Abstract
Damage to the adult CNS often leads to persistent deficits due to the inability of mature axons to regenerate after injury. Mounting evidence suggests that the glial environment of the adult CNS, which includes inhibitory molecules in CNS myelin as well as proteoglycans associated with astroglial scarring, might present a major hurdle for successful axon regeneration. Here, we evaluate the molecular basis of these inhibitory influences and their contributions to the limitation of long-distance axon repair and other types of structural plasticity. Greater insight into glial inhibition is crucial for developing therapies to promote functional recovery after neural injury.
The nervous system has the remarkable ability to adapt and respond to various stimuli, ranging from physiological experiences associated with learning and memory, to pathological insults such as traumatic injury, stroke or neurodegenerative diseases. In addition to plasticity at the functional level, nervous system responses might also occur in the form of structural remodelling. In vivo imaging studies have shown that sensory experience can drive the formation and elimination of synapses, and that these changes might underlie the adaptive remodelling of neural circuits1,2. Similarly, neural injury is often accompanied by a transient period of anatomical remodel ling in the form of local sprouting at the lesion site3. However, although many CNS neurons can survive for years after axotomy, the severed axons ultimately fail to regenerate beyond the lesion site, in contrast to those in the PNS or embryonic nervous system. Recent evidence is beginning to reveal intriguing parallels between some of the molecular mechanisms that affect the different forms of structural plasticity, including both short-range remodelling and long-distance axon regrowth. Therefore, targeting these mechanisms might not only promote the regeneration of damaged nerve fibres, but might also enhance axon sprouting and plasticity after CNS injury. Here, we describe recent progress in under standing the inhibitory components of the adult glial environment, as well as the neuronal receptor complexes and downstream signals that mediate their effects. The limited success in targeting these pathways in vivo will then be evaluated. Finally, we discuss the physiological roles of glial inhibition in the intact nervous system, and their implications for the development of strategies to promote functional recovery after adult CNS injury.
The role of extrinsic inhibition
The regeneration failure in the adult CNS might be partly attributed to the gradual decline in the intrinsic growth ability of neurons as the animal matures. Ramón y Cajal described that, after injury, the ends of lesioned axons become swollen into ‘dystrophic endballs’, which he believed were no longer capable of regeneration4. However, recent studies revealed that these dystrophic growth cones are not quiescent at all, and might actually be highly active structures that are stalled in the hostile injured environment5. Early studies have demonstrated that some injured axons retain a limited capacity for regrowth, and can extend over long distances in the permissive environment of a peripheral nerve graft6. Furthermore, neurons such as those in dorsal root ganglia (DRG) have axons in both the CNS and PNS, but can only regenerate their peripheral processes. These observations suggest that interactions with different environments contribute to the differential regenerative responses.
Increasing evidence suggests that many inhibitory or repulsive guidance cues involved in axon pathfinding during development actually persist into adulthood and might restrict axon regeneration after injury. In addition, the glial environment of the adult CNS is very different from the PNS or embryonic nervous system. The myelin structure formed by oligodendrocytes that normally ensheaths nerve fibres can become damaged after injury, exposing severed axons to myelin-associated inhibitors7–9. In addition, reactive astrocytes form a glial scar at the lesion site and might act as an additional barrier to axon regrowth10 (FIG. 1).
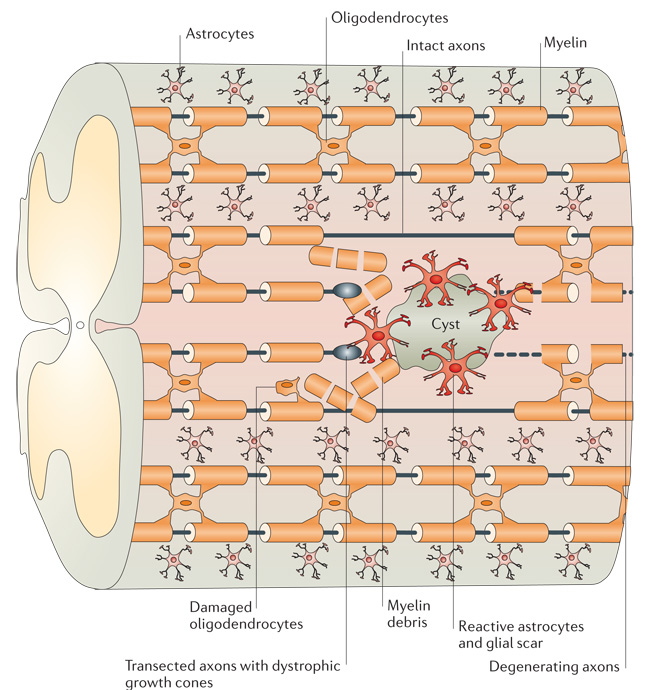
Figure 1. Schematic representation of the CNS injury site.
Injury to the adult CNS often results in the transection of nerve fibres and damage to surrounding tissues. The distal ends of the severed axons form characteristic dystrophic growth cones that are exposed to the damaged glial environment4. During the early phase of injury, myelin-associated inhibitors from intact oligodendrocytes and myelin debris can restrict axon regrowth7–9. Recruitment of inflammatory cells and reactive astrocytes over time leads to the formation of a glial scar, often accompanied by a fluid-filled cyst10. This scarring process is associated with the increased release of chondroitin sulphate proteoglycans, which can further limit regeneration43. Together, these molecular inhibitors of the CNS glial environment present a hostile environment for axon repair.
Many of the initial efforts to identify the molecular components of these inhibitory influences have relied on in vitro assays similar to those used to examine axon guidance molecules in early development. Inhibitory fractions or molecules were characterized by their ability to restrict neurite outgrowth or induce growth cone collapse. However, these studies often rely on immature CNS or PNS neurons, which are more easily grown in culture, and therefore might not truly represent effects in the mature nervous system. Nevertheless, these studies have led to the identification of a growing list of inhibitory molecules that are either constitutively expressed or induced after injury in the glial environment of the adult CNS.
Myelin-associated inhibitors
CNS myelin was first postulated as a major source of inhibition when immobilized CNS myelin, but not PNS myelin, was found to inhibit axon outgrowth11. To identify individual molecules, an antibody to an inhibitory fraction of myelin, termed IN-1, was isolated for its ability to neutralize myelin inhibition in vitro12,13. Subsequent identification of the putative antigen led to the discovery of Nogo14–16. Nogo is a member of the reticulon family of membrane proteins, and at least three isoforms (Nogo-A, -B and -C) are generated by alternative splicing and promoter usage. Among these, Nogo-A is best characterized, owing to its high expression in CNS oligodendrocytes17. Structure–function analyses support the presence of two inhibitory domains: a unique amino-terminal region (amino-Nogo) that is not shared by Nogo-B and Nogo-C18, and a 66 amino acid loop (Nogo-66) that is common to all three isoforms14. However, the topology of Nogo-A remains controversial. Although initial evidence places Nogo-66 on the extracellular surface and amino-Nogo facing intracellularly, at least some amino-Nogo can be detected on cell surfaces and both domains are inhibitory to neurite outgrowth14,15,18,19 (FIG. 2). Furthermore, Nogo-A contains an endoplasmic reticulum (ER)-retention motif, and appears to be largely restricted to tubular ER20. As axon-derived signals might also influence membrane trafficking in oligodendrocytes21, it remains to be clarified whether Nogo-A has different membrane topologies in different contexts, or is exposed only after oligodendrocyte membrane damage during injury.
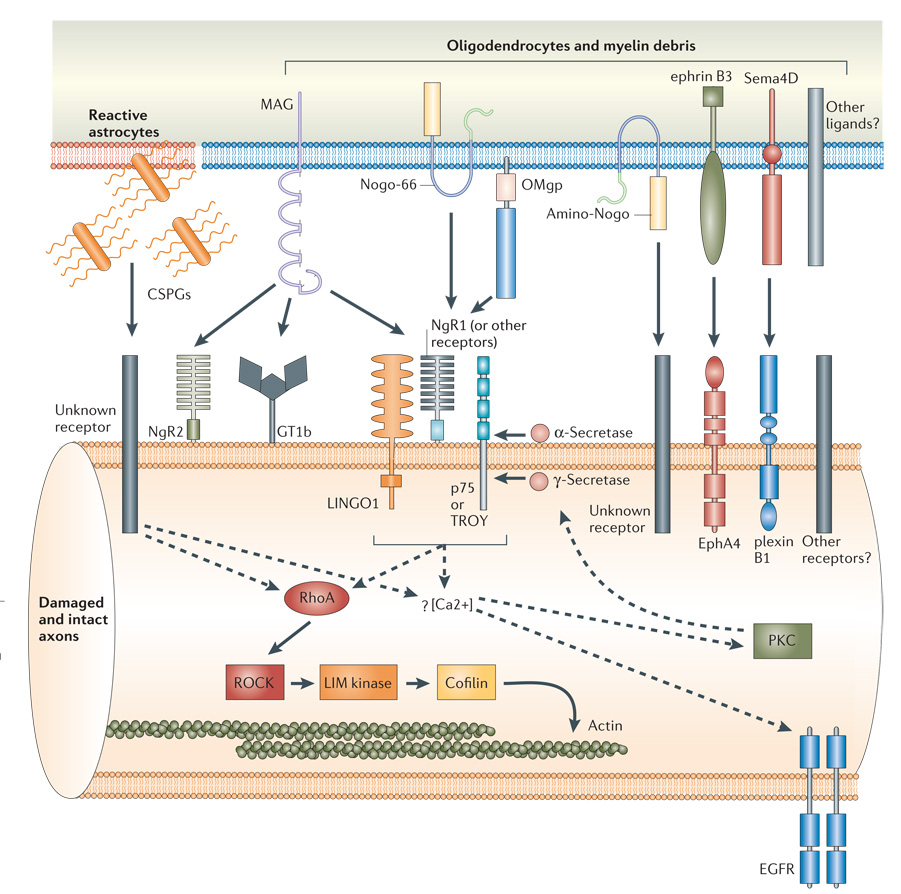
Figure 2. Glial inhibitors and intracellular signalling mechanisms.
The molecular inhibitors of the adult CNS glial environment include chondroitin sulphate proteoglycans (CSPGs) associated with reactive astrocytes from the glial scar43, and myelin-associated inhibitors from intact oligodendrocytes and myelin debris, including myelin-associated glycoprotein (MAG)22,23, Nogo-A14–16, oligodendrocyte myelin glycoprotein (OMgp)24, ephrin B3 (REF. 26) and the transmembrane semaphorin 4D (Sema4D)25. Although the topology of Nogo-A remains unclear, both the 66 amino acid loop (Nogo-66) and the amino-terminal domain (amino-Nogo) are known to be inhibitory to axon outgrowth14,15,18,19. The neuronal receptors and downstream signalling pathways known to be involved in transducing these inhibitory signals are shown. Among the signalling components that are common to both CSPG and myelin inhibition are the activation of RhoA82 and the rise in intracellular calcium65,89,92,93. Whereas the signals downstream of RhoA that lead to the actin cytoskeleton are well characterized (solid arrows), the relationship between components upstream of RhoA and the role of calcium influx are still ambiguous (dashed arrows). For example, calcium transients might activate protein kinase C (PKC)88,89, which is required for p75 cleavage by γ-secretase66, or trigger the transactivation of epidermal growth factor receptor (EGFR)90.
Other studies led to the identification of several other myelin-associated components that can inhibit axon outgrowth in vitro, including myelin-associated glycoprotein (MAG)22,23, oligodendrocyte myelin glycoprotein (OMgp)24, the transmembrane semaphorin 4D (Sema4D/CD100)25 and ephrin B326 (FIG. 2). MAG is a transmembrane protein with five immunoglobulin-like domains in its extracellular region27,28, although myelin disruption might lead to its release as a soluble proteo lytic fragment29. Unlike Nogo-A, it is expressed by both CNS oligodendrocytes and PNS Schwann cells, and has also been implicated in myelin formation and mainte nance30. Interestingly, embryonic and neonatal neurite outgrowth is promoted by MAG, which suggests that this protein can act as a bi-functional cue, with a sharp transition from promotion to inhibition at around the time of birth31–33. OMgp is a GPI-anchored protein that contains a leucine-rich repeat (LRR) domain34. Recent evidence suggests that it is enriched in membranes of oligodendroglia-like cells that encircle nodes of Ranvier, and might act to prevent collateral sprouting35.
In addition to these myelin components, repulsive guidance cues with roles in axon pathfinding during development — such as ephrin B3 and Sema4D/CD100 — have also been found in CNS myelin and implicated as inhibitors of axon repair in the adult. Ephrin B3 functions as a midline repellent during corticospinal tract (CST) for mation36, but continues to be expressed during postnatal stages in myelinating oligodendrocytes26. Sema4D/CD100 is similarly expressed on mature oligodendrocytes, can be induced by injury and triggers growth cone collapse25. Therefore, although many guidance cues that are involved in the initial formation of the nervous system become downregulated when development is complete, at least some persist into adulthood to exert an inhibitory effect in the mature CNS.
Given the remarkable diversity among these myelin components, their respective contributions to myelin inhibition remain unclear. Genetic deletion of Nogo and enzymatic depletion of OMgp or other GPI-linked proteins can reduce the inhibitory activity of myelin24,37–39. However, in vitro neurite outgrowth assays do not measure myelin inhibition in a linear fashion, and removal of individual components might be complicated by compensatory responses by other inhibitors. Nevertheless, it is clear that CNS myelin exerts multiple layers of inhibitory influences with a significant degree of overlap and cross-regulation.
CSPGs and the glial scar
In addition to degenerating myelin, another important source of inhibition is the glial scar, which forms after CNS injury40. The glial reaction to injury results in the recruitment of microglia, oligodendrocyte precursors,meningeal cells and astrocytes to the lesion site. Some of these responses could have beneficial effects; they could isolate the injury site and minimize the area of inflammation and cellular degeneration. Some populations of astrocytes might even support axon regrowth41. However, many astrocytes in the injured area often become hypertrophic and adopt a reactive phenotype, releasing inhibitory extracellular matrix molecules known as chondroitin sulphate proteoglycans (CSPGs)42. CSPGs (aggrecan, brevican, neurocan, versican, phosphacan and NG2) are a family of molecules characterized by a protein core to which large, highly sulphated glycosaminoglycan (GAG) chains are attached43. The spatiotemporal expression of CSPGs correlates with glial boundaries in the developing CNS, such as the spinal cord roof plate, optic tectum and dorsal root entry zone (DREZ)44,45. After injury, CSPG expression is rapidly upregulated by reactive astrocytes, forming an inhibitory gradient that is highest at the centre of the lesion and diminishes gradually into the penumbra.
There is ample evidence to suggest that the inhibitory activity of CSPGs depends on the GAG components, as treatment with chondroitinase ABC (ChABC), an enzyme that removes GAG chains from the protein core, eliminates this inhibition10,46. Other studies have shown that the core proteins of at least some CSPGs (for example, NG2) can also inhibit outgrowth47. However, the mechanisms by which these CSPGs exert their inhibitory effects are still not entirely clear. One model suggests that the proteoglycans serve as a mechanical barrier, indirectly masking neurons from neurite-promoting components of the extracellular matrix48. Indeed, interactions between various proteoglycans with molecules such as laminin, fibronectin and neural cell adhesion molecules have been widely documented49–51. Antibody-mediated blockade of laminin can also reduce growth promotion by ChABC treatment52. Interestingly, unlike the NG2 molecule itself, NG2-expressing glial cells can promote axon growth53. Given the delicate balance between growth-promoting and inhibitory components in the extracellular matrix, it is not surprising that CSPGs might exert their effects by interacting with growth-promoting substrates. Nevertheless, there is also evidence to support the presence of unique intracellular pathways in neurons that mediate CSPG inhibition; for example, a specific neuronal surface receptor has been identified for NG2 (REF. 54). Signalling pathways involving the small GTPase RhoA have also been implicated55. As described below, many of these intracellular signals might share similarities with those triggered by myelin-associated inhibitors. Interestingly, exposure to CSPGs can also stimulate the formation of dystrophic growth cones on lesioned sensory axons similar to those described by Ramón y Cajal5. These observations suggest that CSPG-mediated inhibition could severely affect both the cytoskeletal and membrane components of growth cone architecture5.
Although it is clear that both CNS myelin and the glial scar can inhibit axon outgrowth, their relative importance in vivo remains uncertain. After dorsal rhizotomy, DRG axons treated with neurotrophin 3 are able to overcome the CSPG-enriched glial barrier at the DREZ, but not the degenerative white matter myelin56. This sup ports a hierarchy of inhibitory influences, with myelin being more potent than the glial scar. However, DRG neurons microtransplanted into the spinal cord with minimal scarring can project axons over long distances along degenerating white matter tracts, stopping only on contact with CSPGs at the glial scar. These results indicate that it is the astroglial barrier that is the major impediment to adult CNS regeneration57,58.
Despite these conflicting reports, both CSPGs and myelin-associated inhibition are likely to be involved in regenerative failure, with some overlap and differences in function attributable to their spatial and temporal regulation. For example, whereas myelin inhibitors are constitutively expressed and are not significantly changed after trauma17, CSPGs can be strongly upregulated following injury, with different time courses of expression ranging from 24 hours to 6 months post-lesion59. An interesting possibility is that the two sources of inhibition could have effects on each other. Physical interactions with CSPGs, for example, can convert Sema5A from an attractive to a repulsive guidance cue60. Therefore, the observed effects from ChABC treatment might be partially attributable to the effects on molecules that are associated with CSPGs. Combinatorial treatments targeting these different sources of inhibition in vivo will be crucial to evaluate their relative contribution to nerve fibre regeneration failure.
Receptor mechanisms for myelin inhibition
So far, the receptor mechanisms for CSPG inhibition remain elusive. By contrast, much effort has been made to elucidate the neuronal signalling pathways triggered by myelin-associated inhibitors. The Nogo-66 receptor (NgR) is a GPI-linked protein that is expressed in many types of CNS neuron and can interact with the putative extracellular domain of Nogo-A19. Surprisingly, it was later found to also bind with high affinity to MAG61,62 and OMgp24, which are structurally different from Nogo. In initial studies, the removal of GPI-linked molecules such as NgR from axonal surfaces could reduce or abolish neuronal responses to myelin inhibitors. In addition, overexpression of NgR could confer responsiveness to these inhibitors in embryonic neurons, which are normally unresponsive19.
As NgR is GPI-linked and lacks an intracellular domain, efforts have been made to identify additional transmembrane co-receptors that can transduce the myelin inhibitory signals. So far, two classes of molecule have been implicated, including members of the tumour necrosis factor receptor (TNFR) family, such as p75 and TROY, as well as LINGO1 (FIG. 2). The first such co-receptor to be identified was p75, which was originally characterized as a neurotrophin receptor. Neurons from p75-mutant mice showed dramatically reduced responses to MAG, OMgp and Nogo-66 (REFS 63,64). Subsequently, p75 was found to form a physical receptor complex with NgR64,65, and activation of downstream signals might require its intramembrane proteolysis by α- and γ-secretases66. However, p75 is not expressed in most populations of mature neurons. This led to the identification of another TNFR family member TROY (also known as Taj), which is widely expressed across the adult CNS and can functionally substitute for p75. Similar to p75, TROY can form a receptor complex with NgR. Genetic deletion or overexpression of a dominant-negative form of TROY can also promote robust axon outgrowth on a myelin substrate67,68. Nevertheless, the relative roles of these two molecules remain unknown, and other TNFR family members might also be involved. Other lines of evidence have revealed the requirement for another co-receptor known as LINGO1, a transmem brane protein that can bind to both NgR and p75 (REF. 69). Co-expression of all three receptor components, including NgR, p75 or TROY, and LINGO1, is sufficient to allow non-neuronal cells to respond to myelin inhibitors by activating the downstream signal RhoA67,68.
As enzymatic cleavage or overexpression of dominant-negative versions of NgR render neurons unresponsive to inhibition by myelin19,24,61,62,70, the NgR complex was initially postulated to be the major receptor for myelin-associated signals. However, this oversimplified scenario has been challenged by recent studies using NgR-mutant mice generated by two groups. In one study, Kim and colleagues used a growth cone collapse assay to show that NgR-deficient neurons showed reduced sensitivity to myelin-associated inhibitors71. By contrast, Zheng et al. found that neurite outgrowth from neurons lacking NgR is still limited by these inhibitors72. These data provide evidence for the existence of other receptor mechanisms that are independent of NgR. Two additional human homologues for NgR (NgR2 and NgR3) that are expressed in CNS neurons have been identified73,74. Although neither binds to Nogo-66 (REF. 75), some evidence suggests that NgR2 can bind to MAG76. Additional receptor mechanisms that are unrelated to NgR might also exist, as neurons treated with an enzyme that cleaves GPI-linked proteins can still respond to MAG77. For example, some evidence has implicated the involvement of the ganglioside GT1b in MAG inhibition78,79. Together, these results indicate that the remarkable diversity of inhibitory influences from the glial environment might also be reflected at the receptor level in neurons.
Intracellular signalling pathways
Considering the many ligands and receptors that mediate inhibition by CNS myelin and the glial scar, targeting individual components might not be the most efficient approach to overcome these inhibitory influences. Instead, identifying intracellular pathways that are common to multiple sources of inhibition could offer a greater prospect for promoting axon regeneration. So far, the best-characterized pathway involves RhoA and its effector, RhoA-associated kinase (ROCK). Small GTPases of the Rho family such as RhoA, Rac1 and Cdc42 (cell division cycle 42) are known regulators of the actin cytoskeleton80. In particular, RhoA activation has been shown to correlate with signals that induce growth cone collapse and axon guidance repulsion81. Evidence suggests that this pathway also mediates myelin inhibition. RhoA can be directly activated in response to myelin-associated inhibitors82, possibly by displacing a Rho-guanine dissociation inhibitor (Rho-GDI)83. Inhibiting RhoA using C3 transferase or a dominant-negative approach also promotes axon outgrowth on inhibitory substrates, as can pharmacological inhibition of ROCK77,84–86. More recent evidence has highlighted the mechanisms further downstream, with Nogo-66 signalling through LIM (Lin-11, Isl-1 and Mec-3) kinase and Slingshot (SSH) phosphatase to regulate the actin depolymerization factor cofilin87. Together, these reports support a model in which myelin-based inhibitory signals might trigger the activation of RhoA and ROCK, leading to the phosphorylation of cofilin by LIM kinase to stabilize the growth cone cytoskeleton of damaged axons, restricting regenerative outgrowth (FIG. 2).
As we learn more about the intracellular signals that mediate myelin inhibition, it is becoming evident that many of these components are also triggered by CSPGs. Outgrowth inhibition by CSPGs, for example, can be neutralized by blocking RhoA/ROCK signalling55,84. By systematically screening libraries of small molecules for their ability to promote outgrowth on inhibitory sub strates, two additional signal mediators were found to be common to both CSPG and myelin inhibition: protein kinase C (PKC) and epidermal growth factor receptor (EGFR). Pharmacological inhibition of conventional PKC isoforms attenuates outgrowth inhibition and RhoA activation by both CSPGs and myelin88,89. In addition, intramembrane proteolysis of p75 is PKC depend ent66. Similarly, EGFR activation is also required by both inhibitory influences. Although a direct interaction with NgR components was not detected, EGFR appears to be transactivated by calcium influx90. Local calcium transients are intimately related to growth cone motility, affecting both axon extension and guidance during development91. Intriguingly, both myelin components and CSPGs have been shown to trigger local elevations in calcium at the growth cone65,89,92,93. Given the involvement of calcium-related signals such as PKC and EGFR, it will be important to further explore the role of local calcium changes, the signals that they trigger and their relationship to the actin cytoskeleton.
Regeneration failure in vivo
The relative success from targeting inhibitory signals in vitro has led to much anticipation for the promotion of regeneration and functional recovery after CNS injury in vivo. Both genetic deletion and pharmacological interventions have been used towards this aim to block the inhibitory pathways, including ligands, receptor components and signalling intermediates (TABLE 1). These studies have primarily focused on the regeneration of long axonal fibres, such as those in the CST, dorsal columns or optic nerve in various nerve injury models.
Table 1.
Summary of in vivo studies targeting inhibitory signals in the adult CNS
| Target | Intervention method | Injury model | Result |
|---|---|---|---|
| Myelin-associated inhibitors | |||
| Nogo | IN-1 (MAb secretion from tumour or intrathecal infusion of humanized Fab fragment) | Spinal cord dorsal hemisection (rat) | CST regeneration13,94 |
| Genetic deletion | Spinal cord dorsal hemisection (mouse) | CST regeneration in Nogo-A/B mutants and improved functional recovery37 | |
| Spinal cord dorsal hemisection (mouse) | Slight CST regeneration in Nogo-A mutants38 | ||
| Spinal cord dorsal hemisection (mouse) | No regeneration of CST in Nogo-A/B or Nogo-A/B/C mutants39 | ||
| MAG | Genetic deletion | Spinal cord dorsal hemisection (mouse) | No regeneration of CST95 |
| Optic nerve crush (mouse) | No regeneration of RGC fibres95 | ||
| Glial scar | |||
| GFAP and vimentin | Genetic deletion | Spinal cord lateral hemisection (mouse) | Ventral serotonin-containing fibre and CST regeneration, and improved functional recovery103 |
| CSPGs | ChABC (intrathecal delivery) | Dorsal column crush (rat) | Dorsal column and CST regeneration, and improved motor and sensory functions104 |
| Nigrostriatal tract axotomy (rat) | Nigrostriatal tract regeneration105 | ||
| EphA4 | Genetic deletion | Spinal cord lateral hemisection (mouse) | CST and rubrospinal tract regeneration, and improved functional recovery106 |
| Receptors | |||
| NgR | NEP1–40 antagonist peptide (intrathecal or delayed subcutaneous injection) | Spinal cord dorsal hemisection (rat) | CST regeneration and improved functional recovery96,97 |
| NgR(310)ecto function-blocking peptide (intrathecal delivery or transgenic expression) | Spinal cord dorsal hemisection (rat) | CST and raphespinal regeneration and improved functional recovery98,99 | |
| Truncated dominant-negative NgR (AAV-mediated expression) | Optic nerve crush (rat) | RGC fibre regeneration when combined with lens injury to induce active growth state70 | |
| Genetic deletion | Spinal cord transection or dorsal hemisection (mouse) | Raphespinal and rubrospinal fibre regeneration with improved motor function, but no regeneration of CST71 | |
| Spinal cord dorsal hemisection (mouse) | No regeneration of CST72 | ||
| p75 | Genetic deletion | Spinal cord dorsal hemisection or crush (mouse) | No regeneration of CST or dorsal columns102 |
| Signalling mediators | |||
| RhoA | C3 transferase (intrathecal delivery by polymer release (mouse) or infusion pump (rat)) | Spinal cord dorsal hemisection (mouse) | CST regeneration and improved functional recovery84 |
| Spinal cord dorsal hemisection (rat) | No CST regeneration85 | ||
| C3 transferase (gelfoam delivery) | Optic nerve crush (rat) | RGC fibre regeneration86 | |
| ROCK | Y-27632 (intrathecal delivery by polymer release (mouse) or infusion pump (rat)) | Spinal cord dorsal hemisection (mouse) | CST regeneration and improved functional recovery84 |
| Spinal cord dorsal hemisection (rat) | CST regeneration and improved functional recovery85 | ||
| PKC | Gö6976 (intrathecal infusion) | Spinal cord dorsal hemisection (rat) | Dorsal column regeneration, but no CST regeneration88 |
| EGFR | PD168393 (gelfoam delivery) | Optic nerve crush (mouse) | RGC fibre regeneration90 |
The varied outcomes of these in vivo studies highlight the extent of redundancy and compensation between the different inhibitory pathways, as well as the heterogeneous nature of the methods used to assess regeneration and recovery in CNS injury paradigms. AAV, adeno-associated virus; ChABC, chondroitinase ABC; CSPGs, chondroitin sulphate proteoglycans; CST, corticospinal tract; EGFR, epidermal growth factor receptor; EphA4, the cognate neuronal receptor for ephrin B3; Fab, portion of an immunoglobulin molecule that binds the antigen; GFAP, glial fibrillary acidic protein; IN-1, antibody to an inhibitory fraction of myelin; MAb, monoclonal antibodies; MAG, myelin-associated glycoprotein; NgR, Nogo-66 receptor; PKC, protein kinase C; RGC, retinal ganglion cell; RhoA, a small GTPase; ROCK, RhoA-associated kinase.
At the ligand level, initial attempts to block Nogo-A activity met with some success. Treatment with IN-1 antibody induced some sprouting after a CST lesion13,94. However, when CST regeneration was examined in Nogo-knockout mutants that were generated independently by three groups, the results were ambiguous. Whereas one group reported extensive sprouting of lesioned axons in young adult mice lacking Nogo-A/B37, the other two observed little (in mice lacking only Nogo-A38) to no (in two mouse lines lacking either NogoA/B or Nogo-A/B/C39) improvement in regeneration. Studies using MAG-mutant mice also showed little to no detectable regeneration of optic nerve and CST fibres95. Interestingly, although OMgp-knockout animals have not been examined in any models of injury, the animals were found to have abnormally wide nodes of Ranvier and signs of collateral sprouting35, supporting the importance of this molecule in limiting aberrant sprouting under physiological conditions.
Despite the limited success achieved by genetic deletions of individual myelin components, targeting the NgR complex should neutralize all three major inhibitors. Indeed, early experiments involving the delivery of a Nogo-66 antagonist peptide (NEP1–40)96,97 or the function-blocking NgR ectodomain (NgR(310)ecto)98,99 after spinal cord hemisection promoted some axon regeneration and functional recovery. In addition, transgenic expression of a truncated NgR lacking its co-receptor binding site can enhance optic nerve regeneration if the retinal ganglion cells are in an active growth state70. However, the recent characterization of two strains of mutant mice lacking NgR once again produced mixed outcomes. Although in one of the studies some regeneration was observed in the raphespinal tract and rubrospinal tract, no CST regeneration could be detected in either mutant strain71,72. Similarly, initial studies showed that sympathetic neurons from p75-mutant mice overexpressing NGF can grow in extensively myelinated portions of the cerebellum100 or optic nerve101. However, no CST or dorsal column repair could be detected in these p75 knockouts after spinal cord injury102. Nevertheless, as neurons from both p75- and NgR-deficient mice still retain at least partial responses to myelin inhibitors in vitro67,72, these in vivo results might not faithfully reflect the contribution of myelin inhibition to regeneration failure. In fact, published results from genetic deletion studies have largely been less consistent than function-blocking or dominant-negative approaches in enhancing axon regeneration. The reason for such diver gent regenerative responses remains unclear, although it is probably attributable to compensatory upregulation of other inhibitory pathways. Neutralizing agents might also affect other mediators of inhibition that have not yet been identified. Nevertheless, these studies are beginning to help us to understand the relative contribution of myelin-associated inhibitors and their receptor components to regeneration failure.
Studies that target the glial scar have also shown some promise for the promotion of regeneration and recovery after CNS injury. Mutant mice that are deficient in both glial fibrillary acidic protein (GFAP) and vimentin (important cytoskeletal proteins that are induced in reactive astrocytes) show reduced astroglial reactivity, increased supraspinal sprouting and improved functional recovery after spinal cord hemisection103. Intrathecal administration of ChABC following spinal cord injury promoted the regeneration of various axon tracts as well as some recovery of function104,105. Interestingly, mutants with genetic deletion of EphA4, the cognate neuronal receptor for ephrin B3, also showed regrowth of corticospinal and rubrospinal tract fibres after spinal cord hemisection, although the phenotype was attributed to reduced astrocytic gliosis rather than a loss of outgrowth inhibition by ephrin B3 from oligodendrocytes106.
Additional studies have been used to target intra cellular pathways common to both myelin and CSPGs. Application of C3 transferase, which ADP-ribosylates and inhibits RhoA, increases axon sprouting in the optic nerve after crush injury and in CST fibres after spinal cord hemisection84,86. Pharmacological inhibition of Rho’s downstream effector ROCK using Y-27632 also promoted significant regeneration of CST fibres and an improvement in function84,85. However, not all CNS fibre tracts may respond in the same way. Intrathecal infusion of the PKC inhibitor Gö6976 after dorsal hemisection promoted increased regeneration of only dorsal column, but not CST, fibres88. Nevertheless, pharmacological treatments might ultimately be more useful than genetic approaches in the clinical context. For example, local application of EGFR kinase inhibitors such as PD168393 can promote dramatic retinal ganglion axon regeneration after optic nerve crush90. Incidentally, the EGFR antagonist erlotinib (Tarceva) has already been approved for the treatment of lung cancer, and might therefore be readily tested in clinical trials for its efficacy in nerve repair.
It is difficult to reconcile the varying outcomes from these in vivo studies. The effects of genetic deletions might be obscured by variations in the genetic back ground and differential compensatory mechanisms from the different mouse strains. Pharmacological treatments using small molecules, enzymes or function-blocking antibodies or peptides might depend on the pharma-cokinetic properties of the compound or method of delivery. In addition, evaluating these results could also be complicated by the heterogeneous nature of the complex surgical techniques used in these injury para digms. Even the criteria used to distinguish bona fide regeneration from collateral sprouting or fibre sparing lack standardization107 (BOX 1).
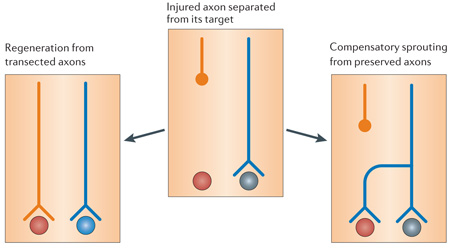
Standardized criteria to assess true axon regeneration
In vivo studies on the promotion of nervous system repair often fail to distinguish regeneration of transected axons from compensatory sprouting that arises from preserved fibres (see figure). Although both types of axon growth might potentially enhance functional recuperation, local sprouting might be more important in physiological plasticity or conditions such as stroke, whereas long-distance regeneration of axons is required for recovery from spinal cord injury Quantifying the number of regenerated fibres is further plagued by the presence of spared fibres, which are easily mistaken for regenerated fibres107. A potentially useful approach could be to monitor the sprouting and/or regenerative behaviours of lesioned axons by in vivo imaging122–125.
Nevertheless, the mixed success from these studies has taught us much more about the complex nature of the mechanisms that prevent CNS axon regeneration. In particular, it is becoming clear that nerve fibre tracts have different intrinsic regenerative potentials, and might respond to various methods of intervention in different ways. For example, PKC inhibitor treatment failed to enhance CST regeneration, but might promote the repair of dorsal column fibres71,88. In addition, treatments that show little effect in one injury paradigm could still have potential benefits under other conditions. This can be seen in a model of middle cerebral artery occlusion, in which both IN-1 antibody treatment and genetic deletion of NgR or Nogo-A/B have been shown to improve local axonal sprouting and stroke recovery108–111. In fact, ischaemic or contusion injuries occur much more frequently in the clinical setting than complete nerve transection, highlighting the importance of testing different injury paradigms when evaluating the efficacy of a particular approach.
It is clear from these studies that no single comp onent is solely responsible for regeneration failure in the adult CNS. Although we cannot exclude the possibility of other key inhibitory molecules that have not yet been identified, a reasonable next step would be to use com binatorial approaches to simultaneously target multiple inhibitory pathways. In addition, treatments to enhance the intrinsic regenerative machinery of the damaged neurons, such as neurotrophin treatment112,113, preconditioning injury114,115 or macrophage activation115, might also be required. Studies combining ChABC treatment with a preconditioning lesion have already revealed markedly improved regeneration across the DREZ after dorsal root crush injury compared with either treatment alone116. Further investigation along these lines will provide valu able information regarding the relative importance of different factors, as well as their therapeutic potential in the clinical setting.
Physiological roles in the intact CNS
Given such remarkable diversity in the structure and expression patterns of the various inhibitory components, it is unlikely that the different mechanisms evolved together solely to limit CNS repair after injury. Evidence indicates that these inhibitory mechanisms have resulted as a by-product of normal physiological processes that are regulated by neuron–glia interactions. For example, monocular deprivation can lead to shifts in ocular dominance in the visual cortex during a postnatal critical period, but not in adults117 (BOX 2). Interestingly, both ChABC treatment118 and genetic deletion of NgR119 have recently been found to either reactivate or extend the duration of this critical period. These results support a crucial role for both CSPGs and myelin in closing the critical period for experience-dependent plasticity. Although the underlying mechanisms for these observations remain unknown, it is conceivable that physiological stimuli could act in a similar manner to injury signals to trigger local structural plasticity. In this way, the removal of extrinsic constraints such as CSPGs and/or myelin inhibitors would allow a greater degree of local anatomical remodelling in the mature CNS. This is exemplified by results showing enhanced short-range plasticity and stroke recovery in the absence of Nogo or NgR expression108, increased sprouting at nodes of Ranvier in OMgp-deficient mice35, and improved collateral sprouting with ChABC treatment after denervation of the superior colliculus120 or spinal cord injury121.
Critical period for ocular dominance plasticity
In the early postnatal period, neural connections in the visual cortex are fine-tuned by external stimuli. However, this experience-dependent plasticity is available only for a short time. As the animal matures, this ‘critical period’ ends and further changes can no longer take place. Interestingly, dark-rearing can prolong the duration of this period, implicating the involvement of sensory input in the maturation process. Ocular dominance in the visual cortex is a commonly used model for studying experience-driven plasticity. The brain receives two images, one from each eye, that are combined in the binocular zone of the visual cortex. Monocular deprivation by suturing one eye closed during the critical period causes the open eye to take control of the binocular zone. In other words, neurons that would normally subserve both visual fields lose connections with the closed eye and gain synaptic inputs from the open eye117. Once this critical period ends (about 3–4 weeks after birth in mice and 7–8 years in humans), the synaptic connections are fixed, and no further changes can occur. An earlier study showed that the injection of immature astrocytes into the cat brain can restore plasticity in the visual cortex126. In two recent studies, treatment with chondroitinase ABC118 and genetic deletion of NgR or Nogo-A/B119 were shown to reactivate or extend the duration of the critical period. These results provide a molecular basis for critical period closure, suggesting that both chondroitin sulphate proteoglycans and myelin have important roles in stabilizing the visual circuitry as the organism matures.
Together, these reports support a model in which different astrocyte and oligodendrocyte-derived inhibitory factors have evolved to promote the maturation and stabilization of the complex neural circuitry in higher vertebrates (BOX 3). During development, embry onic axons are unmyelinated and respond to various guidance cues such as netrins, ephrins, SLITs and semaphorins to fine-tune the neural circuitry. As the nervous system matures, oligodendrocytes ensheath the nascent axons to prevent aberrant sprouting, while astrocytes express CSPGs to further limit structural changes in the adult. Therefore, these inhibitory sig nals might not only prevent axon regeneration in the context of injury, but might also serve as a protective mechanism to preserve the complex neural networks formed during development (FIG. 3).
Glial inhibition in evolution
Why have such diverse mechanisms evolved to limit axon regeneration in the adult CNS? Unlike most higher vertebrates, primitive organisms such as newts and salamanders can regenerate after spinal cord injury. Regeneration also occurs in some mammals such as opossums, but only in the days immediately following birth127. Assuming that ontogeny recapitulates phylogeny, an analogy can be drawn with the increased ability of the embryonic nervous system to regenerate when compared with adults. This evidence suggests that the loss of CNS regeneration might have resulted from recent evolutionary adaptations, and is not hard-wired into the nervous system. Therefore, although glial inhibition is still likely to be crucial for preserving higher-order processes, removing these inhibitory influences after injury could potentially recover some basic, more primitive functions in the clinical setting, such as the ability to breathe without a respirator or improved bowel or bladder control.
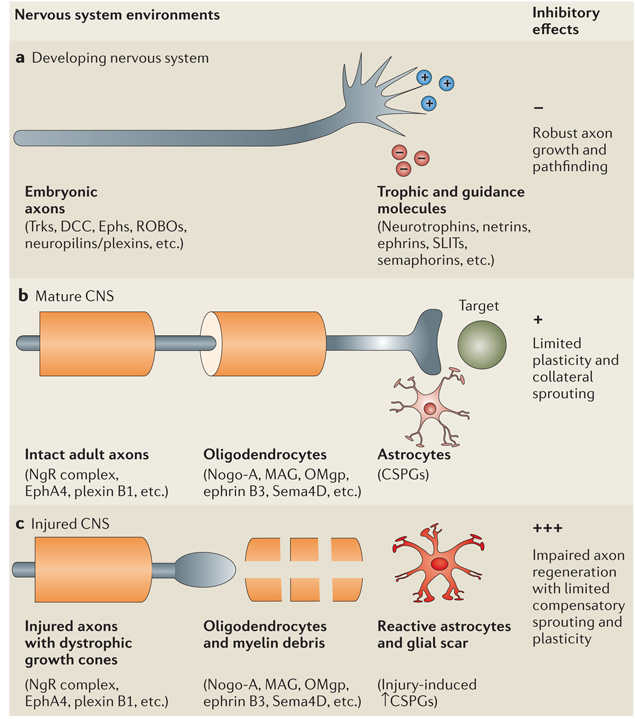
Figure 3. Changes in CNS environments after maturation and injury.
During embryonic development, unmyelinated axons with motile growth cones can extend, retract and respond to various trophic and guidance molecules. This dynamic process allows the neural circuitry to be fine-tuned (a). As the nervous system matures after birth, myelination is finalized with oligodendrocytes ensheathing axons to prevent aberrant sprouting and astrocytes secreting chondroitin sulphate proteoglycans (CSPGs) to further limit structural plasticity in the adult (b). After CNS injury, axons become transected and reactive astrocytes further upregulate their secretion of CSPGs. The distal endings of severed axons form dystrophic growth cones and become exposed to CSPGs from the glial scar, as well as myelin-associated inhibitors from oligodendrocytes and myelin debris (c). As similar mechanisms prevent short-range plasticity in the adult and long-distance axon repair after injury, relieving these inhibitory influences might not only enhance the regeneration of severed axons, but might also promote compensatory sprouting. EphA4, the cognate neuronal receptor for ephrin B3; MAG, myelin-associated glycoprotein; NgR, Nogo-66 receptor; OMgp, oligodendrocyte myelin glycoprotein; Trk, tyrosine receptor kinase.
Concluding remarks
Advances in recent decades have led to the identification of many inhibitory molecules in the adult CNS environment that might be responsible for regenerative failure after injury. These molecular inhibitors are largely distinct from the trophic and guidance cues that regulate the initial formation of the nervous system. Instead, they are mainly associated with the later stages of nervous system development, including myelin formation and termination of the critical period for experience-driven plasticity. During CNS injury, the damaged axons might be initially exposed to various myelin-associated inhibitors from oligodendrocytes and myelin debris. Over time, reactive astrocytes are recruited to the glial scar, releasing gradients of inhibitory CSPGs that further prevent axon repair. As the same set of mechanisms limits both axon repair after injury and local plasticity in the intact adult, alleviating these inhibitory influences might not only promote the regrowth of damaged axons, but might also enhance compensatory sprouting from preserved fibres.
Recent studies using in vivo injury models have provided interesting perspectives on the involvement of glial inhibition in limiting both long-distance and short-range structural remodelling in the mature CNS. In conventional spinal cord injury paradigms, efforts to block these inhibitory influences have generally met with limited success. This not only alludes to the tremendous degree of overlap and cross-compensation between the various inhibitory signals, but might also reflect the reduced intrinsic ability of mature axons for long-distance regeneration. By contrast, removing these inhibitory influences could induce short-range rear rangements of neuronal processes to enhance stroke recovery or experience-dependent plasticity. Such evidence suggests that strategies to overcome glial inhibition could be more useful for conditions such as stroke in which local short-range sprouting is important. But in cases such as spinal cord injury, in which long-distance regeneration of nerve fibres is necessary, it could be important to consider combinatorial approaches to block extrinsic inhibition while also enhancing the intrinsic growth programme of the neurons.
Today, treatment options for CNS injury remain limited to minimizing inflammation and swelling in the acute setting to preserve intact fibres, and physical therapy in the long term to stimulate the little plasticity that is available in adults. Attempts to promote axon repair by neutralizing these inhibitory mechanisms could potentially shift the current treatment paradigm from palliative care to actual restoration of function. Furthermore, experimental results and clinical data have shown that functional recovery after CNS injury does not always correlate with observable regrowth of nerve fibres. In the absence of long-distance regeneration, even a small improvement in compensatory sprouting and local plasticity by reducing the inhibitory environment of the CNS could translate to significant improvements in clinical outcomes. Future work to better characterize the mechanisms that prevent adult CNS regeneration are imperative for developing clinical therapeutic strategies to promote functional recovery after CNS injury.
Acknowledgements
We wish to thank M. Hou for critical reading of this manuscript. Our work is supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS), the McKnight foundation and the US National Multiple Sclerosis Society.
Glossary
- Dystrophic growth cones
Unusually shaped nerve terminals that are characterized by small globular clusters or multivesicular sacs found on the distal ends of regenerating axons in a glial scar environment.
- Dorsal root ganglia
(DRG). Ganglia that are found beside the spinal cord in which the cell bodies of sensory neurons are located. The bipolar neurons send a central axon through the spinal cord and another process to the PNS.
- Oligodendrocytes
Glial cells that elaborate myelin in the CNS. Unlike Schwann cells in the PNS that myelinate single axons, oligodendrocytes typically ensheath several processes at once.
- Astrocytes
The most abundant glial cell in the CNS, with a star-shaped cell body and broad end-feet on their processes. Astrocytes are thought to have nutritive functions, as well as roles in maintaining the blood–brain barrier and extracellular milieu.
- Glial scar
A physical and molecular barrier to regeneration that develops at CNS lesion sites, consisting primarily of reactive astrocytes, along with extracellular matrix molecules such as CSPGs.
- Growth cone
A motile actin-supported extension of a developing axon that can respond to external cues to guide its movement. Exposure to some repulsive guidance cues and many myelin-associated inhibitors leads to the collapse of this broad-shaped structure.
- Alternative splicing
A post-transcriptional process through which a pre-mRNA molecule, containing several introns and exons, can lead to different functional mRNA molecules, and consequently proteins, that originate from a single gene.
- GPI-anchor
A glycosylphosphatidylinositol (GPI) linkage, located at the carboxy termini of proteins without hydrophobic transmembrane regions, that can insert into the cell membrane. They might be released from the membrane by treatment with phospholipase C.
- Corticospinal tract
(CST). Axon fibres that originate from pyramidal neurons in layer 5 of the cerebral cortex and synapse on motor and interneurons in the spinal cord. This tract mediates motor functions and is commonly used for CNS injury models.
- Dorsal root entry zone
(DREZ). The interface between the CNS and PNS where sensory afferents from dorsal root ganglia enter the spinal cord during development.
- Penumbra
The area of secondary injury surrounding a CNS lesion epicentre.
- Dorsal rhizotomy
A transection of sensory nerve fibres in the dorsal root at its point of entry into the spinal cord.
- Rho-guanine dissociation inhibitor
(Rho-GDI). An inhibitory regulator of the Rho small G-protein family that can bind to RhoA and maintain it in an inactive state.
- Dorsal columns
Axon fibres that consist of the central processes of medium-diameter sensory dorsal root ganglia neurons that project up to dorsal column nuclei in the medulla.
- Raphespinal tract
Serotonin-containing fibres originating from caudal raphe nuclei in the brainstem to modulate sensory inputs such as pain.
- Rubrospinal tract
Axon fibres that are functionally related to corticospinal tracts, that originate from the caudal red nucleus and terminate on motor neurons in the spinal cord.
- Preconditioning injury
A lesion of the peripheral branch of bipolar sensory neurons in dorsal root ganglia that can promote the subsequent regeneration of their central axons in the spinal cord after nerve transection at a later time point.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
The following terms in this article are linked online to:
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
EGFR | EphA4 | ephrin B3 | LINGO1 | MAG | NgR | Nogo | OMgp | p75 | RhoA | ROCK | Sema4D/CD100 | TROY
References
- 1.Zito K, Svoboda K. Activity-dependent synaptogenesis in the adult mammalian cortex. Neuron. 2002;35:1015–1017. doi: 10.1016/s0896-6273(02)00903-0. [DOI] [PubMed] [Google Scholar]
- 2.Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
- 3.Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- 4.Ramón y Cajal S. Degeneration and Regeneration of the Nervous System. London: Oxford Univ. Press; 1928. [Google Scholar]
- 5. Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 2004;24:6531–6539. doi: 10.1523/JNEUROSCI.0994-04.2004. An interesting study analysing the morphological changes that constitute the dystrophic growth cones of lesioned axons in the hostile CNS environment.
- 6. David S, Aguayo AJ. Axonal elongation into peripheral nervous system ‘bridges’ after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. A seminal paper that attributes axon regeneration failure to the non-permissive CNS environment by showing that some lesioned axons can regrow through a transplanted peripheral nerve graft.
- 7.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nature Rev. Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 8.He Z, Koprivica V. The Nogo signaling pathway for regeneration block. Annu. Rev. Neurosci. 2004;27:341–368. doi: 10.1146/annurev.neuro.27.070203.144340. [DOI] [PubMed] [Google Scholar]
- 9.Yiu G, He Z. Signaling mechanisms of the myelin inhibitors of axon regeneration. Curr. Opin. Neurobiol. 2003;13:545–551. doi: 10.1016/j.conb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Silver J, Miller JH. Regeneration beyond the glial scar. Nature Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 11.Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J. Neurosci. 1985;5:2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- 13.Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- 14.Prinjha R, et al. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–284. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 15.Chen MS, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 16.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 17.Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oertle T, et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J. Neurosci. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 20.Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 21.Trajkovic K, et al. Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. J. Cell Biol. 2006;172:937–948. doi: 10.1083/jcb.200509022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 23.McKerracher L, et al. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang KC, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 25.Moreau-Fauvarque C, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J. Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benson MD, et al. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc. Natl Acad. Sci. USA. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai C, Watson JB, Bloom FE, Sutcliffe JG, Milner RJ. Neural protein 1B236/myelin-associated glycoprotein (MAG) defines a subgroup of the immunoglobulin superfamily. Immunol. Rev. 1987;100:129–151. doi: 10.1111/j.1600-065x.1987.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 28.Salzer JL, Holmes WP, Colman DR. The amino acid sequences of the myelin-associated glycoproteins: homology to the immunoglobulin gene superfamily. J. Cell Biol. 1987;104:957–965. doi: 10.1083/jcb.104.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang S, Qiu J, Nikulina E, Filbin MT. Soluble myelin-associated glycoprotein released from damaged white matter inhibits axonal regeneration. Mol. Cell. Neurosci. 2001;18:259–269. doi: 10.1006/mcne.2001.1020. [DOI] [PubMed] [Google Scholar]
- 30.Schachner M, Bartsch U. Multiple functions of the myelin-associated glycoprotein MAG (siglec-4a) in formation and maintenance of myelin. Glia. 2000;29:154–165. doi: 10.1002/(sici)1098-1136(20000115)29:2<154::aid-glia9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Johnson PW, et al. Recombinant myelin-associated glycoprotein confers neural adhesion and neurite outgrowth function. Neuron. 1989;3:377–385. doi: 10.1016/0896-6273(89)90262-6. [DOI] [PubMed] [Google Scholar]
- 32.DeBellard ME, Tang S, Mukhopadhyay G, Shen YJ, Filbin MT. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialo-glycoprotein. Mol. Cell. Neurosci. 1996;7:89–101. doi: 10.1006/mcne.1996.0007. [DOI] [PubMed] [Google Scholar]
- 33.Turnley AM, Bartlett PF. MAG and MOG enhance neurite outgrowth of embryonic mouse spinal cord neurons. Neuroreport. 1998;9:1987–1990. doi: 10.1097/00001756-199806220-00013. [DOI] [PubMed] [Google Scholar]
- 34.Mikol DD, Gulcher JR, Stefansson K. The oligodendrocyte-myelin glycoprotein belongs to a distinct family of proteins and contains the HNK-1 carbohydrate. J. Cell Biol. 1990;110:471–479. doi: 10.1083/jcb.110.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang JK, et al. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310:1813–1817. doi: 10.1126/science.1118313. A unique study that provides a spatial explanation for how the myelin-associated inhibitor OMgp can limit collateral sprouting at nodes of Ranvier.
- 36.Kullander K, et al. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 2001;15:877–888. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 38.Simonen M, et al. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 39.Zheng B, et al. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 40.Rudge JS, Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J. Neurosci. 1990;10:3594–3603. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faulkner JR, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgenstern DA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 2002;137:313–332. doi: 10.1016/s0079-6123(02)37024-9. [DOI] [PubMed] [Google Scholar]
- 44.Snow DM, Steindler DA, Silver J. Molecular and cellular characterization of the glial roof plate of the spinal cord and optic tectum: a possible role for a proteoglycan in the development of an axon barrier. Dev. Biol. 1990;138:359–376. doi: 10.1016/0012-1606(90)90203-u. [DOI] [PubMed] [Google Scholar]
- 45.Pindzola RR, Doller C, Silver J. Putative inhibitory extracellular matrix molecules at the dorsal root entry zone of the spinal cord during development and after root and sciatic nerve lesions. Dev. Biol. 1993;156:34–48. doi: 10.1006/dbio.1993.1057. [DOI] [PubMed] [Google Scholar]
- 46.Carulli D, Laabs T, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr. Opin. Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Ughrin YM, Chen ZJ, Levine JM. Multiple regions of the NG2 proteoglycan inhibit neurite growth and induce growth cone collapse. J. Neurosci. 2003;23:175–186. doi: 10.1523/JNEUROSCI.23-01-00175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bovolenta P, Fernaud-Espinosa I. Nervous system proteoglycans as modulators of neurite outgrowth. Prog. Neurobiol. 2000;61:113–132. doi: 10.1016/s0301-0082(99)00044-1. [DOI] [PubMed] [Google Scholar]
- 49.Cole GJ, Loewy A, Glaser L. Neuronal cell–cell adhesion depends on interactions of N-CAM with heparin-like molecules. Nature. 1986;320:445–447. doi: 10.1038/320445a0. [DOI] [PubMed] [Google Scholar]
- 50.Grumet M, Flaccus A, Margolis RU. Functional characterization of chondroitin sulfate proteoglycans of brain: interactions with neurons and neural cell adhesion molecules. J. Cell Biol. 1993;120:815–824. doi: 10.1083/jcb.120.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedlander DR, et al. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J. Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp. Neurol. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 53.Yang Z, et al. NG2 glial cells provide a favorable substrate for growing axons. J. Neurosci. 2006;26:3829–3839. doi: 10.1523/JNEUROSCI.4247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dou CL, Levine JM. Identification of a neuronal cell surface receptor for a growth inhibitory chondroitin sulfate proteoglycan (NG2) J. Neurochem. 1997;68:1021–1030. doi: 10.1046/j.1471-4159.1997.68031021.x. [DOI] [PubMed] [Google Scholar]
- 55.Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol. Cell. Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 56.Ramer MS, Duraisingam I, Priestley JV, McMahon SB. Two-tiered inhibition of axon regeneration at the dorsal root entry zone. J. Neurosci. 2001;21:2651–2660. doi: 10.1523/JNEUROSCI.21-08-02651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davies SJ, et al. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 58.Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J. Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang X, Davies JE, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J. Neurosci. Res. 2003;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- 60.Kantor DB, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Domeniconi M, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–990. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 62.Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 63.Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J. Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- 65.Wong ST, et al. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nature Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- 66.Domeniconi M, et al. MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron. 2005;46:849–855. doi: 10.1016/j.neuron.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 67.Park JB, et al. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 68.Shao Z, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 69.Mi S, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nature Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 70.Fischer D, He Z, Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J. Neurosci. 2004;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. A critical paper demonstrating that NgR-deficient mice show enhanced regeneration from raphespinal and rubrospinal tract fibres, but not CST fibres, suggesting that the contribution by NgR to regeneration failure might be different in different nerve fibre tracts.
- 72. Zheng B, et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc. Natl Acad. Sci. USA. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. An important study showing that genetic deletion of NgR cannot overcome myelin inhibition or promote CST regeneration, supporting the presence of NgR-independent mechanisms mediating myelin inhibition and regeneration failure.
- 73.Lauren J, Airaksinen MS, Saarma M, Timmusk T. Two novel mammalian Nogo receptor homologs differentially expressed in the central and peripheral nervous systems. Mol. Cell. Neurosci. 2003;24:581–594. doi: 10.1016/s1044-7431(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 74.Pignot V, et al. Characterization of two novel proteins, NgRH1 and NgRH2, structurally and biochemically homologous to the Nogo-66 receptor. J. Neurochem. 2003;85:717–728. doi: 10.1046/j.1471-4159.2003.01710.x. [DOI] [PubMed] [Google Scholar]
- 75.Barton WA, et al. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J. 2003;22:3291–3302. doi: 10.1093/emboj/cdg325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Venkatesh K, et al. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J. Neurosci. 2005;25:808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vinson M, et al. Myelin-associated glycoprotein interacts with ganglioside GT1b.A mechanism for neurite outgrowth inhibition. J. Biol. Chem. 2001;276:20280–20285. doi: 10.1074/jbc.M100345200. [DOI] [PubMed] [Google Scholar]
- 79.Vyas AA, Blixt O, Paulson JC, Schnaar RL. Potent glycan inhibitors of myelin-associated glycoprotein enhance axon outgrowth in vitro. J. Biol. Chem. 2005;280:16305–16310. doi: 10.1074/jbc.M500250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maekawa M, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 81.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 82.Winton MJ, Dubreuil CI, Lasko D, Leclerc N, McKerracher L. Characterization of new cell permeable C3-like proteins that inactivate Rho and stimulate neurite outgrowth on inhibitory substrates. J. Biol. Chem. 2002;277:32820–32829. doi: 10.1074/jbc.M201195200. [DOI] [PubMed] [Google Scholar]
- 83.Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nature Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- 84.Dergham P, et al. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lehmann M, et al. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hsieh SH, Ferraro GB, Fournier AE. Myelin-associated inhibitors regulate cofilin phosphorylation and neuronal inhibition through LIM kinase and Slingshot phosphatase. J. Neurosci. 2006;26:1006–1015. doi: 10.1523/JNEUROSCI.2806-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sivasankaran R, et al. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nature Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- 89.Hasegawa Y, et al. Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G. J. Neurosci. 2004;24:6826–6832. doi: 10.1523/JNEUROSCI.1856-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koprivica V, et al. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- 91.Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 92.Bandtlow CE, Schmidt MF, Hassinger TD, Schwab ME, Kater SB. Role of intracellular calcium in NI-35-evoked collapse of neuronal growth cones. Science. 1993;259:80–83. doi: 10.1126/science.8418499. [DOI] [PubMed] [Google Scholar]
- 93.Snow DM, Atkinson PB, Hassinger TD, Letourneau PC, Kater SB. Chondroitin sulfate proteoglycan elevates cytoplasmic calcium in DRG neurons. Dev. Biol. 1994;166:87–100. doi: 10.1006/dbio.1994.1298. [DOI] [PubMed] [Google Scholar]
- 94.Brosamle C, Huber AB, Fiedler M, Skerra A, Schwab ME. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J. Neurosci. 2000;20:8061–8068. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bartsch U, et al. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. doi: 10.1016/0896-6273(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 96.GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 97.Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J. Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li S, Kim JE, Budel S, Hampton TG, Strittmatter SM. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol. Cell. Neurosci. 2005;29:26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li S, et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walsh GS, Krol KM, Crutcher KA, Kawaja MD. Enhanced neurotrophin-induced axon growth in myelinated portions of the CNS in mice lacking the p75 neurotrophin receptor. J. Neurosci. 1999;19:4155–4168. doi: 10.1523/JNEUROSCI.19-10-04155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hannila SS, Kawaja MD. Nerve growth factor-induced growth of sympathetic axons into the optic tract of mature mice is enhanced by an absence of p75NTR expression. J. Neurobiol. 1999;39:51–66. [PubMed] [Google Scholar]
- 102.Song XY, Zhong JH, Wang X, Zhou XF. Suppression of p75NTR does not promote regeneration of injured spinal cord in mice. J. Neurosci. 2004;24:542–546. doi: 10.1523/JNEUROSCI.4281-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Menet V, Prieto M, Privat A, Gimenez y Ribotta M. Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc. Natl Acad. Sci. USA. 2003;100:8999–9004. doi: 10.1073/pnas.1533187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. A pioneering study describing the use of ChABC to neutralize glial scar-based inhibition as a therapeutic means to promote recovery after CNS injury. This paper spawned many subsequent studies using similar techniques in other injury paradigms.
- 105.Moon LD, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nature Neurosci. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- 106.Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J. Neurosci. 2004;24:10064–10073. doi: 10.1523/JNEUROSCI.2981-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steward O, Zheng B, Tessier-Lavigne M. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J. Comp. Neurol. 2003;459:1–8. doi: 10.1002/cne.10593. [DOI] [PubMed] [Google Scholar]
- 108. Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J. Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. An interesting paper describing the potential application of blocking inhibitory influences to promote local sprouting and plasticity to enhance stroke recovery.
- 109.Papadopoulos CM, et al. Dendritic plasticity in the adult rat following middle cerebral artery occlusion and Nogo-a neutralization. Cereb. Cortex. 2006;16:529–536. doi: 10.1093/cercor/bhi132. [DOI] [PubMed] [Google Scholar]
- 110.Markus TM, et al. Recovery and brain reorganization after stroke in adult and aged rats. Ann. Neurol. 2005;58:950–953. doi: 10.1002/ana.20676. [DOI] [PubMed] [Google Scholar]
- 111.Seymour AB, et al. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J. Cereb. Blood Flow Metab. 2005;25:1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- 112.Oudega M, Hagg T. Nerve growth factor promotes regeneration of sensory axons into adult rat spinal cord. Exp. Neurol. 1996;140:218–229. doi: 10.1006/exnr.1996.0131. [DOI] [PubMed] [Google Scholar]
- 113.Oudega M, Hagg T. Neurotrophins promote regeneration of sensory axons in the adult rat spinal cord. Brain Res. 1999;818:431–438. doi: 10.1016/s0006-8993(98)01314-6. [DOI] [PubMed] [Google Scholar]
- 114.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 115.Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J. Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steinmetz MP, et al. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J. Neurosci. 2005;25:8066–8076. doi: 10.1523/JNEUROSCI.2111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 118. Pizzorusso T, et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. The first report describing the involvement of CSPGs in forming a perineuronal net that limits experience-driven plasticity in the adult.
- 119. McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. Another important paper that further demonstrates the parallels between CNS myelin and CSPGs. It shows that myelin-associated inhibitors, such as CSPGs, are also involved in critical period closure.
- 120.Tropea D, Caleo M, Maffei L. Synergistic effects of brain-derived neurotrophic factor and chondroitinase ABC on retinal fiber sprouting after denervation of the superior colliculus in adult rats. J. Neurosci. 2003;23:7034–7044. doi: 10.1523/JNEUROSCI.23-18-07034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Massey JM, et al. Chondroitinase ABC digestion of the perineur onal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J. Neurosci. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. A study demonstrating that removal of CSPG inhibition also promotes functional collateral sprouting after injury, providing a potential mechanistic link between structural remodelling and functional plasticity.
- 122.Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nature Med. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- 123.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 124.Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nature Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- 125.Lichtman JW, Sanes JR. Watching the neuromuscular junction. J. Neurocytol. 2003;32:767–775. doi: 10.1023/B:NEUR.0000020622.58471.37. [DOI] [PubMed] [Google Scholar]
- 126.Muller CM, Best J. Ocular dominance plasticity in adult cat visual cortex after transplantation of cultured astrocytes. Nature. 1989;342:427–430. doi: 10.1038/342427a0. [DOI] [PubMed] [Google Scholar]
- 127.Ferretti P, Zhang F, O’Neill P. Changes in spinal cord regenerative ability through phylogenesis and development: lessons to be learnt. Dev. Dyn. 2003;226:245–256. doi: 10.1002/dvdy.10226. [DOI] [PubMed] [Google Scholar]