Abstract
The first International Caenorhabditis elegans Experiment (ICE-First) was carried out using a Russian Soyuz spacecraft from April 19-30, 2004. This experiment was a part of the program of the DELTA (Dutch Expedition for Life science Technology and Atmospheric research) mission, and the space agencies that participate in the International Space Station (ISS) program formed international research teams. A Japanese research team that conducted by Japan aerospace Exploration Agency (JAXA) investigated the following aspects of the organism: (1) whether meiotic chromosomal dynamics and apoptosis in the germ cells were normal under microgravity conditions, (2) the effect of the space flight on muscle cell development, and (3) the effect of the space flight on protein aggregation. In this article, we summarize the results of these biochemical and molecular biological analyses.
Introduction
The nematode Caenorhabditis elegans is a model organism for the study of most biological functions involved in development and differentiation. Further, it is advantageous for space-flight experiments due to its compact body size and easy handling.
The first International C. elegans Experiment (ICE-First) involved an international cooperative research between Canadian Space Agency (CSA), Centre National d’Etudes Spatiales (CNES), European Space Agency (ESA), National Aeronautics and Space Administrations (NASA), Japan Aerospace Exploration Agency (JAXA), and the Space Research Organization of the Netherlands (SRON). This flight experiment was a part of the program of the Dutch Expedition for Life science, Technology, and Atmospheric research (DELTA) and was carried out using a Russian Soyuz spacecraft from April 19-30, 2004. The research teams of each nation analyzed several aspects of the space-flown nematodes, such as gene expression, motility, apoptosis, muscular atrophy, etc. In this article, we summarize the results of the biochemical and molecular biological analyses that were carried out on the nematodes in Japan. The experimental team comprised four research groups, namely, those of Dr. Ishioka (JAXA), Dr. Higashitani (Tohoku University), Dr. Kagawa (Okayama University), and Dr. Honda (Tokyo Metropolitan Institute of Gerontology). The mission was accomplished successfully, and several results have already been published [1-4].
Experimental Objective of ICE-First
In this cooperative experiment, we investigated the following aspects of C. elegans: (1) whether meiotic chromosomal dynamics and apoptosis in the germ cells were normal under microgravity conditions, (2) the effect of the space flight on muscle cell development, and (3) the effect of the space flight on sarcomere integrity and age-related protein aggregation.
Flight Overview and Sample Preparation
The nematodes used in this experiment were prepared in Toulouse, France. After preparation, they were transported to Moscow. The ground control groups were kept in Moscow, and the experimental flight groups were transported to the launch site located at Baikonur, Kazakhstan. The Soyuz spacecraft was launched 5 days after the preparation of the samples, and it was docked 2 days after the launch. The flight experimental groups were at the International Space Station (ISS) for 10 days. The nematodes were cultured in a chemically defined liquid medium (CeMM: C. elegans Maintenance Medium) in order to exclude the surface tension incident on them [5]. The samples were maintained at 12°C in KUBIK until the spacecraft docked at the ISS. KUBIK is an incubator developed by ESA to perform biological experiments. In this experiment, two KUBIKs were uploaded: KUBIK Amber with an insert with a microgravity plate and a centrifuge, and KUBIK Topaz with only a microgravity plate. The uploaded worms were cultured in KUBIKs throughout the experiment. After docking, the samples were exposed to a temperature of 20°C. The samples were frozen in liquid nitrogen after the spacecraft was landed.
During the flight, the population size more than doubled for the space-flown as well as the ground control nematodes. Statistical analysis revealed no significant difference in the number of the ground control and flight groups of nematodes (p = 0.083). The total RNA and protein content per nematode were identical in the flight and ground samples. These results demonstrate that the two populations of nematodes developed equally well; no gross defects in growth had occurred due to the space flight.
Results
Summary of apoptosis analyses
During the development of C. elegans hermaphrodites, 131 of the 1090 somatic cells and approximately 300 germ cells undergo programmed cell death as physiological apoptosis [6, 7]. In addition, DNA damage induced apoptosis can occur at the meiotic pachytene nucleus stage of the germ cells [8]. Checkpoint-induced apoptosis is involved in maintaining genomic stability through the elimination of cells that have failed to repair DNA damage. However, the occurrence of checkpoint-induced and other types of physiological apoptosis in animals during or as a result of space flight has not been documented. In this experiment, we have analyzed the occurrence of checkpoint-induced and other types of physiological apoptosis in germ cells during space flight by using a well-characterized metazoan, the nematode C. elegans. Both DNA damage checkpoint-induced apoptosis and physiological apoptosis are mediated by the core apoptotic machinery (CED-9, CED-4, and CED-3) and the engulfment machinery (CED-1 and CED-2); however, the former apoptosis is differentially controlled by the checkpoint genes (atl-1, mrt-2, rad-5, and him-7). Most of these C. elegans genes are highly conserved in mammals [8, 9]. Comparison of the number of cell corpses in wild-type or ced-1 mutants, grown under either ground or space flight conditions, showed that both pachytene-checkpoint-induced apoptosis and physiological apoptosis in germ cells occurred normally under space flight conditions. In addition, apoptosis-related genes were expressed normally in the space-flown nematodes. This is the first report that documents the normal occurrence of several kinds of apoptosis, including checkpoint-induced apoptosis, in the space environment. This finding supports the hypothesis that humans would retain the ability to eliminate cells that have failed to repair cosmic radiation-induced DNA lesions during space flight. The details of these results have already been reported in another journal [1].
Summary of the molecular biological analyses of muscle development in the space-flown nematodes
The molecular mechanisms underlying muscle atrophy during space flight are not well understood. We analyzed the effects of a 10 day space flight on muscle development in wild-type C. elegans (Bristol N2) using biochemical and molecular biological techniques such as DNA microarray, real-time quantitative PCR, and quantitative western blot. The results of these analyses revealed that the amount of myosin heavy chains (MHC) in both the body-wall and pharyngeal muscles decreased in response to the space flight. Furthermore, decreased transcription of the body wall myogenic transcription factor HLH-1 (CeMyoD) and the three pharyngeal myogenic transcription factors PEB-1, CEH-22, and PHA-4 were also observed. Following their return to Earth, microscopic observation of the nematodes revealed reduced motility rates, indicating a functional defect. These results demonstrate that muscle development in C. elegans was altered in response to the space flight. This altered development occurred at the level of gene transcription and was observed in the presence of innervation, not simply in isolated cells. This important finding coupled with past reports of decreased transcription levels of the same myogenic transcription factors in space-flown vertebrates raises the possibility that altered muscle development is a contributing factor to space flight-induced muscle atrophy in vertebrates. The details of these results have already been reported in another journal [2].
On the other hand, the analyses of paramyosin mutant unc-15(e73) nematodes showed results that were different from those of the wild-type organisms. After the space flight, atrophy of the body-wall muscle and an increase in thick filament proteins were observed in the unc-15 nematodes. This result indicated that the mutant with abnormal muscles responded to microgravity by increasing the total amount of muscle protein in order to compensate for the loss of muscle function (manuscript in preparation).
Summary of the DNA microarray analysis
DNA microarray is a powerful technique to analyze the microgravity effect on gene expressions. We compared the gene expression levels between the ground control worms and the space-flown worms. As shown in Table 1, the number of genes transcriptionally altered was listed up by gene ontology (GO) terms. In the space-flown worms, the up-regulated genes were dominant in the GOs related to embryonic and larval development, gametogenesis, and reproduction, and the down-regulated genes were dominant in the GOs related to locomotory behavior, G-protein coupled receptor protein, and ion transport. myo-3, unc-54, and hlh-1 genes described in previous section are categorized as the down-regulated genes in “locomotory behavior”. These results indicate that microgravity especially plays an important role of locomotory regulation, early embryo-genesis and the regeneration process in C. elegans.
Table 1.
The genes that showed significant change in the expression
| GO (biological process) | total genes* | Flags | |
|---|---|---|---|
| I (increase) | D (decrease) | ||
| embryonic development | 1632 | 194 | 51 |
| larval development | 1266 | 103 | 55 |
| gametogenesis | 667 | 68 | 16 |
| reproduction | 761 | 86 | 20 |
| morphogenesis | 431 | 57 | 25 |
| locomotory behavior | 875 | 54 | **90 |
| regulation of transcription | 509 | 20 | 40 |
| proteolysis and peptidolysis | 312 | 14 | 50 |
| ion transport | 246 | 7 | 33 |
| G-protein coupled receptor | 149 | 2 | 20 |
The total number of expressing genes flagged “present” are indicated.
myo-3, unc-54, and hlh-1 are categorized.
Protein mapping of the space-flown worms
The alterations of entire protein expression of the space-flown worms were analyzed by a combination of two-dimensional gel electrophoresis. Over 1000 protein spots were detected with SYPRO Ruby stain (Table 2, Fig. 1), and approximately 200 phosphoprotein spots were detected with Pro-Q Diamond stain (Fig. 1). Approximately 10-15% spots significantly increased or decreased in the flight samples compared with the ground control (Table 2).
Table 2.
The number of spots detected by SYPRO Ruby stain
| Categories | Number of spots |
|---|---|
| Total spots detected | 1205 |
| Specific spots on “control” | 41 |
| Specific spots on “flight” | 21 |
| Increase on “flight” (more than twice) | 167 |
| Decrease on “flight” (more than twice) | 87 |
Fig. 1.
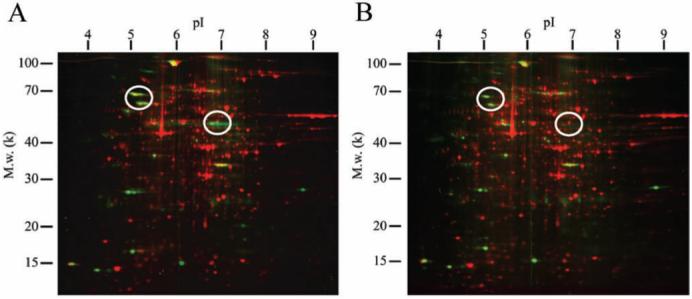
Multi-channel image of 2D electrophoresis gel of C. elegans proteins from ground control and flight experiment. Panel (A) and (B) are 2D protein mapping images of the ground control and the flight experiment, respectively. The whole lysate was extracted by protein extraction solution containing 7M urea, 2M thiourea, 4% (w/v) CHAPS, 40mM DTT. Isoelectric focusing was performed using an immobilized pH3-10 linear gradient strip (Bio-Rad). SDS-PAGE was performed using a ready made gel (PROTEAN II ReadyGel, 8-16%, Bio-Rad). Phosphoproteins (green spots) and total proteins (red spots) were detected by Pro-Q Diamond and SYPRO Ruby staining (Invitrogen), respectively. The spot patterns were analyzed using PDQuest software (Bio-Rad). Circles indicate decreasing phosphoprotein spots in flight samples.
In addition, several phosphoprotein spots drastically decreased in the flight samples (Fig. 1, indicated in circles).
Summary of the effect of space flight on protein aggregation
In order to examine the effect of space flight on protein aggregation, we examined the change in the polyglutamine (polyQ) aggregation in the transgenic C. elegans of (CAG)35 with yellow fluorescent protein under muscle myosin heavy chain promoter. The space-flown and ground control nematodes were compared during the larval and young adult stages. The polyQ aggregation in the space-flown organisms was less than that in the ground control ones. These findings suggest that the protein aggregation rate of the space-flown nematodes was slower than that of the ground control ones (manuscript in preparation).
Conclusion
The ICE-First is one of the first international collaboration that was performed at the International Space Station using a genetic model species. A Japanese research team participated in this program, and we concentrated on three investigations, namely, apoptosis, muscle development, protein phosphorylation, and protein aggregation. The results of these investigations revealed important evidence with regard to the effect of space flight on the nematode and validated that C. elegans is a valuable organism for space experiments. In vertebrates, MyoD, a basic helix-loop-helix protein (bHLH), has been identified as a myogenic factor playing a critical role in the determination and differentiation of skeletal muscle. CeMyoD, which has been also identified as a body wall muscle specific transcription factor in C. elegans, is highly conserved in MyoD of vertebrates. Both of CeMyoD and MyoD has same function regulating MHC gene expression [10, 11]. From this point, we suppose that C. elegans is suitable to extrapolate one of the good answers against the muscle declination under microgravity. As compared to other experimental species, this organism has advantages for space experiments that include various limitations due to its compact size and easy handling and also because abundant genetic information is available about this organism. The variety of space experiments would increase if an automated experimental system were to be developed.
This is a good opportunity for researchers who are interested in space life sciences; opportunities for flight experiments since the Columbia tragedy are few. We hope to continue to execute such international cooperative experiments in the future.
Acknowledgements
This flight experiment was the part of the DELTA mission sponsored by the Space Research Organization of the Netherlands. We are grateful to Dr. M. Viso (CNES: Centre National d’Etudes Spatiales) for his management and support. We acknowledge all the crew that assisted in this flight experiment in the Soyuz spacecraft and at the ISS. We thank Drs. D. Chaput (CNES), L. Granger, B. Echet, and G. Gasset (Universite Paul Sabatier) for their kind support. We also thank the NASA Ames Research Center for providing the liquid medium for C. elegans (CeMM). We appreciate the European Space Agency supplying the KUBIKs. The strains used in this experiment were obtained from the Caenorhabditis Genetics Center funded by the National Institute of Health, National Center for Research Resources.
Contributor Information
Akira Higashibata, Institute of Space and Astronautical Science, Japan Aerospace Exploration Agency, 2-1-1 Sengen, Tsukuba, Ibaraki 305-8505, JAPAN.
Atsushi Higashitani, Graduate School of Life Sciences, Tohoku University, 2-1-1 Katahira, Aoba, Sendai, Miyagi 980-8577, JAPAN.
Ryota Adachi, Department of Welfare Engineering, Faculty of Engineering, Iwate University, 4-3-5 Ueda, Morioka, Iwate 020-8551, JAPAN.
Hiroaki Kagawa, Department of Biology, Faculty of Science, Okayama University, 3-1-1 Tsushima-naka, Okayama, Okayama 700-8530 JAPAN.
Shuji Honda, Tokyo Metropolitan Institute of Gerontology, 35-2 Sakae-cho, Itabashi, Tokyo 173-0015, JAPAN.
Yoko Honda, Tokyo Metropolitan Institute of Gerontology, 35-2 Sakae-cho, Itabashi, Tokyo 173-0015, JAPAN.
Nahoko Higashitani, Graduate School of Life Sciences, Tohoku University, 2-1-1 Katahira, Aoba, Sendai, Miyagi 980-8577, JAPAN.
Yohei Sasagawa, Graduate School of Life Sciences, Tohoku University, 2-1-1 Katahira, Aoba, Sendai, Miyagi 980-8577, JAPAN.
Yutaka Miyazawa, Graduate School of Life Sciences, Tohoku University, 2-1-1 Katahira, Aoba, Sendai, Miyagi 980-8577, JAPAN.
Nathaniel J. Szewczyk, Department of Biological Sciences, University of Pittsburgh, Pittsburgh, PA 15260, USA
Catharine A. Conley, Ames Research Center, National Aeronautics and Space Administration, M/S 239-11, Moffett Field, CA 94035-1000, USA
Nobuyoshi Fujimoto, Institute of Space and Astronautical Science, Japan Aerospace Exploration Agency, 2-1-1 Sengen, Tsukuba, Ibaraki 305-8505, JAPAN.
Keiji Fukui, Japan Space Forum, 2-2-1 Otemachi, Chiyoda, Tokyo 100-0004, JAPAN.
Toru Shimazu, Japan Space Forum, 2-2-1 Otemachi, Chiyoda, Tokyo 100-0004, JAPAN.
Kana Kuriyama, Japan Space Forum, 2-2-1 Otemachi, Chiyoda, Tokyo 100-0004, JAPAN.
Noriaki Ishioka, Institute of Space and Astronautical Science, Japan Aerospace Exploration Agency, 2-1-1 Sengen, Tsukuba, Ibaraki 305-8505, JAPAN.
References
- [1].Higashitani A, Higashibata A, Sasagawa Y, Sugimoto T, Miyazawa Y, Szewczyk NJ, Viso M, Gasset G, Eche B, Fukui K, Shimazu T, Fujimoto N, Kuriyama K, Ishioka N. Checkpoint and physiological apoptosis in germ cells proceeds normally in spaceflown Caenorhabditis elegans. Apoptosis. 2005;10:949. doi: 10.1007/s10495-005-1323-3. [DOI] [PubMed] [Google Scholar]
- [2].Higashibata A, Szewczyk NJ, Conley CA, Imamizo-Sato, Atsushi Higashitani A, Ishioka N. Decreased expression of myogenic transcription factors and myosin heavy chains in Caenorhabditis elegans muscles developed during spaceflight. J. Exp. Biol. 2006;209:3209. doi: 10.1242/jeb.02365. [DOI] [PubMed] [Google Scholar]
- [3].Zhao Y, Lai K, Cheung I, Youds J, Tarailo M, Tarailo S, Rose A. A mutational analysis of Caenorhabditis elegans in space. Mutat. Res. 2006;601:19. doi: 10.1016/j.mrfmmm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- [4].Kagawa H, Adachi R. Summary of spaceflight of mutant animal in the first international Caenorhabditis elegans experiment: ICE-first. Biol. Sci. Space. 2005;19:74. [Google Scholar]
- [5].Szewczyk NJ, Kozak E, Conley CA. Chemically defined medium and Caenorhabditis elegans. BMC Biotechnol. 2003;3:19. doi: 10.1186/1472-6750-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sulstone JE, Schierenberg E, White JG, Thomson N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 1983;100:64. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- [7].Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999;126:1011. doi: 10.1242/dev.126.5.1011. [DOI] [PubMed] [Google Scholar]
- [8].Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO. A conserved checkpoint pathway mediates DNA damage-induced apoptosis and cell cycle arrest in C. elegans. Mol. Cell Biol. 2000;5:435. doi: 10.1016/s1097-2765(00)80438-4. [DOI] [PubMed] [Google Scholar]
- [9].Stergiou L, Hengartner M. Death and more: DNA damage response pathways in the nematode C. elegans. Cell death differ. 2004;11:21. doi: 10.1038/sj.cdd.4401340. [DOI] [PubMed] [Google Scholar]
- [10].Chen L, Krause M, Sepanski M, Fire A. The Caenorhabditis elegans MYOD homologue HLH-1 is essential for proper muscle function and complete morphogenesis. Development. 1994;120:1631. doi: 10.1242/dev.120.6.1631. [DOI] [PubMed] [Google Scholar]
- [11].Krause M. MyoD and myogenesis in C. elegans. Bioessays. 1995;17:219. doi: 10.1002/bies.950170308. [DOI] [PubMed] [Google Scholar]


