Abstract
The use of “non-standard” metallic radionuclides continues to be an expanding field of investigation. Radiolabeling small molecules, peptides, proteins, and up to nano-particles are all areas of active investigation for both diagnostic and therapeutic applications. All require a common variable – the need for appropriate chelation chemistry for adequate sequestration of the metallic radionuclide that is equal to the intended application. A brief overview of the array of the chelation chemistry options available to researchers and the means for their selection is provided.
Introduction
Nature has provided a vast array of radionuclides with emission properties that that makes them valuable reagents for investigating basic problems in chemistry, biology, and medicine. These properties include γ-, β+-, β--, α-, and Auger emissions just to list some of those useful for medical diagnostic (γ–scintigraphy, SPECT, PET) and therapeutic applications. In addition to their radionuclidic properties, there is an even wider array of fundamental chemical properties that are available for researchers to exploit. However, the use of these same radionuclides is then constrained by limits of half-life, decay chain, production and availability, realistic chemical usage, and matching all of these properties appropriately to the intended biological application(s) which severely diminishes the number of choices to a select few (Table 1).1 As much of these aspects have been well reviewed, the focus herein then is on those properties and the chemistry required for the use of those metallic radionuclides that have generally been accepted to be within those boundary conditions set forth above.
Table 1.
Selected Properties of “Non-Standard” Radionuclides
| Radionuclide | Ionic Radius* | Charge | T½ |
|---|---|---|---|
| 66Ga/68Ga | 62.0 | +3 | 9.5 h/68 min |
| 86Y/90Y | 90.0 | +3 | 14.7 h/2.67 d |
| 111In | 80.0 | +3 | 2.8 d |
| 212Pb | 119.0 | +2 | 10.64 h |
| 212Bi/213Bi | 103.0 | +3 | 1.01 h/ 45.6 min |
| 89Zr | 72.0 | +4 | 3.27 d |
| 177Lu | 86.1 | +3 | 6.71 d |
| 225Ac | 112.0 | +3 | 10 d |
The utility of these metallic radionuclides has necessitated the development of metal chelating agents to effectively provide a handle over their behavior. These chelating agents have been termed “bifunctional chelating agents” since they have a metal binding moiety function and then also possess a chemically reactive functional group. The former then provides for the sequestration of the metallic radionuclide while the latter aspect provides the requisite chemistry for covalent attachment to a targeting vector of interest, such as a small molecules peptides (octreotide),2 proteins (monoclonal antibody, Zevalin),3 or nano-particles.4
There are a number of fundamental criteria that have to be met in the design of bifunctional chelating agents for such applications. Foremost seems based on the stability of the metal complex. Clearly, the consequences of loss or dissociation of the radionuclide are associated with toxicity in the case of therapeutics and poor image qualities for diagnostics. Fundamental coordination chemistry criteria such as: (1) charge; (2) matching cavity size of the chelating agent with the ionic radius of the radionuclide; (3) providing the appropriate chelate denticity or number of donor binding groups; and (4) providing donor binding groups of appropriate chemical character are all key elements. Two additional properties are also critical to consider: the rate at which the metal complex forms and the rate of dissociation. All of these criteria are interrelated. Cavity size must accommodate the ionic radius of the radionuclide such that all of required donor groups can be properly aligned for optimal binding to the metal ion in such a way to adequately encapsulate the ion thereby providing high stability and limiting dissociation. A listing of those metallic radionuclides that will be discussed here along with their selected properties of ionic radius, charge, and half-life is provided in Table 1. The suitable radiometals are diverse in their properties and coordination chemistry, so, unfortunately there is no bifunctional chelating agent suitable for all radionuclides.5 Lastly, there are a number of copper radionuclides. These are not included here and are left for discussion in other papers in this issue.
Having then created a bifunctional chelating agent, validation of its suitability for biological applications still remains to be executed. There are a number of properties that can be used to validate acceptability of a novel bifunctional chelating agent, including: (1) thermodynamic stability constants; (2) transchelation studies; (3) acid catalyzed dissociation constants; and (4) serum stability studies. All of these properties do provide some information that can be used to suggest potential in vivo suitability. Serum stability can be a very useful tool and model that serves to predict and eliminate from contention those bifunctional chelating agents that are unsuitable for in vivo applications. None of these properties or models is predictive of actual in vivo stability of the metal complex. To assess real in vivo stability of the metal complex, evaluation in an appropriate animal model is necessary. The definition of appropriate animal model is variable, however clearly it should really reflect very closely the ultimate intended biological application. As yet, no in vitro model system replicates all of the ongoing processes and components of a living organism just as the therapeutic efficacy of a macromolecule can not be predicted from in vitro results.
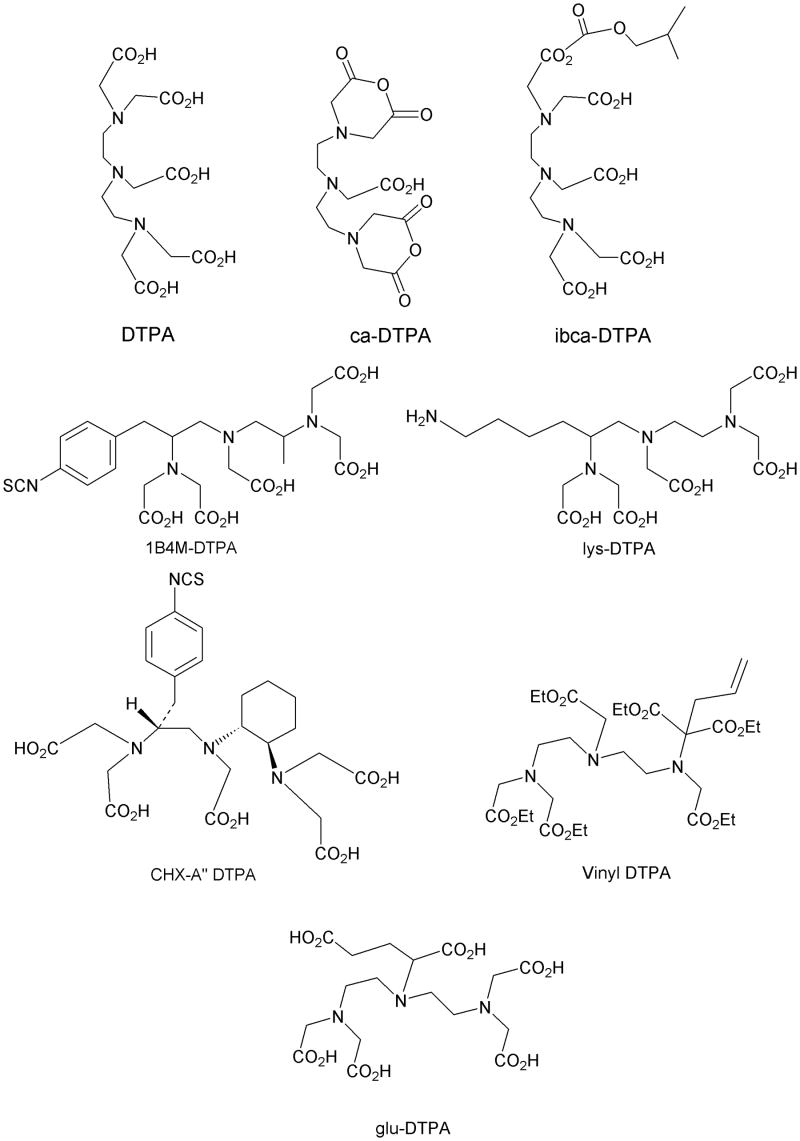
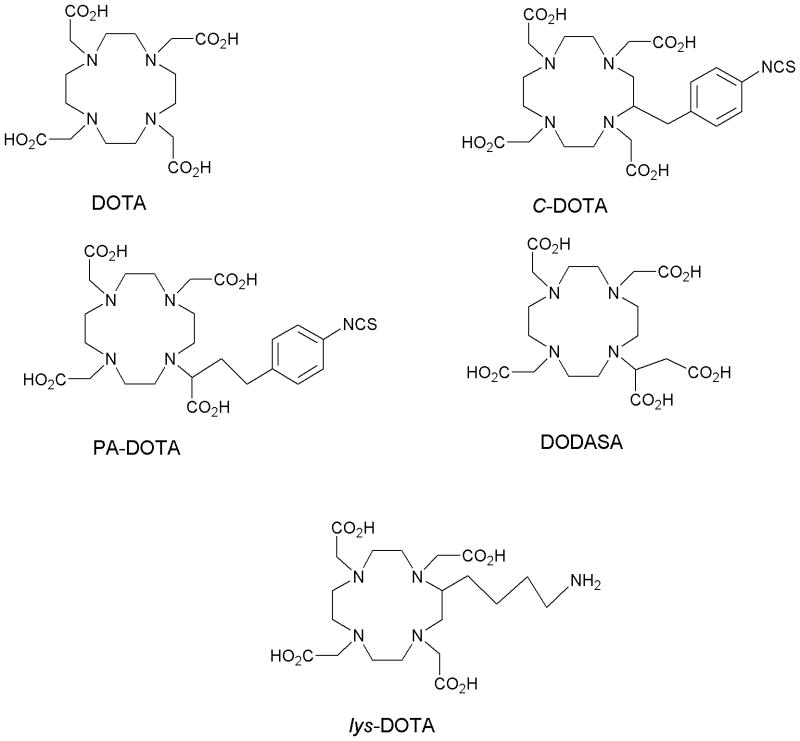
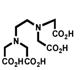
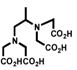
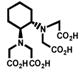
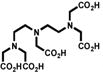
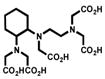
Despite all those considerations, the development of bifunctional chelating agents has been rooted in making derivations from well established and defined inorganic chemistry chelating agents, e.g., ethylenediamine tetraacetic acid (EDTA), diethylenetriamine pentaacetic acid (DTPA) (Figure 1), and 1,4,7,10-tetra-azacylcododecane-N,N′,N″,N‴-tetraacetic acid (DOTA) (Figure 2), all of which as polyaminocarboxylate ligands vary coordination number from 6 to, and also cover acyclic and macrocyclic options to encapsulate the metal ion. Fundamental thermodynamic stability constants are known for these ligands with a variety of metal ions that have provided a starting point for their derivation into an array of bifunctional chelating agents (Table 2).6 In addition to that data, actual biological data for some of these ligands complexing radionuclides of medical interest, both animal and human use, is available in the literature and an example of such is provided in Table 3.7 Such data can be used to extrapolate to the creation of bifunctional chelating agents.
Figure 1.
Structures of DTPA, ca-DTPA, ibca-DTPA, 1B4M-DTPA, lys-DTPA, vinyl DTPA, glu-DTPA, and CHX-A” DTPA
Figure 2.
Structures of DOTA, C-DOTA, PA-DOTA, DODASA, and lys-DOTA
Table 2.
Selected Stability Constants for Acyclic Polyaminocarboxylate Chelates6a
 |
 |
 |
 |
 |
|
|---|---|---|---|---|---|
| EDTA | Me-EDTA | CHX-EDTA | DTPA | CHX-DTPA | |
| Y(III) | 18.09 | 18.78 | 19.85 | 22.13 | ------ |
| In(III) | 24.9 | ------ | 28.8 | 29.0 | ------ |
| Bi(III) | 27.8 | ------ | 32.4 | 35.6 | ------ |
Table 3.
Plasma Level and Cumulative Urinary Excretion of Yttrium Chelates7
| Ligand | 4 hr
Plasma Urine |
8 hr
Plasma Urine |
24 hr
Plasma Urine |
|||
|---|---|---|---|---|---|---|
| EDTA | 5.3 | 36.5 | 0.9 | 43.7 | 0.5 | 46.7 |
| CDTA | 5.7 | 68.4 | 2.3 | 87.1 | 0 | 97.6 |
| DTPA | 5.0 | 75.0 | 1.8 | 93.0 | 0 | 101.3 |
Historically, one of the earliest reports of a bifunctional chelating agent conjugated to an antibody made use of a natural product, desferrioxamine, for radiolabeling with 111In.8 Desferrioxamine and related compounds are well known chelators of Fe(III), and as such their derivation for use with In(III) and Ga(III) has precedence. Interestingly, more recently desferrioxamine has been investigated for sequestering 89Zr through a somewhat complicated, yet elegant protocol that exploits that same Fe(III)/(II) chemistry for antibody labeling in support of immunoPET applications.9,10 The Fe(III) complex was formed first with the desferrioxamine then activated for conjugation through extension of the terminal amine with succinic anhydride followed by conversion of the formed carboxylate into an active ester.10 After conjugation, the Fe(III) was reduced and displaced with the 89Zr.
Returning to polyaminocarboylate ligands, one can recognize that while bifunctional EDTA derivatives were initially reported for use with 111In and 90Y, their limited stability forced a move to develop bifunctional DTPA derivatives that would provide a more appropriate coordination number.11,12 Concurrently, direct derivatives of DTPA, the cyclic anhydride (ca-DTPA) and the isobutylcarbonic anhydride (carb-DTPA) were also routinely in use (Figure 1).13,14 Despite their products routinely being termed DTPA conjugates these conjugation products, in fact, are not DTPA chelators. This is due to the utilization of one carboxylate in the conjugation forming an amide that may or may not then bind to the metal effectively. Studies to determine the impact of this change in coordination number and donor character very clearly defined decreased in vitro and in vivo stability of these products.12,15 Additionally, the cyclic anhydride with two reactive conjugation moieties suffered from potential cross-linking issues.
Full octadentate bifunctional DTPA derivatives addressed these deficiencies.12,16-19 The numbers and variations on structure combined with variations in conjugation strategies that have been developed exceed the scope of this paper. However, a selection of representative structures is provided in Figure 1. The 1B4M-DTPA, also known as MX-DTPA or tiuxetan, has been developed as the chelating agent component of Zevalin for radiolabeling with either 111In or 90Y.3 Further refinements in the pre-organization geometry of the DTPA donor elements ultimately led to the creation of the CHX-A” DTPA which has been reported to form stable complexes with 111In, 177Lu, 213Bi, and to also be significantly more stable than the 1B4M-DTPA for sequestering 90Y.20-22 The CHX-A” DTPA is notable for being the chelating agent component in the first clinical antibody trial using an α-emitter, 213Bi,23 as well also being a commercially available product.
Despite the successes achieved with bifunctional DTPA derivatives, their overall stability complexing radionuclides such as 90Y was noted as being less than perfect and could potentially contribute to toxicity.24
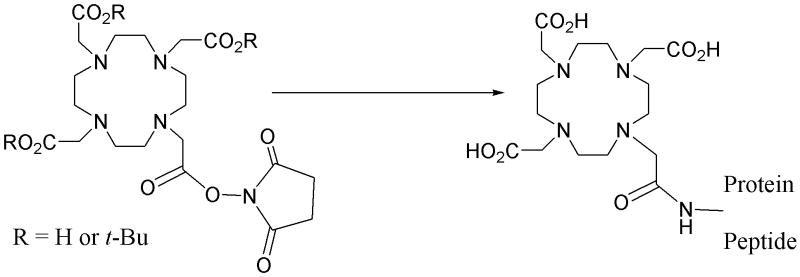
In response to that deficiency, full octadentate macrocyclic bifunctional DOTA derivatives have been developed for complexing 111In, 86Y, 90Y, radio-lanthanides, 213Bi, 212Pb, and 225Ac.25-29 Again, the numbers and variations on structure combined with variations in conjugation themes that have been developed exceed the scope of this paper. However, a selection of representative structures is provided in Figure 2. Concurrently, a bifunctional DOTA that makes use of one carboxylate in an active ester form for protein conjugation has also been developed and is also a commercially available product (Figure 3).30 As with analogous DTPA derivatives, the conjugation product really is not DOTA, but again a mono-amide product wherein the amide may or may not bind to the metal. While this DOTA mono-amide product provides convenience (another commercial product), the impact on the fundamental chemical characteristics by this change in donor number and character have not been well studied with the array of radionuclides that have been used with this agent. Considerable benefit on metal ion complex stability is no doubt conferred by the macrocyclic effect, yet actual stability constants remain to be reported for those metallic radionuclides with which this chemistry have been employed.
Figure 3.
Structures of DOTA hydroxysuccinimide active ester and its peptide or protein conjugation product.
Limitations to the use of the DOTA derivatives is directly related to their exquisite stability; slower complex formation rates compromise radiolabeling yields, efficiency, and specific activity. The multi-step mechanism of complex formation severely limits the actual use of bifunctional DOTA agents and in fact may have contributed to some questionable results using this agent.31 The slow formation rates can be in part traversed if the conjugate product is tolerant of being heated transiently.2 Conversely, use of bifunctional DTPA ligands is not hampered by complex formation rates. Thus, one must very carefully choose which class of ligands is most appropriate for each specific application.
Bifunctional DOTA has also been used for 225Ac, however, the reported radiolabeled conditions to force complexation are not acceptable for protein conjugates, hence formation of the complex has been performed first followed by conjugation via isothiocyanate chemistry with concomitant low efficiencies in both complexation and conjugation.32
Other macrocyclic ligands have been reported. There include a number of bifunctional NOTA agents (Figure 4).33-36 NOTA is well established to form an exceedingly stable complex with Ga(III), and as such, one might think that PET agents would feature its use. Surprisingly, DOTA seems to be preferred despite there being no real substantiation as to the stability of the DOTA Ga(III) complex. One might speculate that use of DOTA in this specific instance may be directly linked to the commercial availability of the DOTA active ester derivative (Figure 3). One variant on DOTA that has seen significant use with 203Pb and 212Pb is the tetra-primary amide of DOTA termed TCMC (Figure 4).37,38
Figure 4.
Structures of C-NOTA, N-NOTA, NODASA DTPA, and TCMC
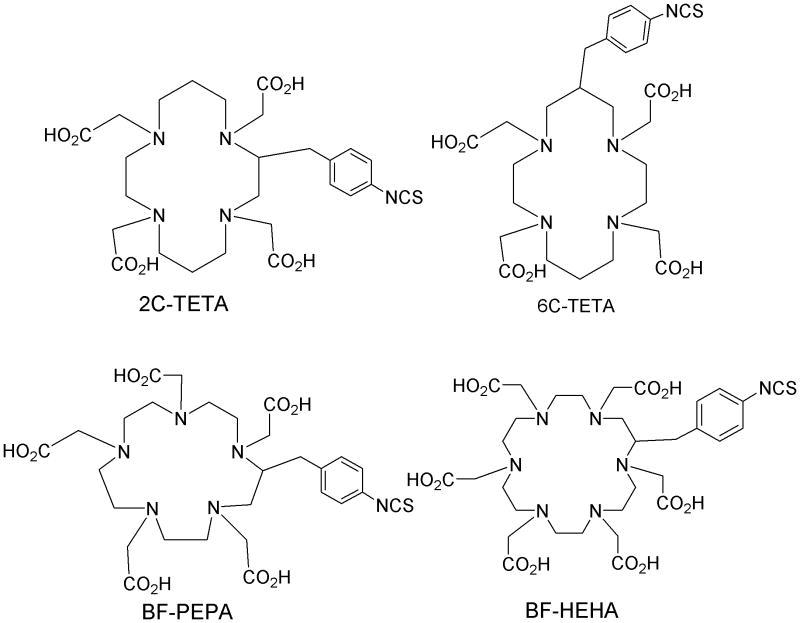
Bifunctional macrocyclic chelating agents with larger than the 12-membered ring DOTA, 14-membered, 15-membered, and 18-membered ring agents have also been developed (Figure 5). At least two different geometrically substituted 14-membered ring bifunctional TETA agents have been reported.26,39 TETA had been promoted as being stable for copper radionuclides and that topic will be discussed in other papers in this issue. Oddly, TETA appears to have had no use with any other radionuclides. One 15-membered ring bifunctional PEPA has been investigated for complexing 213Bi stably in vivo without success.40 Lastly, one 18-membered ring bifunctional HEHA has been investigated for complexing 225Ac and superior stability versus DOTA reported, yet still not adequate for in vivo use with this element.41,42
Figure 5.
Structures of 2C-TETA, 6C-TETA, BF-PEPA and BF-HEHA
An area in the development of bifunctional chelating agents that has seen a surprising small level of investigation has been impact on radio-metal complex stability due to the stereochemical constraints of the chelating agent. Clearly, stereochemistry plays a serious role in the three dimensional geometry and arrangement of donor elements directed towards the metal ion and that optimization of these variables should equally lead to optimized chelation chemistry. The study that led to the development of the CHX-A” DTPA in fact investigated whether differences might even exist between radiolabeled enantiomeric forms of chelating agents post-conjugation.21 Both serum stability and transchelation studies indicated that radiolabeled enantiomers behaved identically to their corresponding racemates, however in vivo studies that examined bone deposition as an indicator of 88Y loss clearly demonstrated significant differences between enantiomeric conjugates. One might be tempted to attribute this result to stereochemical resolution due to complex formation except that the in vitro studies failed to, or were inadequate to detect this condition. This result does reflect two very critical aspects of bifunctional chelate development: stereochemistry can be important and should be studied, and that the importance of in vivo studies can not be discounted.
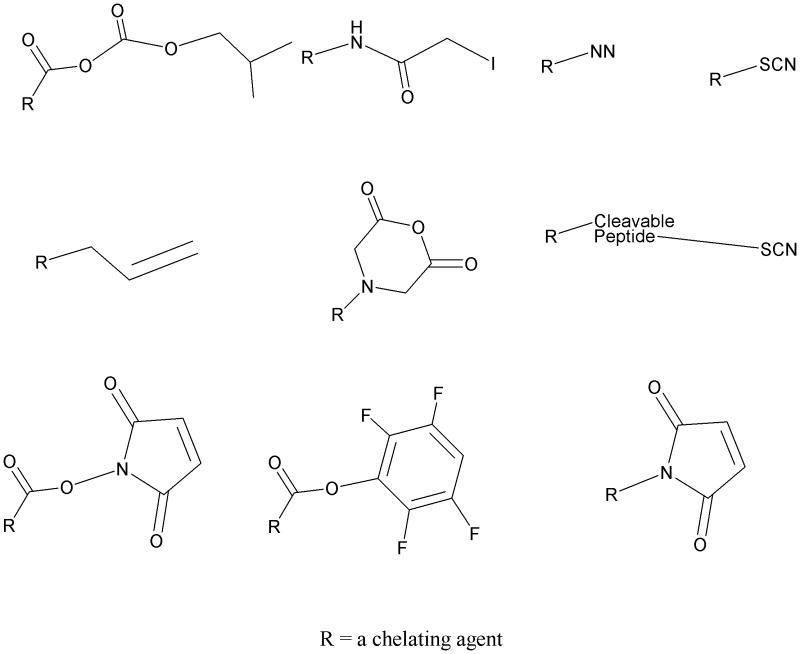
An array of reactive functional groups for conjugation of bifunctional chelating agents has also been reported in the literature of which a selection is depicted in Figure 6. Beyond just the simple amine or carboxylate for use in conjugation chemistry protocols, haloacetamide or maleimide have been reported for reaction with sulfhydryl moieties that are either extant of introduced,43,44 isothiocyanates have been readily available for reaction with amine groups,11,12 azides have been familiar as photoaffinity reagents,11 a wide array of active ester chemistry for reacting with amines have been perhaps only partly explored,30,45,46 and even an alkene derivative for use in Suzuki coupling chemistry has been reported.18 The breadth of choices of conjugation reactive functional groups has explored all of these listed possibilites and more, however, the need for refinement of these choices and their actual usage remains an area where opportunities remain. Currently, active esters and isothiocyanate chemistry dominate the use of bifunctional chelating agents, primarily for peptides and proteins, respectively, perhaps more from convenience than actually having arrived at the terminus of development. Clearly, protein conjugation remains inefficient regardless of conjugation chemistry employed, resulting in random product distributions, and with radiolabeling yields that achieve far lower than theoretical specific activities, critical to both imaging and therapeutic applications. All of these areas continue to call for improvement and refinement of more than the bifunctional chelate itself, but rather how they are actually employed.
Figure 6.
Structure of some of the reactive functional groups that have been used for conjugation of bifunctional chelating agents to peptides and / or proteins
On a related note, if one considers the numbers of both bifunctional DTPA and DOTA that have been reported in the literature, one must really begin to question the need for further permutations of the fundamental structure of either ligand. How many more structural variants of these as well as many of the others are really advancing the field of use of bifunctional chelating agents? There seems little chance that any further advances in stability with DTPA or DOTA will be forthcoming. This is particularly relevant to DOTA since no measurable advances in either stability or formation rate enhancement has been achieved. Needs remain to select new permutations in conjugation chemistry as noted above, however, the fundamental base bifunctional ligands for this purpose do seem to be well in hand for nearly every metallic radionuclide that may be required to ask and answer the vast majority of research and clinical questions relevant to their use. Exceptions to this are obvious and tend towards the more “exotic” metallic radionuclides such as 225ac or 223Ra. Clearly, unequivocally stable bifunctional chelating agents for these radionuclides remain to be developed that would permit their precise therapeutic benefits to be determined.
Lastly, the continued pursuit of exceptionally stable complexes has to be put into the context of actual use, i.e., just what is “good enough”. One must balance the variable of radionuclide half-life with biological half-life versus actual biological application to assess just what is acceptable stability further balanced against potential toxicity. All of those parameters must yet again be weighed against actual feasibility of use of the chemistry. Clearly, we already have a great many of the requisite tools of bifunctional chelates with which to move forward to accomplished those refinements needed to develop both better and more effective imaging and therapeutic agents using metallic radionuclides.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. I would also thank Kwamena Baidoo for his critical reading of the manuscript and Julia Brechbiel for her assistance in assembly of this manuscript.
References
- 1.Milenic DE, Brady ED, Brechbiel MW. Antibody-Targeted Radiation Cancer Therapy. Nature Rev Drug Disc. 2004;3:488–498. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 2.Norenberg JP, Krenning BJ, Konings IRHM, Kusewitt TKN, Anderson TL, de Jong M, Garmestani K, Brechbiel MW, Kvols LK. 213Bi-DOTATOC Peptide Receptor Radionuclide Therapy of Pancreatic Tumors in a Pre-Clinical Animal Model. Clin Cancer Res. 2006;12:897–903. doi: 10.1158/1078-0432.CCR-05-1264. [DOI] [PubMed] [Google Scholar]
- 3.Chinn P, Braslawsky G, White C, Hanna N. Antibody therapy of non-Hodgkin's B-cell lymphoma. Cancer Immunol Immunother. 2003;52:257–280. doi: 10.1007/s00262-002-0347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Wartchow CA, Danthi SN, Shen Z, Dechene N, Pease J, Choi S, Doede T, Chu P, Ning S, Lee DY, Bednarski MD, Knox SJ. A Novel Antiangiogenesis Therapy using an Integrin Antagonist or anti-FLK-1 Antibody Coated 90Y-Labeled Nanoparticles. Int J Radiat Oncol Biol Phys. 2004;58:1215–1227. doi: 10.1016/j.ijrobp.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 5.Packard AB, Kronauge JF, Brechbiel MW. In: Metalloradiopharmaceuticals II, Diagnosis and Therapy. Clarke MJ, Sadler PJ, editors. Springer; New York: 1999. pp. 45–116. [Google Scholar]
- 6.Martell AE, Smith RM. Critical Stability Constants. Vol. 1 Plenum; New York: 1974. [Google Scholar]
- 7.Kroll H, Korman S, Siegel E, Hart HE, Rosoff B, Spencer H, Laszlo D. Excretion of yttrium and lanthanum chelates of cyclohexane 1,2-trans diamine tetraacetic acid and diethylenetriamine pentaacetic acid in man. Nature. 1957;180:919–920. doi: 10.1038/180919b0. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard JH, Ackerman M, Tubis M, Blahd WH. Indium-III-labeled Antibody Heavy Metal Chelate Conjugates: A Potential Alternative to Radioiodination. Proc Soc Expt Biol Med. 1976;151:297–302. doi: 10.3181/00379727-151-39196. [DOI] [PubMed] [Google Scholar]
- 9.Meijs WE, Herscheid JDM, Haisma HJ, Pinedo HM. Evaluation of deferal as a bifunctional chelating agent for labeling antibodies with Zr-89. Appl Rad Isot. 1992;43:1443–1447. doi: 10.1016/0883-2889(92)90170-j. [DOI] [PubMed] [Google Scholar]
- 10.Verel I, Visser GWM, Boellaard R, Stigter M, Snow GB, van Dongen GAMS. 89Zr Immuno-PET: Comprehensive Procedures for the Production of 89Zr-Labeled Monoclonal Antibodies. J Nucl Med. 2003;44:1271–1281. [PubMed] [Google Scholar]
- 11.Meares CF, Wensel TG. Metal Chelates as Probes of biological Systems. Acc Chem Res. 1984;17:202–209. [Google Scholar]
- 12.Brechbiel MW, Gansow OA, Atcher RW, Schlom J, Esteban J, Simpson DE, Colcher D. Synthesis of 1-(p-Isothiocyanatobenzyl) Derivatives of DTPA and EDTA. Antibody Labeling and Tumor-Imaging Studies. Inorg Chem. 1986;25:2772–2781. [Google Scholar]
- 13.Krejcarek GE, Tucker KL. Covalent attachment of chelating groups to macromolecules. Biochem Biophys Res Commun. 1977;77:581–585. doi: 10.1016/s0006-291x(77)80018-1. [DOI] [PubMed] [Google Scholar]
- 14.Hnatowich DJ, Layne WW, Childs RL, Lanteigne D, Davis MA, Griffin TW, Doherty PW. Radioactive Labeling of Antibody: A Simple and Efficient Method. Science. 1983;220:613–615. doi: 10.1126/science.6836304. [DOI] [PubMed] [Google Scholar]
- 15.Brockmann J, Rosch F. Determination of Stability Constants in Y-DTPA-Peptide-Systems: Evaluation of a Radiochemical Method Using n.c.a. Yttrium-88. Radiochim Acta. 1999;87:79–85. [Google Scholar]
- 16.Carney PL, Rogers PE, Johnson DK. Dual Isotope Study of Iodine-125 and Indium-111-Labeled Antibody in Athymic Mice. J Nucl Med. 1989;30:374–384. [PubMed] [Google Scholar]
- 17.Parker D. Tumour Targeting with Radiolabelled Macrocycle-Antobody Conjugates. Chem Soc Rev. 1990;19:271–291. [Google Scholar]
- 18.Anelli PL, Fedeli F, Gazzotti O, Lattuada L, Lux G, Rebasti F. L-Glutamic Acid and L-Lysine as Useful building Blocks for the Preparation of Bifunctional DTPA-like Ligands. Bioconjugate Chem. 1999;10:137–140. doi: 10.1021/bc970212u. [DOI] [PubMed] [Google Scholar]
- 19.Nemoto H, Cai J, Yamamoto Y. A New Synthetic Method of All Carboxylate-free DTPA Derivatives and its Application to the Synthesis of Gd-Carborane Complex. Tetrahedron Lett. 1996;37:539–542. [Google Scholar]
- 20.Brechbiel MW, Gansow OA. Synthesis of C-Functionalized Derivatives of trans-Cyclohexyldiethylenetriaminepenta-acetic Acid for Labelling of Monoclonal Antibodies with the 212Bi Alpha-particle Emitter. J Chem Soc Perkin Trans 1. 1992:1173–1178. [Google Scholar]
- 21.Wu C, Kobayashi H, Sun B, Yoo TM, Paik CH, Gansow OA, Carrasquillo JA, Pastan I, Brechbiel MW. Stereochemical Influence on the Stability of Radio-Metal Complexes In Vivo. Synthesis and Evaluation of the Four Stereoisomers of 2-(p-nitrobenzyl)-trans-CyDTPA. Bioorg Med Chem. 1997;5:1925–1934. doi: 10.1016/s0968-0896(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 22.Milenic DE, Garmestani K, Chappell LL, Dadachova E, Yordanov A, Ma D, Schlom J, Brechbiel MW. In Vivo Comparison of Macrocyclic and Acyclic Ligands for Radiolabeling of Monoclonal Antibodies with 177Lu for Radioimmunotherapeutic Applications. Nucl Med Biol. 2002;29:431–442. doi: 10.1016/s0969-8051(02)00294-9. [DOI] [PubMed] [Google Scholar]
- 23.Jurcic JG, Larson SM, Sgouros G, McDevitt MR, Finn RD, Divgi CR, Ballangrud AM, Klaus KA, Ma D, Humm JL, Brechbiel MW, Molinet R, Scheinberg DA. Targeted α Particle Immunotherapy in Humans. Blood. 2002;100:1233–1239. [PubMed] [Google Scholar]
- 24.Carrasquillo JA, White JD, Le N, Rotman M, Brechbiel MW, Gansow OA, Top LE, Perentesis P, Reynolds JC, Nelson DL, Waldmann TA. Similarities and Differences in 111In- and 90Y-Labeled 1B4M-DTPA AntiTac Monoclonal Antibody. J Nucl Med. 1999;40:268–76. [PubMed] [Google Scholar]
- 25.Moi MK, Meares CF. The Peptide Way to Macrocyclic Bifunctional Chelating Agents: Synthesis of 2-(p-Nitrobenzyl)-1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic Acid and Study of Its Yttrium(III) complex. J Am Chem Soc. 1988;110:6266–6267. doi: 10.1021/ja00226a063. [DOI] [PubMed] [Google Scholar]
- 26.Kline SJ, Betebenner DA, Johnson DK. Carboxymethyl-Substituted Bifunctional Chelators: Preparation of Aryl Isothiocyanate Derivatives of 3-(Carboxymethyl)-3-azapentanedioic Acid, 3,12-Bis (carboxymethyl) -6,9-dioxa-3,12-diazatetradecanedioic Acid, and 1,4,7,10-Tetraazacyclododecane-N,N′,N″,N‴-tetraacetic Acid for Use as Protein Labels. Bioconjugate Chem. 1991;2:26–31. doi: 10.1021/bc00007a005. [DOI] [PubMed] [Google Scholar]
- 27.McMurry TJ, Brechbiel MW, Kumar K, Gansow OA. Convenient Synthesis of Bifunctional Tetraaza Macrocycles. Bioconjugate Chem. 1992;3:108–117. doi: 10.1021/bc00014a004. [DOI] [PubMed] [Google Scholar]
- 28.Chappell LL, Rogers BE, Khazaeli MB, Mayo MS, Buchsbaum DJ, Brechbiel MW. Synthesis of the Bifunctional Chelating agent 1,4,7,10-tetraaza-N-(1-carboxy-3-(4-nitrophenyl)propyl)-N′,N″,N‴-tris(acetic acid)cyclododecane (PA-DOTA), Conjugation to HuCC49 and HuCC49ΔCH2, and Radiolabeling with 177Lu. Bioorg Med Chem. 1999;7:2313–2320. doi: 10.1016/s0968-0896(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 29.Eisenwiener KP, Powell P, Macke HR. A Convenient Synthesis of Novel Bifunctional Prochelators for Coupling to Bioactive Peptides for Radiometal Labelling. Biorg Med Chem Lett. 2000;10:2133–2135. doi: 10.1016/s0960-894x(00)00413-3. [DOI] [PubMed] [Google Scholar]
- 30.Lewis MR, Kao JY, Anderson AL, Shively JE, Raubitschek A. An Improved Method for Conjugating Monoclonal Antibodies with N-Hydroxysulfosuccinimidyl DOTA. Bioconjugate Chem. 2001;12:320–324. doi: 10.1021/bc0000886. [DOI] [PubMed] [Google Scholar]
- 31.Moreau J, Guillon E, Pierrard JC, Rimbault J, Port M, Aplincourt M. Complexing Mechanism of the Lanthanide Cations Eu3+, Gd3+, and Tb3+ with 1,4,7,10-Tetrakis(carboxymethyl)-1,4,7,10-tetraazacyclododecane(dota)--Characterization of Three Successive Complexing Phases: Study of the Thermodynamic and Structural Properties of the Complexes by Potentiometry, Luminescence Spectroscopy, and EXAFS. Chem Eur J. 2004;10:5218–5232. doi: 10.1002/chem.200400006. [DOI] [PubMed] [Google Scholar]
- 32.McDevitt MR, Ma D, Lai LT, Simon J, Borchardt P, Frank RK, Wu K, Pellegrini V, Curcio MJ, Miederer M, Bander NH, Scheinberg DA. Tumor Therapy with Targeted Atomic Nanogenerators. Science. 2001;294:1537–1540. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 33.Studer M, Meares CF. Synthesis of Novel 1,4,7 - Triazacyclononane-N,N′,N″ -triacetic Acid Derivatives Suitable for Protein Labeling. Bioconjugate Chem. 1992;3:337–341. doi: 10.1021/bc00016a013. [DOI] [PubMed] [Google Scholar]
- 34.McMurry TJ, Brechbiel M, Wu C, Gansow OA. Synthesis of 2-(p-NCS-Bz)-NOTA: Application of the 4-methoxy-2,3,6-trimethylbenzenesulfonamide (Mtr) Protecting Group in the Synthesis of Macrocyclic Polyamines. Bioconjugate Chem. 1993;4:236–245. doi: 10.1021/bc00021a009. [DOI] [PubMed] [Google Scholar]
- 35.Brechbiel MW, McMurry TJ, Gansow OA. A Direct Synthesis of a Bifunctional Chelating Agent for Radiolabeling Proteins. Tetrahedron Lett. 1993;34:3691–3694. [Google Scholar]
- 36.Andre JP, Maecke HR, Zehnder M, Macko L, Akyel KG. 1,4,7-Triazacyclononane-1-succinic acid-4,7-diacetic acid (NODASA): a new bifunctional chelator for radio gallium-labelling of biomolecules. J Chem Soc Chem Commun. 1998:1301–1302. [Google Scholar]
- 37.Chappell LL, Dadachova E, Milenic DE, Garmestani K, Wu C, Brechbiel MW. Synthesis, Characterization, and Evaluation of a Novel Bifunctional Chelating Agent for the Lead Isotopes 203Pb and 212Pb. Nucl Med Biol. 2000;27:93–100. doi: 10.1016/s0969-8051(99)00086-4. [DOI] [PubMed] [Google Scholar]
- 38.Milenic DE, Garmestani K, Brady ED, Albert PS, Abdulla A, Flynn J, Brechbiel MW. Potentiation of High LET Radiation by Gemcitabine: Targeting of HER2 with Trastuzumab for the Treatment of Disseminated Peritoneal Disease. Clin Cancer Res. 2007;13:1926–1935. doi: 10.1158/1078-0432.CCR-06-2300. [DOI] [PubMed] [Google Scholar]
- 39.Moi MK, Yanuck M, Deshpande SV, Hope H, DeNardo SJ, Meares CF. X-ray Crystal Structure of a Macrocyclic Copper Chelate Stable Enough for Use in Living Systems: Copper (II) Dihydrogen 6-(p-Nitrobenzyl)-1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetate. Inorg Chem. 1987;26:3458–3463. [Google Scholar]
- 40.Garmestani K, Yao Z, Zhang M, Wong K, Park CW, Pastan I, Carrasquillo JA, Brechbiel MW. Synthesis and Evaluation of a Macrocyclic Bifunctional Chelating Agent for use with Bismuth Radionuclides. Nucl Med Biol. 2001;28:409–418. doi: 10.1016/s0969-8051(00)00203-1. [DOI] [PubMed] [Google Scholar]
- 41.Deal KA, Davis IA, Mirzadeh S, Kennel SJ, Brechbiel MW. J Med Chem. 1999;42:2988–2992. doi: 10.1021/jm990141f. [DOI] [PubMed] [Google Scholar]
- 42.Kennel SJ, Chappell LL, Dadachova K, Brechbiel MW, Lankford TK, Davis LL, Stabin M, Mirzadeh S. Cancer Biother Radiopharm. 2000;15:235–244. doi: 10.1089/108497800414329. [DOI] [PubMed] [Google Scholar]
- 43.McCall MJ, Diril H, Meares CF. Simplified Method for Conjugating Macrocyclic Bifunctional Chelating Agents to Antibodies via 2-Iminothiolane. Bioconjugate Chem. 1990;1:222–226. doi: 10.1021/bc00003a007. [DOI] [PubMed] [Google Scholar]
- 44.Xu H, Regino CAS, Bernardo M, Koyama Y, Kobayashi H, Choyke PL, Brechbiel MW. Towards Improved Syntheses of Dendrimer-Based MR Contrast Agents: New Bifunctional DTPA Ligands and Non-aqueous Conjugation Chemistry. J Med Chem. doi: 10.1021/jm061324m. In Press. [DOI] [PubMed] [Google Scholar]
- 45.Xu H, Hama Y, Regino CAS, Gunn AJ, Bernardo M, Koyama Y, Wong KJ, Kobayashi H, Choyke PL, Brechbiel MW. Preparation and Preliminary Evaluation of a Lectin-Targeted Biotinylated Dendrimer for Dual-Modality Magnetic Resonance and Fluorescence Imaging. Bioconjugate Chem. doi: 10.1021/bc0701085. In Press. [DOI] [PubMed] [Google Scholar]
- 46.Clifford T, Boswell CA, Biddlecombe GB, Lewis JS, Brechbiel MW. Small Animal PET/CT Imaging using 86Y-CHX-A”-Octreotide: Validation of a Novel CHX-A” Derivative Suitable for Peptide Conjugation. J Med Chem. 2006;49:4297–4303. doi: 10.1021/jm060317v. [DOI] [PubMed] [Google Scholar]