SUMMARY
Background
A 17-year-old male presented with pain in his lower-left chest. He had no significant medical history and was previously in good health. He had a fractured ninth left anterior rib and the tenth, eleventh and twelfth ribs were absent, which was thought to be a congenital anomaly. Several months later, he presented again with back pain, an enlarging mass in the lower-left chest wall, erosion of the lateral pedicles of the lower thoracic vertebrae and pleural effusion.
Investigations
Physical examination, chest X-ray, MRI of the spine, incisional biopsy, serial CT imaging of the hemithorax, immunohistochemistry, flow cytometry, and enzyme-linked immunosorbent assays.
Diagnosis
Gorham’s lymphangiomatosis with expression of platelet-derived growth factor receptor-β and elevated circulating platelet-derived growth factor-BB.
Management
Spine stabilization, thalidomide, celecoxib, interferon-α2b, pamidronate, zoledronate, thoracotomy, pleurectomy, talc pleurodesis, and imatinib mesylate.
Keywords: Gorham’s disease, imatinib mesylate, lymphangiomatosis, pediatric, platelet-derived growth factor (PDGF)
This article offers the opportunity to earn one Category 1 credit toward the AMA Physician’s Recognition Award.
THE CASE
A 17-year-old male presented to his doctor in June 2000 with pain in his lower-left chest. The patient had no significant medical history and was in good health and participating in athletic activities when the pain began, shortly before his presentation. A chest X-ray revealed a nondisplaced fracture of the ninth left anterior rib and absence of the tenth, eleventh, and twelfth ribs, which was thought to be a congenital anomaly. The pain continued, prompting multiple medical visits. Five months later, the patient noticed a small, slowly enlarging mass in the lower-left chest wall accompanied by moderate, but worsening back pain. After a further 4 months, a repeat chest X-ray showed a large, left pleural effusion and erosion of lateral spine pedicles from the T10 to the T12 vertebrae. MRI demonstrated involvement of the lower cervical spine as well as the lower thoracic spine. In March 2001, a CT scan demonstrated the absence of the ninth to twelfth left ribs, erosion of the pedicles of the lower thoracic vertebrae, tumor invasion into the spine and pleural effusion (Figure 1A–C).
Figure 1.
CT and MRI scans showing disease progression in the patient. (A) Scout image from CT in March 2002 showing absence of left ribs 9–12 (purple arrowhead) and pleural effusion (green arrowhead). (B,C) Axial CT images from March 2001 showing erosion of spinal pedicles and invasion into bone (red arrowhead) by the tumor (blue arrowhead). The pleural effusion (green arrowhead) and absence of ribs (purple arrowhead) are also apparent. (D,E) Sagittal MRI scans from February 2002 showing destruction of the lower thoracic vertebrae (red arrowheads).
The patient underwent an incisional biopsy in March 2001, which revealed lymphangiomatosis. Several weeks later, a spine stabilization procedure was performed to prevent vertebral body collapse of the T10 to T12 vertebrae. Resection of bone and soft tissue from the T9 to T10 vertebrae demonstrated angiomatous proliferation, consistent with Gorham’s lymphangiomatosis.
The patient was started on a 2-year course of thalidomide (200 mg/day) and celecoxib (200 mg twice daily). In February 2002, an MRI scan showed the presence of persistent disease in the spine (Figure 1D,E). Progressive pain prompted evaluation by the Pediatric Hematology/ Oncology service at the authors’ institution in July 2002 and the patient commenced interferon (IFN)-α2b (3 million units/day) at this time. In January 2003, pamidronate (90 mg/month) was started in an attempt to inhibit the debilitating osteolysis. In February 2003, the doses of thalidomide, IFN-α2b, and celecoxib were doubled. In March 2003, the patient showed signs of muscle wasting and complained of fatigue and generalized aches. As no evidence of substantial benefit from IFN-α2b therapy had been observed, this drug was discontinued, and pamidronate was switched to intravenous zoledronate (4 mg/month). Radiotherapy was considered at this point, but the volume of normal lung tissue in required radiation fields, and the patient’s already compromised respiratory status, precluded the safe delivery of a radiation dose sufficient to achieve local-disease control (40–45 Gy1).
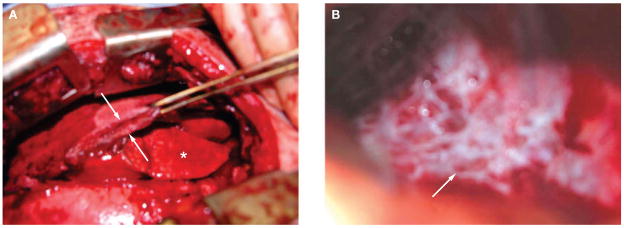
Despite pharmacological intervention, the patient’s clinical status continued to deteriorate, with dyspnea and worsening pain. The patient subsequently developed a right-sided chylothorax and his dyspnea became disabling. In early August 2004 he underwent a thoracotomy, pleurectomy, and talc pleurodesis to re-expand the right lung, but could not be extubated after surgery. During surgery, a massively thickened parietal pleura (Figure 2A) and abundant, leaky lymphatic vessels on the inner chest wall (Figure 2B) were observed. In September 2004, the patient was transferred from the intensive care unit following prolonged admission due to profound muscle weakness, ascites and pseudomonal colonization of his lungs.
Figure 2.
Images from the patient’s thoracotomy. (A) A massively thickened parietal pleura (between arrows; asterisk denotes normal lung). (B) Numerous lymphatic vessels visible on the interior surface of the chest wall (arrow).
To determine the presence of blood and lymphatic vessel markers on the endothelial cells of the abnormally proliferating vasculature in this patient’s Gorham’s lymphangiomatosis, specimens taken during surgery were stained for the panendothelial marker CD31 (platelet endothelial cell adhesion molecule), lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) and VEGF receptor (VEGFR)-3. All vessel structures expressed CD31 (Figure 3A) and approximately 90% of the CD31- positive endothelial cells also expressed LYVE-1, indicating that the proliferating vasculature associated with Gorham’s lymphangiomatosis consisted mainly of lymphatic endothelium (Figure 3B). Staining detected VEGFR-3, the receptor for VEGF-C and VEGF-D, in immune cells/macrophages in the tissue and in less than half of the proliferating vessels (Figure 3C).
Figure 3.
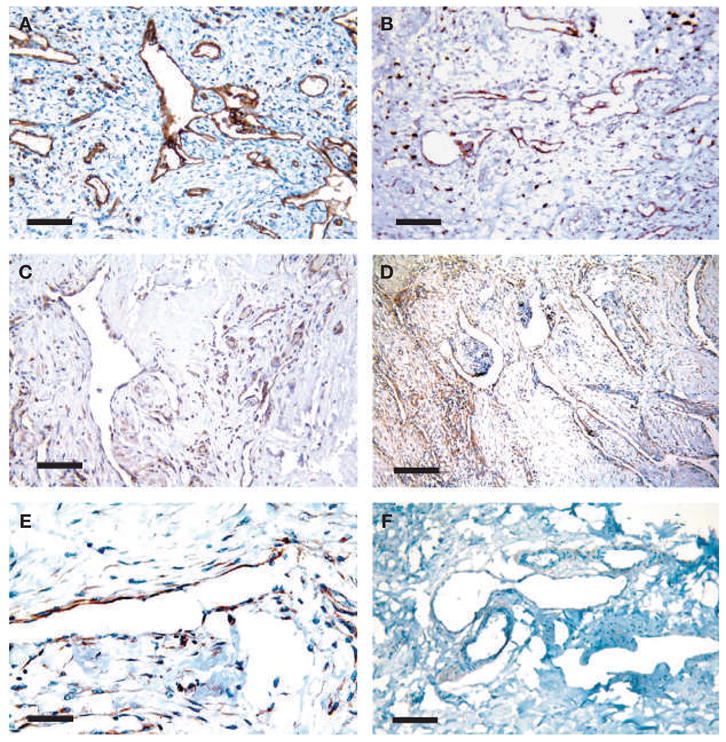
Immunohistochemical analysis of the patient’s tumor tissue. (A) All proliferating microvascular structures in the tumor stroma express the panendothelial marker CD31. (B) 90% of these vessels express the lymphatic endothelial cell marker LYVE-1. (C) VEGFR-3, a receptor for the lymphangiogenic factor VEGF-C, is expressed by immune cells and some of the vessels. (D,E) PDGFR-β is expressed around these proliferating lymphatic vessels. (F) Control lymphangiomatosis does not express PDGFR-β. Scale bars: (A), (B), (C), (F) 100 μm, (D) 200 μm, and (E) 50 μm.
The lymphatic endothelium was also examined for expression of lymphangiogenic or angiogenic growth factors that might drive the vessel proliferation in Gorham’s lymph-angiomatosis, and thus form a potential therapeutic target for blockade with therapeutic inhibitors. The patient’s Gorham’s lymphangiomatosis tissue, archived non-Gorham’s lymph-angiomatosis (control) and several normal archived tissues, were stained immunohistochemically for VEGF, VEGF-C, platelet-derived endothelial growth factor receptor (PDGFR)-β and epidermal growth factor (EGF). VEGF, VEGF-C and EGF did not stain strongly in Gorham’s lymphangiomatosis or in control lymphangiomatosis compared with normal human skin. PDGFR-β staining was present in endothelial cells and cells directly adjacent to most vessels in the patient’s lymphangiomatosis (Figure 3D, E), but not in control lymphangiomatosis (Figure 3F), normal skin or normal pleura.
To confirm the potential role of the PDGFR-β receptor pathway in Gorham’s lymphangiomatosis, enzyme-linked immunosorbent assays (ELISAs) for PDGF-BB and VEGF were performed on the patient’s plasma. The circulating level of PDGF-BB was elevated 7-fold (108 ± 4 pg/ml) compared with control samples (15 ± 4 pg/ml), while VEGF level was within normal limits (100 ± 14 pg/ml). A multiplexed ELISA found that plasma basic fibro-blast growth factor was also slightly elevated (27 ± 1 pg/ml; normal range 0–14 pg/ml), while placental growth factor (24 ± 1 pg/ml; normal range 0–24 pg/ml) and soluble VEGFR-1 (157 ± 11 pg/ml, normal range 55–123 pg/ml) were within normal limits. Circulating endothelial cells, which are thought to be mobilized by angiogenic factors such as VEGF, were relatively abundant, representing 2.3% of all mononuclear cells (compared to an average of 1.7% measured in ten rectal cancer patients2 and almost undetectable levels in healthy individuals3). Collectively, these data suggested the diagnosis of Gorham’s lymph angiomatosis with expression of PDGFR-β and elevated circulating PDGF-BB.
As a result of the elevated circulating levels of PDGF-BB and the presence of PDGFR-β in the tumor, the patient was started on imatinib mesylate (400 mg/day) in early October 2004. After 6 days of this treatment, however, his respiratory status declined with reaccumulation of pleural fluid. Imatinib mesylate was discontinued, and the patient’s respiratory status improved over the following week. The patient was discharged from hospital in late October 2004. A second trial of imatinib mesylate (200 mg/day) was commenced 4 weeks later when the patient’s overall function had improved, but this was aborted when he reported increasing respiratory difficulty within 3 days of starting medication. During subsequent months, the patient’s respiratory status and overall condition gradually deteriorated as a result of disease progression and he died 46 months after the initial diagnosis.
DISCUSSION OF DIAGNOSIS
Lymphatic malformations are rare, benign lesions consisting of abnormally formed lymphatic vessels.4 These malformations generally arise in the skin and soft tissues, predominantly in the head and neck region, axilla, and visceral organs, and most are clinically apparent by 5 years of age. Multifocal or diffuse growth of abnormal lymphatic vessels is termed lymphangiomatosis. Gorham’s disease is a potentially lethal form of lymphangiomatosis, characterized by a proliferation of lymphatic vessels accompanied by osteolysis.5,6 Gorham’s disease is rare, with less than 200 cases reported in the literature, and usually presents in young adults without gender or race predilection. The survival rate for patients with Gorham’s disease is approximately 60%, based on these published cases. In about 15% of patients, the course of the disease is complicated by thoracic involvement accompanied by chylothorax, as in this patient. Thoracic involvement carries a worse prognosis with a survival rate of less than 40%. To the authors’ knowledge, there are no reports in the literature that describe progression to lymphangiosarcoma. The vascular proliferation of Gorham’s disease can destroy adjacent soft tissues, organs and bones by as yet unknown mechanisms. The etiology and pathogenesis of lymphangiomatosis and Gorham’s disease also remain unknown.
Recently, a number of growth factors that stimulate the proliferation of lymphatic vessels and lymphangiogenesis have been identified,7,8 including VEGF-A, VEGF-C, and PDGF-BB. The PDGF-signaling pathway stimulates tumor-cell proliferation, angiogenesis, and pericyte recruitment to tumor blood vessels.9 Interestingly, a recent report showed that PDGF-BB expression is associated with the formation of abnormal initial lymphatics in human lymphedema disti-chiasis,10 whereas another report suggested that PDGF-BB is directly lymphangiogenic.11 The immunohistochemical profiles of several of these lymphangiogenic factors were examined in tissue specimens from this patient. More than 90% of the proliferating vessels expressed LYVE-1, a relatively specific marker for lymphatic endothelial cells that is also expressed in liver and spleen sinusoids.12 This finding suggests that these vessels were mainly of lymphatic endothelial origin, consistent with what has previously been proposed.13 These vessels also expressed PDGFR-β and this finding, together with the elevated level of circulating PDGF-BB in the patient’s plasma, support a potential role for the PDGF-signaling pathway in Gorham’s lymphangiomatosis. Interestingly, strong staining of PDGFR-β, VEGF, VEGF-C, or EGF was not detected in two other patients with lymph angiomatosis who were used as control tissues for this case. Unfortunately, it was not possible to determine whether PDGFR-β was phosphorylated in this patient, as reliable immunohistochemical staining for phospho-PDGFR-β could not be obtained. It is possible that the pathogenesis of these disorders is different; lymphatic malformations that arise during early childhood might stem from aberrant sequestrations of lymphatic vessels,13 while the acquired proliferation of small lymphatics observed in Gorham’s disease might be driven by lymphangiogenic growth factors, such as PDGF-BB.
The number of molecules known to be involved in lymphangiogenesis is increasing, providing new targets for the treatment of tumors consisting of proliferating lymphatic vessels. Angiopoietin-1, angiopoietin-2, VEGF-D and hepatocyte growth factor are other molecules known to play a role in lymphatic vessel growth and maturation. These molecules were not assessed in this case, as there are no drugs currently available that interrupt their molecular signaling pathways.
TREATMENT AND MANAGEMENT
Current successful therapies for Gorham’s disease are limited to aggressive surgery aimed at preventing reaccumulation of fluid in the pleural cavity, IFN-α2b, which is also used to treat other highly vascular lesions including hemangiomas and giant-cell lesions,14,15 and radiotherapy.1 This patient also received bisphosphonates in the hope that their antiresorptive and antiangiogenic properties might arrest the unremitting progression of his osteolytic disease.6,16 The evidence for expression of PDGF-BB and its receptor PDGFR-β in Gorham’s disease suggests that targeted therapy with PDGFR-blocking agents, such as imatinib mesylate, might also be beneficial in certain cases of lymphangiomatosis. Imatinib mesylate17 and other agents that block the PDGFR pathway are, however, associated with fluid exudates and edema, which can cause pleural effusions, pulmonary edema, ascites, or pericardial effusion. The mechanism by which imatinib mesylate causes these imbalances in tissue-fluid homeostasis is currently unknown. A preclinical animal study is needed to determine the mechanism, the understanding of which would help guide the use of imatinib mesylate in the future. PDGFR blockade also has other possible toxic effects involving the integuementary, hematologic, dermatologic, and gastrointestinal systems. This patient had a pre-existing pleural effusion and compromised pulmonary function, making fluid retention and its deleterious effects on respiratory function the most alarming potential adverse event. Although worsening pleural effusions and deteriorating respiratory function necessitated discontinuation of imatinib mesylate in this patient, this novel therapy could be of benefit to patients with PDGF-driven lymphangiomas in extrapleural sites. Alternatively, if complications related to fluid exudates develop, agents directed at blocking this toxic side effect might be employed. These include molecules that reduce microvascular permeability, such as VEGF inhibiting antibodies and phosphotyrosine kinase inhibitors.18,19
CONCLUSION
Gorham’s disease is a rare and devastating form of lymphangiomatosis with unknown etiology. The data from this patient show that the PDGFR signaling pathway could be implicated in the pathogenesis of this disease. Unfortunately, in this case, declining respiratory function after the start of imatinib mesylate therapy necessitated cessation of the drug before the potential efficacy of PDGFR-β blockade could be determined. Clinicians should be aware of the possibility that PDGFR signaling could be important in Gorham’s disease. Future evaluation of PDGFR-β and its ligands in patients with Gorham’s disease could provide new insight into the pathogenesis of this disease.
As a larger portfolio of specific inhibitors becomes available to clinicians, the ability to identify therapeutics that will positively impact individual patients will become increasingly important. Similarly, the toxicity profiles of therapeutic agents should be considered when tailoring treatments. This case illustrates the potential for individualized, targeted therapy and highlights the need to tailor therapy based on both the presence of therapeutic targets and the complications those therapies might induce in the individual patient.
Acknowledgments
The authors thank R Padera, K Cohen, Y-L Chen and M Booth for their help in obtaining and analyzing samples; K Kozak and J Lahdenranta for help and advice; and C Smith for excellent technical assistance. This study was supported in part by the NIH (Bioengineering Research Partnership grant R01-CA85140).
Footnotes
Competing interests
RK Jain has declared associations with the following companies/ organizations: AstraZeneca, Novartis, SK Bio-Pharmaceuticals and GLG Life Tech Ltd. See the article online for full details of the relationship. The other authors declared they have no competing interests.
References
- 1.Dunbar SF, et al. Gorham’s massive osteolysis: The role of radiation therapy and a review of the literature. Int J Radiat Oncol Biol Phys. 1993;26:491–497. doi: 10.1016/0360-3016(93)90968-2. [DOI] [PubMed] [Google Scholar]
- 2.Willett CG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 3.Mancuso P, et al. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood. 2001;97:3658–3661. doi: 10.1182/blood.v97.11.3658. [DOI] [PubMed] [Google Scholar]
- 4.Davis KK, et al. Thoracic lymphatic disorders. Lymphat Res Biol. 2004;2:131–137. doi: 10.1089/lrb.2004.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37-A:985–1004. [PubMed] [Google Scholar]
- 6.Moller G, et al. The Gorham–Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81:501–506. doi: 10.1302/0301-620x.81b3.9468. [DOI] [PubMed] [Google Scholar]
- 7.Hagendoorn J, et al. Molecular regulation of microlymphatic formation and function: role of nitric oxide. Trends Cardiovasc Med. 2005;15:169–173. doi: 10.1016/j.tcm.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Tammela T, et al. Molecular lymphangiogenesis: new players. Trends Cell Biol. 2005;15:434–441. doi: 10.1016/j.tcb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 10.Petrova TV, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 11.Cao R, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 12.Mouta Carreira C, et al. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084. [PubMed] [Google Scholar]
- 13.Weiss SW, Goldblum JR. Tumors of lymph vessels. In: Weiss SW, et al., editors. Soft Tissue Tumors. St Louis: CV Mosby, Inc; 2001. pp. 955–983. [Google Scholar]
- 14.Ezekowitz RA, et al. Interferon alfa-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med. 1992;326:1456–1463. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- 15.Fujiu K, et al. Chylothorax associated with massive osteolysis (Gorham’s syndrome) Ann Thorac Surg. 2002;73:1956–1957. doi: 10.1016/s0003-4975(02)03413-6. [DOI] [PubMed] [Google Scholar]
- 16.Hammer F, et al. Gorham–Stout disease—stabilization during bisphosphonate treatment. J Bone Miner Res. 2005;20:350–353. doi: 10.1359/JBMR.041113. [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. [accessed 12 October 2005];Gleevec® revised product label. [ http://www.fda.gov/cder/foi/label/2003/021588s002lbl.pdf] (online 8 December 2003)
- 18.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 19.Jain RK, et al. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]