supernatants were harvested and used for IgM (panel A) or IgG (panel B) detection by ELISA. Each bar represents the means ± SD (n=3). The statistical significance in reduction of antibody synthesis was determined by one-way ANOVA test, followed by Tukey’s post-hoc test. *, p< 0.05, **, p< 0.001 as compared to control (no drug) sample.
Ibuprofen inhibits Cox-2 activity in a concentration-dependent manner in human PBMC
Due to the fact that ibuprofen had one of the most striking effects on antibody synthesis and that it is widely used, we further investigated its effects. In our initial experiments we used ibuprofen in the form of a racemic mixture which contains both S- and R-ibuprofen enantiomers.
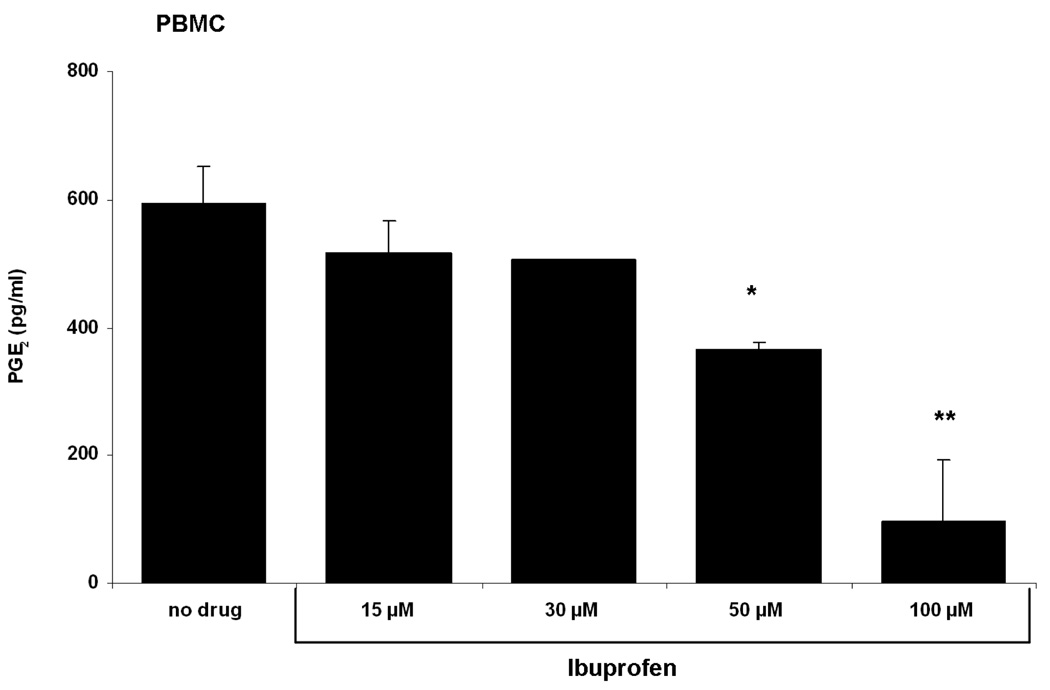
Prostaglandin E2 (PGE2) is synthesized from arachidonic acid via Cox-1 and Cox-2 and is a principal mediator of inflammation and fever [26]. PGE2 production following cell activation derives mainly from Cox-2 activity. In order to determine if ibuprofen impedes PGE2 synthesis, stimulated PBMCs were exposed to increasing concentrations of ibuprofen for 2 days after which PGE2 production was measured. Figure 2 shows that ibuprofen inhibits PGE2 synthesis in a concentration-dependent manner. Doses of 50 µM or 100 µM ibuprofen statistically diminished PGE2 production in human PBMC in vitro.
Figure 2. Ibuprofen inhibits PGE2 synthesis in human PBMC in a concentration-dependent manner.
Human PBMCs were stimulated with anti-IgM plus CpG 2395 for 2 days. Cells were exposed to increasing concentrations of ibuprofen from 15 µM to 100 µM. Ibuprofen was added at the onset of culture. Control cells received only the vehicle (DMSO). There is a concentration-dependent decrease in PGE2 synthesis. PGE2 production was significantly inhibited by 50 µM ibuprofen (~ 300 pg/ml) and further decreased by 100 µM ( ~100 pg/ml). Data are shown as mean ± SD (n=3). *, p< 0.05, **, p< 0.001 were calculated using one-way ANOVA test followed by Tukey’s post-hoc test.
Ibuprofen decreases IgM synthesis in Cox-2−/− splenocytes
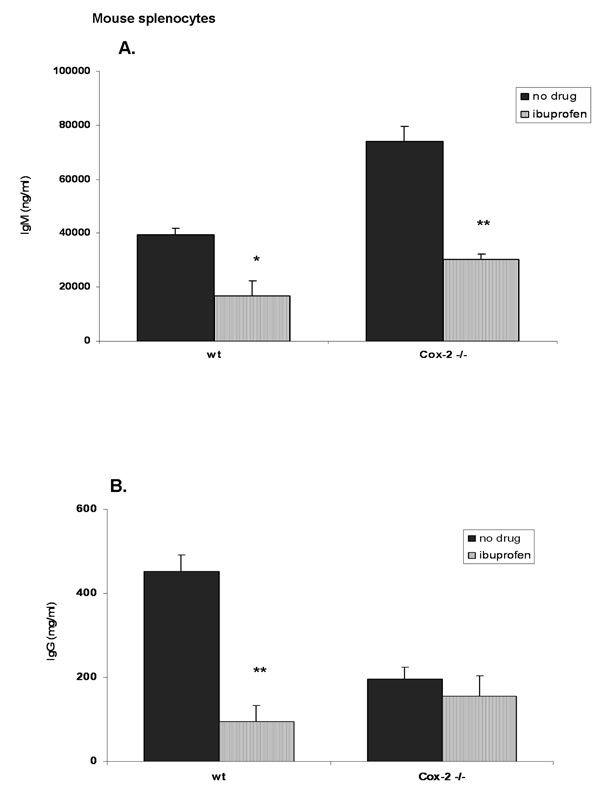
Ibuprofen is a dual Cox-1/Cox-2 inhibitor. Therefore, we determined whether or not the reduction in antibody synthesis was due solely to Cox-2 inhibition or possibly via Cox-1 or Cox-1/Cox-2. For this reason we used Cox-2 knock-out mice. Firstly, we made sure that splenocytes from Cox-2 deficient mice did not show a defect in B cell populations. Spleen cells were stained for IgD, CD19, CD27 and CD38 and analyzed by flow cytometry. These markers are typically used to distinguish between naïve and memory B cells. Our analysis did not show any differences in these surface markers (data not shown). Next, splenocytes were activated in vitro with LPS (10 µg/ml) for 6 days in the presence or absence of ibuprofen (50 µM) after which supernatants were collected and used for IgM and IgG detection by ELISA. Figure 3A shows that in wild-type mice there is a significant reduction in IgM synthesis (60% less) in ibuprofen-treated splenocytes compared to wt control (no drug). Cox-2 deficient mice showed an increased IgM production compared to wild-type control (Figure 3A; black bars). Interestingly, ibuprofen caused a ~ 60% reduction of IgM production in splenocytes of Cox-2 deficient mice. These results imply that Cox-2 is not responsible for IgM inhibition. Ibuprofen significantly inhibited IgG production only in wild-type splenocytes (by ~ 70%) but did not inhibit IgG production in Cox-2 knock-out mice (Figure 3B; striped bars). Furthermore, Cox-2 deficient mice produced less IgG (~200 ng/ml) compared to wild-type controls (~450 ng/ml) (Figure 3B, black bars) suggesting that Cox-2 might be involved in isotype switching. Therefore, we cannot draw a clear conclusion whether Cox-2 is responsible for IgG synthesis or for isotype switching.
Figure 3. Ibuprofen fails to decrease IgG production in Cox-2 deficient mice.
Splenocytes from Cox-2 knock-out mice and their wild-type controls were stimulated with LPS (10 µg/ml) for 6 days and were exposed to ibuprofen (50 µM; added everyday). Control cells (no drug) received only the vehicle (DMSO). Supernatants were harvested and used for determining IgM and IgG synthesis. There is an increase in IgM synthesis in Cox-2 deficient mice (80,000 ng/ml) compared to wild-type controls (~40,000 ng/ml) (Panel A; black bars). Ibuprofen significantly inhibited IgM synthesis in both Cox-2 deficient and wild-type mice (by ~ 60%) (Panel A; striped bars). Cox-2 knock-out mice produce less IgG (~200 ng/ml) compared to wild-type controls (~450 ng/ml) (Panel B; black bars). Ibuprofen significantly inhibited IgG synthesis in wild-type mice (by ~ 70%) but not in Cox-2 deficient mice (Panel B; striped bars). Data are shown as mean ± SD (n=3). *, p< 0.05, **, p < 0.001 were calculated using one-way ANOVA test followed by Tukey’s post-hoc test.
Ibuprofen decreases antibody synthesis in a concentration and time-dependent manner in human PBMC
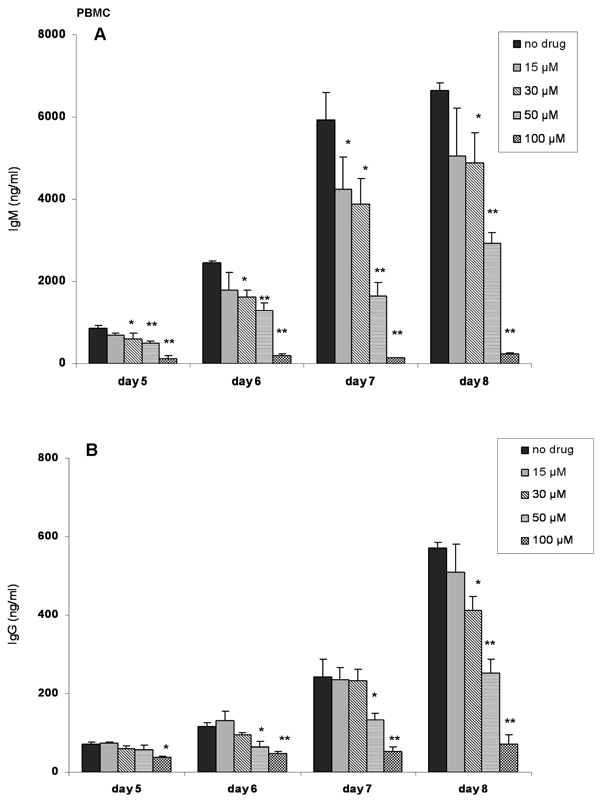
Next, we conducted a detailed experiment to determine the effects of ibuprofen on human PBMC. Anti-IgM+ CpG 2395 stimulated PBMC were exposed to ibuprofen and IgM and IgG levels were determined between day 5 (the earliest time-points at which antibody synthesis could be detected) and day 8. Figure 4A shows that doses as low as 15 µM attenuated IgM synthesis. For IgG, doses as low as 30 µM diminished IgG production (Figure 4B). In general, there was a dose-dependent decrease in IgM and IgG synthesis.
Figure 4. Ibuprofen inhibits antibody synthesis by human PBMC in a concentration-dependent manner.
Stimulated (anti-IgM plus CpG 2395) PBMCs were exposed to ibuprofen (15 µM, 30 µM, 50 µM or 100 µM). Ibuprofen was added everyday. Control cells received only the vehicle (DMSO). Supernatants were harvested on day 5, 6, 7 and 8 and IgM (panel A) and IgG (panel B) synthesis was determined. Ibuprofen (50 or 100 µM) caused a statistically significant decrease in IgM and IgG synthesis from day 5 to day 8. Fifteen µM ibuprofen significantly decreased IgM synthesis only on day 7, whereas 30 µM cause a significant decrease in IgM and IgG synthesis on day 6 and day 7 and day 6 to day 8, respectively. Statistical significance, *, p< 0.05, **, p< 0.001 was calculated using one-way ANOVA test followed by Tukey’s post-hoc test.
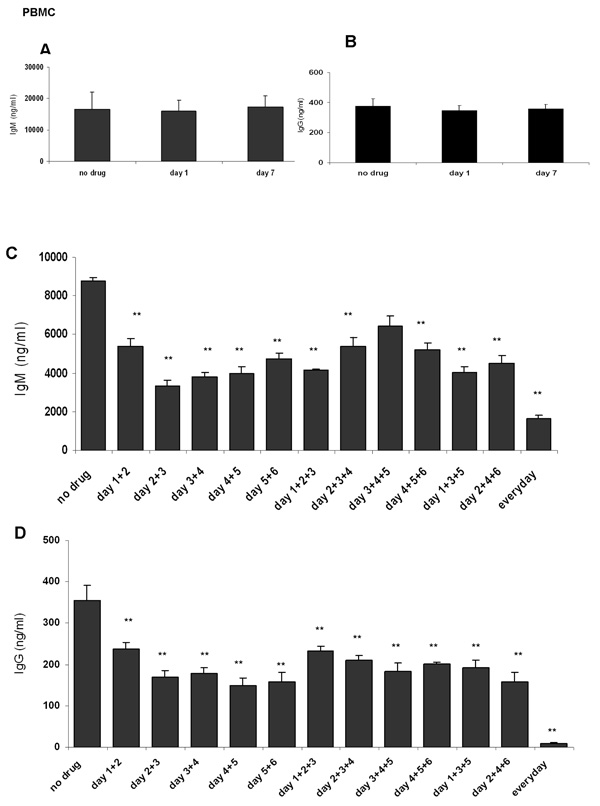
Ibuprofen is prescribed for daily use in patients with rheumatoid arthritis and is also recommended for transitory inflammation or pain, therefore the dosage and administration regimen widely varies. Moreover, patients take widely divergent amounts of ibuprofen. To simulate this, ibuprofen was added to stimulated PBMCs only once, or two or three times during a 7 days culture period and IgM and IgG production was determined. A single dose of ibuprofen (on day 1 or day 7) was not sufficient to inhibit IgM or IgG synthesis (Figure 5A and 5B). Instead, daily ibuprofen treatment resulted in a ~ 4 fold decrease in IgM (Figure 5C) and virtually extinguished IgG (Figure 5D) synthesis. Unexpectedly, exposure to ibuprofen on as few as 2 days of culture decreased both IgM and IgG levels as potently as three exposures. The inhibition of IgM production was more striking when PBMC were exposed to ibuprofen during the first days of culture (days 1, 2 and/or 3), whereas administration of ibuprofen at later time points (day 5 and/or day 6) had a less dramatic effect (Figure 5C).
Figure 5. Time course of ibuprofen addition and inhibition of antibody synthesis.
PBMC were stimulated with anti-IgM plus CpG 2395 for 7 days in the presence or absence of ibuprofen (added on different days). Panel A and panel B show that only one dose of ibuprofen added at the beginning (day 1) or end of culture (day 7) does not affect IgM or IgG synthesis. Panel C and Panel D show that daily doses of ibuprofen (50 µM) caused a ~ 4 fold decrease in IgM and completely abrogated IgG synthesis. Two doses of ibuprofen decreased IgM and IgG production to the same extent as three doses. *, p< 0.05, **, p< 0.001 were calculated using one-way ANOVA test followed by Tukey’s post-hoc test.
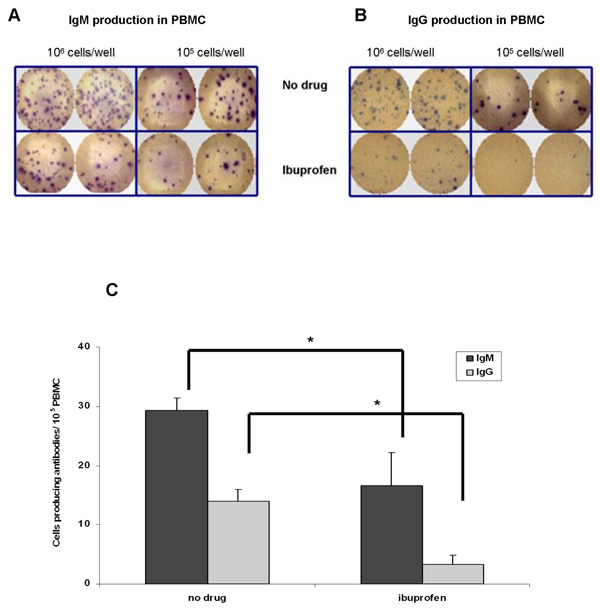
We next determined the frequency of cells which produce antibodies in stimulated PBMC by ELISPOT assay. Figures 6A–6C show that ibuprofen (added everyday) reduced the number of IgM and IgG antibody secreting cells by up to 60% compared to control (no drug) cells.
Figure 6. Ibuprofen decreases the number of antibody producing cells.
PBMC were stimulated with anti-IgM plus CpG 2395 for 7 days in the presence or absence of ibuprofen (50 µM; added everyday). Panel A and panel B show that ibuprofen reduced the numbers of antibody secreting cells. In panels C the number of spots was counted and their means +/− SD were plotted on the graph. There are more IgM-producing cells (~ 30 cells / 105 PBMC) compared to IgG (~18 cells/ 105 PBMC). In the presence of ibuprofen there are fewer IgM (~ 15 cells / 105 PBMC) and IgG (~ 3 cells/ 105 PBMC) producing cells. *, p< 0.05 was calculated using one-way ANOVA test followed by Tukey’s post-hoc test.
Ibuprofen decreases purified B lymphocyte antibody synthesis
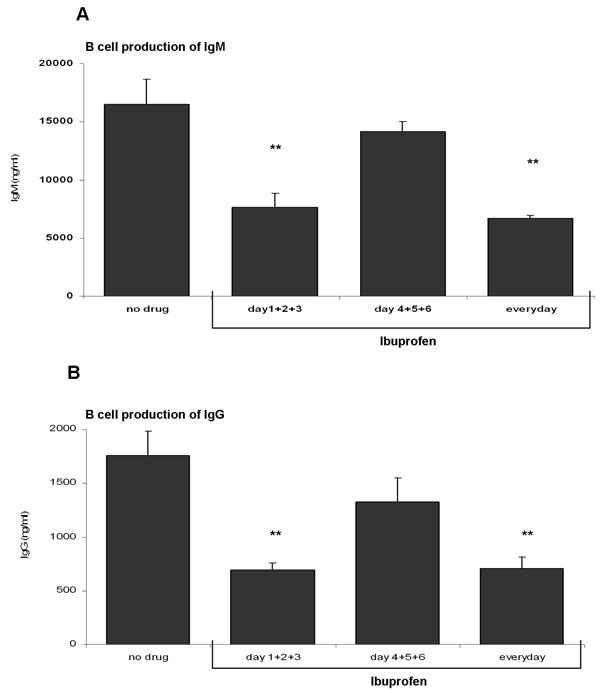
In order to study the effect of ibuprofen directly on B lymphocytes, highly purified peripheral blood B cells were stimulated with anti-IgM (2 µg/ml) + CpG 2395 (1 µg/ml) for 7 days. B cells were exposed to ibuprofen (50 µM) on different days and IgM and IgG synthesis determined. Figure 7A and 7B shows that stimulated B lymphocytes produce ~ 10 fold more IgM compared to IgG. Daily or three consecutive ibuprofen treatments (on days 1+2+3) potently repressed both IgM and IgG synthesis whereas ibuprofen exposures late in culture (days 4+5+6) had relatively little effect on antibody synthesis.
Figure 7. Ibuprofen inhibits antibody synthesis in purified human B lymphocytes.
Human B lymphocytes were stimulated with anti-IgM (2 µg/ml) plus CpG 2395 (1 µg/ml). Cells were exposed to ibuprofen (50 µM; added on various days of culture). Control cells (no drug) received only the vehicle (DMSO). IgG and IgM synthesis was determined on day 7 of culture. There is a decrease in IgM (panel A) and IgG (panel B) synthesis in B lymphocytes exposed to ibuprofen everyday or on the first days of culture (days 1+2+3). Ibuprofen added on the last days of culture (days 4+5+6) did not reach statistical significance. **, p< 0.001 (as determined by one-way ANOVA test followed by Tukey’s post-hoc test).
Ibuprofen only modestly influences B cell viability
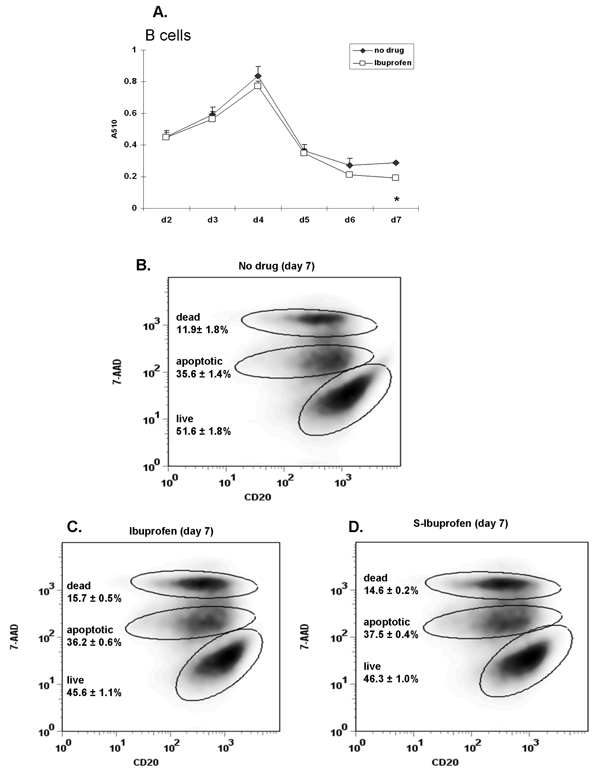
It is possible that ibuprofen decreases cell viability and this could result in decreased antibody production. To test such a scenario, the viability of purified B lymphocytes in the presence of ibuprofen (50 µM; added daily) was determined by MTT (colorimetric assay) and 7-AAD staining in two donors. Figure 8A (MTT assay) shows that viability of ibuprofen-treated B cells was nearly identical to vehicle (no drug) except for a slight decrease on day 7. 7-AAD staining provides the advantage of discriminating between dead (7-AAD bright) and apoptotic cells (7-AAD intermediate); viable cells do not incorporate 7-AAD. Ibuprofen-treated B lymphocytes (Figure 8C) show a small, but not statistically significant reduction in the percentage of living cells (~45% live versus ~51% in control cells) and a non-significant increase in the percentage of dead cells (~15% dead versus ~12% in control cells). These results support the MTT data and show that even daily doses of ibuprofen have only a very modest effect on B cell viability. In corroboration with these findings, inspection of cells by brightfield microscopy also showed no differences in viability (data not shown).
Figure 8. Ibuprofen only modestly inhibits purified B cell viability.
Purified human peripheral blood B cells isolated from 2 different donors were stimulated with anti-IgM (2 µg/ml) plus CpG 2395 (1 µg/ml) and were exposed to ibuprofen (50 µM; added daily) for 7 days. Cell viability was determined by MTT assay (donor 1) and by 7-AAD staining (donor 2). (A) The absorbance (A510 nm) values of ibuprofen-treated samples were similar to control (no drug) samples throughout day 2 to day 7. (B) Cells were surface stained for CD20 followed by 7-AAD staining and were analyzed by flow cytometry. A total of 15,000 events were acquired in each sample. Viable cells do not incorporate 7-AAD compared to apoptotic (7-AAD intermediate) and dead cells (7-AAD bright). The percentage of live, apoptotic and dead cells is similar in ibuprofen- (panel C) and S-Ibuprofen (panel D) treated samples. Cell percentages represent the mean values (n=3) with the corresponding SD. *, p< 0.05 (see panel A) was calculated using one-way ANOVA test followed by Tukey’s post-hoc test.
The anti-inflammatory property of ibuprofen has been mostly ascribed to S-ibuprofen enantiomer [27]. Since in our experiments ibuprofen was used (racemic mixture of S- and R- ibuprofen) we also determined if S-ibuprofen would have a more dramatic effect on B cells. Figure 8D shows that S-ibuprofen was virtually identical to R-ibuprofen.
Discussion
Because NSAIDs are one of the most commonly used class of drugs, we thought it important to investigate whether or not the over-the-counter NSAIDs would interfere with antibody synthesis. Here, we show that aspirin, ibuprofen, tylenol and naproxen (used at pharmacological concentrations) blunt antibody production in human PBMC in vitro. Low-dose aspirin (50–100 mg/day) has been shown to reduce the incidence of myocardial infarction and stroke [28], effects attributed to the irreversible inhibition of Cox-1 in platelets [29]. However, aspirin is also used as an anti-inflammatory agent (1200 mg/day) in which case, in vivo therapeutic levels are between 100– 2000 µM [30]. Tylenol (acetaminophen), frequently recommended as an antipyretic agent and often used for the treatment of osteoarthritis, is a relatively weak, nonselective inhibitor of Cox enzymes [31]. Its mechanism(s) of action remains widely debated [32]. Pharmacological studies show that in patients who received 1000 mg of tylenol (liquid or extra strength caplets), the acetaminophen plasma concentration was sustained between 50–200 µM [33]. Ibuprofen and naproxen are 2-arylpropionic acid derivatives and are used for the treatment of arthritis. Administration of ibuprofen (400 mg three times/day) translates to ~120 µM levels in plasma [21]. Here, we show that NSAIDs of divergent chemical structure and pharmacological properties, and well within the dose range achievable in humans, reduced both IgG and IgM synthesis.
Ibuprofen, available under Motrin, Advil or Nuprin brand names, is better tolerated and causes fewer side-effects compared to other NSAIDs [34, 35]. Despite advantages offered by ibuprofen compared to other NSAIDs, we report that in vitro ibuprofen (50–100 µM) blunts antibody synthesis. Ibuprofen had its major influence on antibody production when administered early (day 2 and 3) in our culture system. Using purified B cells, we also show that ibuprofen added late in culture (day 3+ 4+ 5) had relatively little effect on antibody production (Figure 4 and 6). The half-life of ibuprofen in cell culture is estimated to be ~ 2 hours [36], thus high doses of ibuprofen cannot accumulate in culture.
Ibuprofen might affect B cell viability, proliferation and/or differentiation, all contributing to reduced antibody production. Ibuprofen had relatively little effect on B cell viability (Figure 8). It was reported that ibuprofen can regulate the activity of cell cycle proteins. Ibuprofen decreased the expression of cyclin A and cyclin B (cell cycle promoters), but increased the cell cycle inhibitor protein p27Kip1, which resulted in cell cycle arrest of human colon carcinoma cells [37]. Such a scenario could take place in B lymphocytes as well. The dramatic antibody inhibition caused by ibuprofen addition during the first days of culture indicates that ibuprofen likely targets only small, resting, B lymphocytes and has little or no effect once B cells have divided and have undergone several cycles of division. Therefore, we analyzed the expression of several surface activation markers including CD25, CD69 and CD83 on B cells cultured in the presence of NSAIDs but we were not able to detect any differences in these markers (data not shown).
Ibuprofen is a non-selective Cox-1/Cox-2 inhibitor. In order to identify whether its ability to blunt antibody production was dependent on Cox-1, Cox-2 or both, we used Cox-2−/− spleen cells from mice. While IgM synthesis was inhibited, IgG production was not affected by ibuprofen in Cox-2 deficient mice. These results would suggest that Cox-2 is responsible for ibuprofen-mediated IgG, but not IgM inhibition. However, due to the fact that LPS activated Cox-2 deficient splenocytes produced less IgG raises the possibility that Cox-2 regulates isotype switching not IgG synthesis.
The immunomodulatory effects of NSAIDs are not fully understood and are far from being completely elucidated. Cox-derived prostaglandins, such as PGE2, can alter cytokine production by promoting synthesis of type-2 cytokines (IL-4, IL-5 and IL-10) and inhibit production of type 1 cytokines (IFN-γ and IL-12) [38]. Type-2 cytokines are implicated in humoral immune responses (antibody synthesis) whereas type-1 are generally more involved in cellular immunity. Thus, besides affecting B lymphocyte proliferation, NSAIDs might also influence cytokine production and ultimately antibody synthesis. We have attempted to add back certain PGs to ibuprofen-treated human cells and have yet to discover the key PG(s) that can reverse ibuprofen effects.
Inflammation, with its cardinal features: swelling, pain, redness and fever, constitute the primal response to infections, irritations or injuries and is partly mediated by PGE2 production [26]. Thus, administration of ibuprofen during the first stages of inflammation can have repercussions on the immune system by interfering with optimal antibody production. Vaccinations, designed to evoke an immune response, greatly depend on the functions of B lymphocytes. NSAID administration in patients with rheumatoid arthritis or due to a transitory pain or swelling at the sites of injection may be contraindicated in the first days following vaccination. The importance of Cox-2 activity for antibody production following vaccination was recently reported by us employing Cox-2 knock-out mice [39]. Vaccination of Cox-2-deficient mice with human papillomavirus-like particles (HPV) resulted in an impaired immune response characterized by inhibition of antibody synthesis (mostly IgG isotypes) [39].
The connection between NSAIDs and antibody synthesis is just beginning to be discovered. Given that NSAIDs inhibit Cox activity and Cox-2 is expressed in activated B lymphocytes and is required for optimal antibody production, it is pertinent to predict that NSAID therapy can have repercussions on antibody synthesis. Our preliminary observations (unpublished data) show that human subjects vaccinated with influenza vaccine taking NSAIDs had a decrease in antibody against certain influenza antigens. A full clinical study is required to determine if vaccinated subjects taking NSAIDs, e.g. ibuprofen, at different time points before or after vaccination will show a decrease in antibody synthesis.
In conclusion, we report that a panel of widely used NSAIDs blunts antibody synthesis in human PBMCs and in purified B cells. Ibuprofen’s ability to reduce antibody production was concentration- and time-dependent and likely occurred via Cox-2 inhibition. Our results call for awareness regarding the consequences that NSAIDs can have on immunity. NSAIDs are one of the most commonly used drugs; they are recommended for all age categories, are prescribed for relieving transient pain or in cases of serious inflammatory diseases. By decreasing antibody synthesis, NSAIDs also have the ability to weaken the immune system which can have serious consequences for children, the elderly and the immune-compromised patients.
Acknowledgments
This research was supported by: USPHS grants, DE011390, ES01247, AI071064 and T-32-DE007202.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dai C, Stafford RS, Alexander GC. National trends in cyclooxygenase-2 inhibitor use since market release: nonselective diffusion of a selectively cost-effective innovation. Arch. Intern. Med. 2005;165:171–177. doi: 10.1001/archinte.165.2.171. [DOI] [PubMed] [Google Scholar]
- 2.Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24:985–990. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- 3.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 4.Chiroli S, Chinellato A, Didoni G, Mazzi S, Lucioni C. Utilisation Pattern of Nonspecific Nonsteroidal Anti-Inflammatory Drugs and COX-2 Inhibitors in a Local Health Service Unit in Northeast Italy. Clin. Drug. Investig. 2003;23:751–760. doi: 10.2165/00044011-200323110-00008. [DOI] [PubMed] [Google Scholar]
- 5.FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N. Engl. J. Med. 2001;345:433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- 6.Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998;104:413–421. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 7.Purcell P, Henry D, Melville G. Diclofenac hepatitis. Gut. 1991;32:1381–1385. doi: 10.1136/gut.32.11.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fored CM, Ejerblad E, Lindblad P, Fryzek JP, Dickman PW, Signorello LB, Lipworth L, Elinder CG, Blot WJ, McLaughlin JK, Zack MM, Nyren O. Acetaminophen, aspirin, and chronic renal failure. N. Engl. J. Med. 2001;345:1801–1808. doi: 10.1056/NEJMoa010323. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen HT, Juurlink DN. Recurrent ibuprofen-induced aseptic meningitis. Ann. Pharmacother. 2004;38:408–410. doi: 10.1345/aph.1D329. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler P, Batt M. Do non-steroidal anti-inflammatory drugs adversely affect stress fracture healing? Br. J. Sport Med. 2005;39:65–69. doi: 10.1136/bjsm.2004.012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cryer B, Kimmey MB. Gastrointestinal side effects of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998;105:20S–30S. doi: 10.1016/s0002-9343(98)00071-0. [DOI] [PubMed] [Google Scholar]
- 12.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 13.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 14.Inigues M, Punzon C, Fresno M. Induction of Cyclooxygenase-2 on Activated T Lymphocytes: Regulation of T Cell Activation by Cyclooxygenase-2 Inhibitors. J. Immunol. 1999;163:111–119. [PubMed] [Google Scholar]
- 15.Paccani SR, Boncristiano M, Ulivieri C, D'Elios MM, Del Prete G, Baldari CT. Nonsteroidal anti-inflammatory drugs suppress T-cell activation by inhibiting p38 MAPK induction. J. Biol. Chem. 2002;277:1509–1513. doi: 10.1074/jbc.M110676200. [DOI] [PubMed] [Google Scholar]
- 16.Hartel C, von Puttkamer J, Gallner F, Strunk T, Schultz C. Dose-dependent Immunomodulatory Effects of Acetylsalicylic Acid and Indomethacin in Human Whole Blood: Potential Role of Cyclooxygenase-2 Inhibition. Scand. J. Immunol. 2004;60:412–420. doi: 10.1111/j.0300-9475.2004.01481.x. [DOI] [PubMed] [Google Scholar]
- 17.Ryan EP, Pollock SJ, Murant TI, Bernstein SH, Felgar RE, Phipps RP. Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J. Immunol. 2005;174:2619–2626. doi: 10.4049/jimmunol.174.5.2619. [DOI] [PubMed] [Google Scholar]
- 18.Bernard MP, Phipps RP. CpG oligodeoxynucleotides induce cyclooxygenase-2 in human B lymphocytes: implications for adjuvant activity and antibody production. Clin. Immunol. 2007;125:138–148. doi: 10.1016/j.clim.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emori HW, Bluestone R, Champion GD, Pearson C. Indomethacin serum concentrations in man. Effects of dosage, food, and antacid. Ann. Rheum. Dis. 1973;35:333–338. doi: 10.1136/ard.35.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitlam JB, Brown KF, Crooks MJ, Room GF. Transsynovial distribution of ibuprofen in arthritic patients. Clin. Pharmacol. Ther. 1981;29:487–492. doi: 10.1038/clpt.1981.67. [DOI] [PubMed] [Google Scholar]
- 21.Blain H, Boileau C, Lapicque F, Nedelec E, Loeuille D, Guillaume C, Gaucher A, Jeandel C, Netter P, Jouzeau JY. Limitation of the in vitro whole blood assay for predicting the COX selectivity of NSAIDs in clinical use. Br. J. Clin. Pharmacol. 2002;53:255–265. doi: 10.1046/j.0306-5251.2001.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rainsford KD. Aspirin and Related Drugs. London: CRC Press; 2004. [Google Scholar]
- 23.Pernerstorfer T, Schmid R, Bieglmayer C, Eichler HG, Kapiotis S, Jilma B. Acetaminophen has greater antipyretic efficacy than aspirin in endotoxemia: a randomized, double-blind, placebo-controlled trial. Clin. Pharmacol. Ther. 1999;66:51–57. doi: 10.1016/S0009-9236(99)70053-6. [DOI] [PubMed] [Google Scholar]
- 24.Gelotte CK, Auiller JF, Lynch M, Temple AR, Slattery JT. Disposition of Acetaminophen at 4, 6, and 8 g/day for 3 Days in Healthy Young Adults. Clin. Pharmacol. Ther. 2007;81:840–848. doi: 10.1038/sj.clpt.6100121. [DOI] [PubMed] [Google Scholar]
- 25.Capone ML, Tacconelli S, Di Francesco L, Sacchetti A, Sciulli MG, Patrignani P. Pharmacodynamic of cyclooxygenase inhibitors in humans. Prostag. Other Lipid Mediat. 2007;82:85–94. doi: 10.1016/j.prostaglandins.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Davies P, Bailey PJ, Goldenberg MM, Ford-Hutchinson AW. The role of arachidonic acid oxygenation products in pain and inflammation. Annu. Rev. Immunol. 1984;2:335–357. doi: 10.1146/annurev.iy.02.040184.002003. [DOI] [PubMed] [Google Scholar]
- 27.Neupert W, Brugger R, Euchenhofer C, Brune K, Geisslinger G. Effects of ibuprofen enantiomers and its coenzyme A thioesters on human prostaglandin endoperoxide synthases. Br. J. Pharmacol. 1997;122:487–492. doi: 10.1038/sj.bjp.0701415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antithrombotic Trialists' Collaboration, Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Br. Med. J. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loll PJ, Picot D, Garavito RM. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat. Struct. Biol. 1995;2:637–643. doi: 10.1038/nsb0895-637. [DOI] [PubMed] [Google Scholar]
- 30.Borthwick GM, Johnson AS, Partington M, Burn J, Wilson R, Arthur HM. Therapeutic levels of aspirin and salicylate directly inhibit a model of angiogenesis through a Cox-independent mechanism. Faseb J. 2006;20:2009–2016. doi: 10.1096/fj.06-5987com. [DOI] [PubMed] [Google Scholar]
- 31.Ouellet M, Percival MD. Mechanism of acetaminophen inhibition of cyclooxygenase isoforms. Arch. Biochem. Biophys. 2001;387:273–280. doi: 10.1006/abbi.2000.2232. [DOI] [PubMed] [Google Scholar]
- 32.Lucas R, Warner TD, Vojnovic I, Mitchell JA. Cellular mechanisms of acetaminophen: role of cyclo-oxygenase. Faseb J. 2005;19:635–637. doi: 10.1096/fj.04-2437fje. [DOI] [PubMed] [Google Scholar]
- 33. www.tylenolprofessional.com, published by McNeil Consumer Healthcare, a Division of McNEIL-PPC, Inc.
- 34.Moore N. Diclofenac potassium 12.5mg tablets for mild to moderate pain and fever: a review of its pharmacology, clinical efficacy and safety. Clin. Drug Investig. 2007;27:163–195. doi: 10.2165/00044011-200727030-00002. [DOI] [PubMed] [Google Scholar]
- 35.Hay AD, Costelloe C, Redmond NM, Montgomery AA, Fletcher M, Hollinghurst S, Peters TJ. Paracetamol plus ibuprofen for the treatment of fever in children (PITCH): randomised controlled trial. Br. Med. J. 2008;337:a1302. doi: 10.1136/bmj.a1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanins M, Adams WJ, Kaiser DG, Halstead GW, Baillie TA. Studies on the metabolism and chiral inversion of ibuprofen in isolated rat hepatocytes. Drug Metab. Dispos. 1990;18:527–533. [PubMed] [Google Scholar]
- 37.Janssen A, Maier TJ, Schiffmann S, Coste O, Seegel M, Geisslinger G, Grosch S. Evidence of COX-2 independent induction of apoptosis and cell cycle block in human colon carcinoma cells after S- or R-ibuprofen treatment. Eur. J. Pharmacol. 2006;540:24–33. doi: 10.1016/j.ejphar.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 38.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 39.Ryan EP, Malboeuf CM, Bernard M, Rose RC, Phipps RP. Cyclooxygenase-2 inhibition attenuates antibody responses against human papillomavirus-like particles. J. Immunol. 2006;177:7811–7819. doi: 10.4049/jimmunol.177.11.7811. [DOI] [PubMed] [Google Scholar]