Abstract
In many species, the germ cells, precursors of sperm and egg, migrate during embryogenesis. The signals that regulate this migration are thus essential for fertility. In flies, lipid signals have been shown to affect germ cell guidance. In particular, the synthesis of geranylgeranyl pyrophosphate through the 3-hydroxy-3-methyl-glutaryl coenzyme A reductase (Hmgcr) pathway is critical for attracting germ cells to their target tissue. In a genetic analysis of signaling pathways known to affect cell migration of other migratory cells, we failed to find a role for the Hedgehog (Hh) pathway in germ cell migration. However, previous reports had implicated Hh as a germ cell attractant in flies and suggested that Hh signaling is enhanced through the action of the Hmgcr pathway. We therefore repeated several critical experiments and carried out further experiments to test specifically whether Hh is a germ cell attractant in flies. In contrast to previously reported findings and consistent with findings in zebrafish our data do not support the notion that Hh has a direct role in the guidance of migrating germ cells in flies.
Keywords: Primordial germ cells, Drosophila, Hedgehog, HMGCoA reductase, tout velu
Introduction
The germ cells are the progenitors of the gametes and thus are critical for the passing of the genetic material from generation to generation. In many species, including Drosophila, zebrafish and mouse, germ cells are set aside from somatic cells early in development whilst their somatic gonadal counterparts are specified later at distant locations. Therefore the germ cells undergo a stereotyped migration to find and associate with the somatic gonad (reviewed in Kunwar et al., 2006).
Genetic screens in fish and flies have led to identification of factors that are responsible for guiding germ cells and regulating their survival during their migration. In zebrafish, key players guiding germ cell migration are the ligand stromal derived factor-1 (SDF-1) and its receptor CXCR4b (Doitsidou et al., 2002; Knaut et al., 2003). Mutants in CXCR4b, which is expressed in germ cells, or knock down of SDF1, which is expressed along the migratory route of germ cells, results in germ cell mis-migration. Conversely, mis-expression of SDF-1 is sufficient to attract germ cells to ectopic sites (Doitsidou et al., 2002; Knaut et al., 2003).
In Drosophila the germ cells are formed at the posterior pole through the action of maternally derived germ plasm components such as Oskar, Vasa and Tudor (reviewed in (Santos and Lehmann, 2004a). During gastrulation the germ cells adhere to the underlying somatic cells and become internalized, placing the germ cells inside the posterior midgut pocket by embryonic stage 9. At stage 10 the germ cells begin actively migrating, crossing the midgut epithelium and moving into the overlying mesoderm (Kunwar et al., 2008). The somatic gonadal precursors (SGPs) are specified in bilateral mesodermal clusters in parasegments 10-12 (Boyle and DiNardo, 1995). Once in the mesoderm the germ cells associate with the SGP clusters which migrate towards each other at stage 13, and compact at stage 14, causing the germ cells to coalesce forming the bilateral embryonic gonads.
In flies, a key player that regulates germ cell migration is the enzyme 3-hydroxy-3-methyl-glutaryl coenzyme A reductase (Hmgcr). In hmgcr mutant embryos many germ cells fail to reach the SGPs leading to germ cells being ‘lost’ in the soma by the time the SGPs and germ cells coalesce (Van Doren et al., 1998a). Hmgcr catalyses the rate-limiting step of the pathway that synthesizes the isoprenoid lipids, farnesyl pyrophosphate and geranylgeranyl pyrophsphate. hmgcr is highly expressed in the SGPs and ectopic expression of hmgcr, in the CNS for example, leads to attraction of germ cells to the ectopic tissue (Van Doren et al., 1998a). Thus hmgcr expression leads to an attractive cue for germ cells. Mutants in the downstream genes, farnesyl pyrophosphate synthase (fpps), geranylgeranyl pyrophosphate synthase (quemao) and geranylgeranyl transferase type I (betaGGTI) also show defects in germ cell migration (Santos and Lehmann, 2004b). These data suggest that the germ cell attractant in flies is a geranylgeranyl modified protein or that a geranylgeranyl modified protein promotes the secretion or synthesis of a germ cell attractant.
Several studies have suggested that Hedgehog (Hh) is the germ cell guidance factor in flies (Deshpande et al., 2007; Deshpande et al., 2001) and that this is the critical downstream factor of the Hmgcr pathway (Deshpande and Schedl, 2005). In flies, Hh is produced as a precursor protein that is extensively modified prior to secretion (reviewed in Ingham and McMahon, 2001; Jacob and Lum, 2007; Lum and Beachy, 2004) Following an internal cleavage, a palmitate and cholesterol moiety are added to the N- and C-termini respectively of the N-terminal signaling domain (HhN). Hh release requires the activity of the 12-transmembrane protein Dispatched (Disp) with correct extracellular spreading depending on cell surface heparan sulphate proteoglycans (HSPGs). In the receiving cell, Hh binds the receptor Patched (Ptc), which relieves the inhibitory effect of Ptc on the seven-transmembrane effector Smoothened (Smo). In the absence of Smo activity the transcription factor Cubitus interruptus (Ci) is phosphorylated by protein kinase A (PKA) and other kinases and targeted for processing into a shorter cytoplasmic repressor form. Activation of Smo, which involves phosphorylation by PKA, inhibits Ci phosphorylation and processing resulting in the accumulation of full length Ci that can translocate into the nucleus and activate gene expression. The Ser/Thr kinase Fused (Fu), the protein suppressor of fused [Su(fu)] and the kinesin-like molecule Costal2 (Cos2) are also required to regulate the subcellular distribution of Ci.
Hh plays an important role as a secreted, diffusible signal that controls cell fate specification during Drosophila embryogenesis, oogenesis and imaginal disc development (Basler and Struhl, 1994; Heemskerk and DiNardo, 1994; Lane and Kalderon, 1994). It has been proposed that in addition to its role as a morphogen, Hh also acts as a diffusible chemoattractant that guides Drosophila germ cells to the somatic gonad (Deshpande and Schedl, 2005; Deshpande et al., 2007; Deshpande et al., 2001). Several different types of experiments were used to analyze Hh function in germ cell migration. Firstly, the authors observed that hh-lacZ is expressed in the somatic gonadal mesoderm (Deshpande et al., 2001), a tissue that attracts germ cells and aggregates with them to form the embryonic gonad (Boyle and DiNardo, 1995; Broihier et al., 1998). Second, the authors report that ectopic expression of hh leads to germ cell migration defects (Deshpande et al., 2001). Third, the authors report that mutating maternal components of the Hh signaling pathway leads to germ cell migration defects in the progeny (Deshpande et al. 2001). Fourth, the authors report that Hmgcr promotes Hh signaling in particular through release or transmission of the Hh ligand and postulate that the function of Hmgcr in germ cell migration is to promote Hh signaling (Deshpande and Schedl, 2005). Lastly the authors report that mutants of tout velu (ttv), an enzyme required for heparan sulphate proteoglycan biosynthesis, also have germ cell migration defects (Deshpande et al., 2007).
We have conducted a number of large-scale screens for mutants that affect germ cell migration as well as screens that directly tested the role of signaling pathways known to affect migration of other cell types. In both types of experiments we failed to identify a role in germ cell migration for components of the Hh signaling pathway. Since Hh signaling function is required for normal embryonic patterning, we conducted a number of experiments to probe more directly for a role of Hh in germ cell migration. In contrast to the conclusions drawn previously, our experiments do not support the notion that Hh has a direct role in the guidance of migrating germ cells in flies.
Materials and Methods
Drosophila mutant strains
FRT DCOH2 was provided by Daniel Kalderon (Lane and Kalderon, 1994) but we also tested a line provided by Girish Deshpande. FRT smoX43 (also known as FRT smo2) from Marek Mlodzik and we also tested a line provided by Girish Deshpande. FRT ptcIIW was from Haifan Lin (King et al., 2001) (same line as tested by Girish Deshpande). ptc-lacZ (ptcAT96) is a lacZ enhancer trap line courtesy of Gary Struhl (Struhl et al., 1997). hh-lacZ is an enhancer trap line obtained from Girish Deshpande. FRTG13 ttvl(2)00681 was courtesy of Norbert Perrimon (Bellaiche et al., 1998).
Drosophila UAS and Gal4 lines
elav-Gal4 was a gift from Brad Jones, and we also tested a line provided by Girish Deshpande. UAS-hh was provided by Manfred Frasch (Azpiazu et al., 1996) but we also tested a line provided by Girish Deshpande. UAS-hh-N, encoding the N-terminal signaling domain of the protein but which lacks the cholesterol modification site (Porter et al., 1996), UAS-HACi(m1-4) encoding a HA tagged constitutively active version of Ci due to serine to alanine mutations in four PKA phosphorylation sites (Chen et al., 1999), UAS-Ci76 encoding the N-terminal fragment of Ci which acts as a repressor (Aza-Blanc et al., 1997) and pannier-Gal4 (Heitzler et al., 1996) were gifts from Jessica Treisman. UAS-ptcΔloop2 was a gift from Gary Struhl (Briscoe et al., 2001) and UAS-ttv was a gift from Norbert Perrimon (The et al., 1999). The UAS-ptcΔloop2 transgene is inserted on the X chromosome therefore, when crossed to females carrying the nos-Gal4 driver, only half of the progeny would be expected to inherit the transgene and express the mutant protein. The following stocks were also used: hairy-Gal4 (Brand and Perrimon, 1993), twi-Gal4 (Brand and Perrimon, 1993), twi-Gal4, 24B-Gal4 (Greig and Akam, 1995), nos-Gal4 (Broihier et al., 1998; Van Doren et al., 1998b), UAS-hmgcr (Van Doren et al., 1998a) and moodyEP1631 (Kunwar et al., 2003).
Antibody staining
The following antibodies were used: polyclonal anti β-Gal 1/10,000 (Cappel), monoclonal anti-Clift 1/20 (Eya, DSHB), polyclonal anti-Vasa 1/10,000 (Helene Zinszner, Lehmann Lab), monoclonal anti Ptc 1/150 (Apa1, DSHB). Antibody detection was carried out with horseradish peroxidase using a biotinylated secondary antibody (Jackson Immuno research) and the Vectastain Elite ABC Kit. For fluorescent labeling we used CY3 anti-mouse (Jackson lab) and Alexa 488 anti rabbit (Molecular probes) secondary antibodies. hh cDNA was obtained from Jessica Treisman and in-situ hybridization performed according to (Lehmann and Tautz, 1994).
Results and Discussion
Expression of Hh signaling components does not support a role for Hh in germ cell migration
Given previous reports of the role of Hh as a chemoattractant for Drosophila germ cells (Deshpande and Schedl, 2005; Deshpande et al., 2007; Deshpande et al., 2001) we have re-examined the role of Hh in germ cell migration. We have used RNA in situ hybridization to analyze the expression of hh in the Drosophila embryo. As described previously, hh is expressed in the ectoderm and the hindgut (Mohler and Vani, 1992). We also detected weak hh RNA staining in the mesoderm in a segmental pattern during stages 10-12 (Fig. 1A). Analyzing a hh-lacZ enhancer trap line, we found co-expression of LacZ and the gonadal mesoderm marker Clift in the gonadal mesoderm during stage 11 and 12 as reported by Deshpande et al. (2001). However, while Clift was specifically expressed in parasegments 10-12 (Fig. 1B, arrowhead) (Boyle et al., 1997), the segments that give rise to gonadal mesoderm, hh-lacZ was expressed at similar levels in every segment. Thus Hh is expressed in the lateral mesoderm, where it has been shown to play a role in patterning but its expression is not gonad specific (Riechmann et al., 1998).
Figure 1.
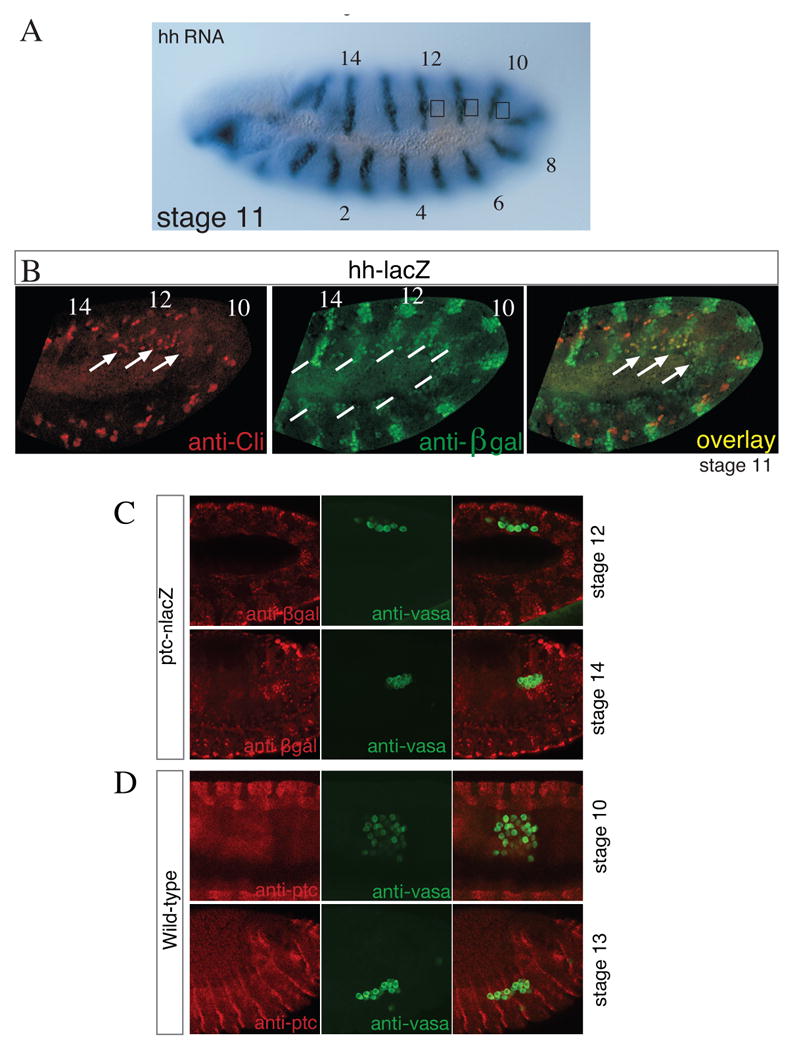
hh is not expressed in the gonadal mesoderm and its receptor ptc is not expressed in germ cells.
(A) Stage 11 embryo hybridized with hh antisense RNA. Note the segmental expression of the RNA in a similar expression pattern in each segment. A square in parasegments 10-12 indicates the position of the gonadal mesoderm.
(B) Stage 11 embryo doubly stained to detect hh expression using a hh-lacZ enhancer line (green) and anti-Clift (red) antibody. In the mesoderm, hh-lacZ is expressed at low levels in every segment, while Clift is expressed only in the gonadal mesoderm in parasegments 10-12 (lines). Clift expression partially overlaps with Hh (arrows).
(C) Stage 12 and 14 embryos carrying a ptc-lacZ enhancer trap stained for LacZ (red) and Vasa to mark the germ cells (green).
(D) Stage 10 and 13 embryos stained for Ptc protein (red) and vasa (green) showing that Ptc is not expressed in germ cells at any stage during germ cell migration. Embryos are oriented anterior to the left and dorsal up.
We next analyzed if the Hh receptor Ptc is expressed in germ cells as would be expected if these cells use Hh as an attractant. Firstly we used a ptc-lacZ enhancer trap line (Struhl et al., 1997) to analyze Ptc expression. Whilst we were able to visualize Ptc expression in stripes of ectodermal cells, as expected, we did not see LacZ expression in germ cells (Fig. 1C). We also used a Ptc antibody and again detected Ptc in segmental stripes but did not detect Ptc in germ cells at any stage (Fig. 1D). In addition, transcriptional profiling of germ cells in late embryos by Shigenobu et al. (2006) also failed to detect ptc expression. Our results confirm previous findings that showed a role for Hh in mesodermal patterning but do not reveal a specific upregulation of Hh in the gonadal mesoderm or expression of signal transduction components of the Hh pathway in germ cells.
Ectopic expression of hh does not lead to germ cell migration defects
Molecules functioning as chemoattractants lead to ectopic cell migration when misexpressed (Dormann and Weijer, 2003). To test whether misexpression of hh causes germ cells to move towards hh expressing cells, we ectopically expressed hh in different tissues: the nervous system (using elav-Gal4) and in the mesoderm (twi-Gal4 or twi-Gal4, 24B-Gal4 double driver). We also tested three different UAS-hh lines, a UAS-hh line from Manfred Frasch, a UAS-hh-N line from Phil Beachy and the UAS-hh line used in experiments by Deshpande et al. 2001. As a positive control for the biological activity of these lines, we tested the UAS lines for their effectiveness in Hh signaling during disc development using a pannier-Gal4 driver. The UAS-hh construct caused notum and thoracic bristle defects as expected, while the UAS-hh-N construct caused lethality with the pannier-Gal4 driver used (data not shown) (Lee and Treisman, personal communication; (Heitzler et al., 1996; Porter et al., 1996). As summarized in Fig. 2, for each experiment we counted the number of germ cells outside of the embryonic gonads per embryo, and compared these results with a negative control (twi-Gal4 driver alone and UAS-hh alone) and with a positive control UAS-hmgcr (Van Doren et al., 1998a). While we occasionally observe an embryo with migration defects, we see similar defects in the experimental and control embryos. Furthermore, we do not see any attraction of germ cells to the particular region/tissue in which hh is mis-expressed.
Figure 2.
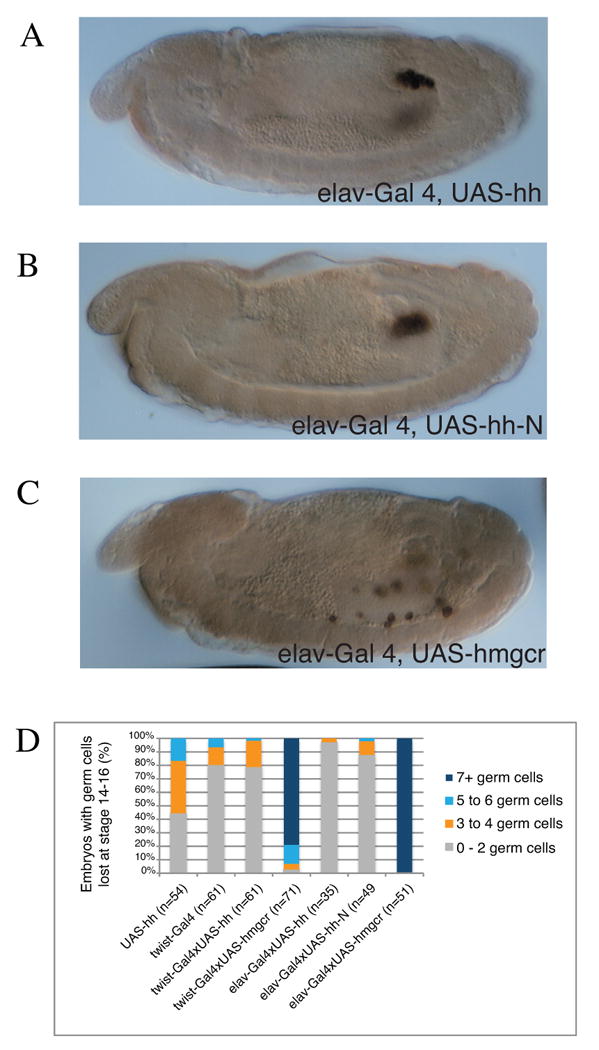
Expression of UAS-hmgcr but not UAS-hh or UAS-hh-N in the nervous system can attract germ cells.
(A-C) Stage 14 embryos stained for Vasa to mark germ cells (brown). The driver elav-GAL4 activates UAS expression in the nervous system. Note that UAS-hmgcr (C) expression in the nervous system leads to attraction of germ cells, while expression of UAS-hh (A) or the more active UAS-hhN (B) has no effect on germ cell migration. Embryos are oriented anterior to left, dorsal up.
(D) Quantification of hh misexpression phenotypes. Percentage of stage 14-16 embryos with indicated number of germ cells outside of the embryonic gonads. Flies homozygous for the mesoderm driver, twist-Gal4, or CNS driver, elav-Gal4, were crossed with flies homozygous for the UAS transgene. Embryos containing the UAS-hh and twist-Gal4 transgenes alone were used as a control to indicate the normal fidelity of germ cell migration.
The failure to observe any effect on germ cell migration after ectopic expression of Hh is in stark contrast to our positive control and previous results with the same type of expression system using UAS-hmgcr (Van Doren et al., 1998a). In these experiments germ cells specifically move to the region of high Hmgcr expression. For example, use of the hairy-Gal4 driver led to ‘striped’ expression of Hmgcr and preferential accumulation of germ cells in the ‘hmgcr expressing stripe’ compared to the intervening region (Van Doren et al., 1998a). Using a nervous system driver (elav-Gal4), we observed germ cells moving to the region of high Hmgcr expression in the CNS (Van Doren et al., 1998a). These experiments provided clear evidence for a role of Hmgcr in germ cell attraction (Van Doren et al., 1998a). For the hairy-Gal4 and elav-Gal4 drivers, Deshpande et al. (2001) noted germ cell migration defects but no specific colocalization of germ cells and ectopic hh expression. The authors reported an association of germ cells with the mesodermal “cells just to the right of the posterior midgut invagination” after pan-mesodermal expression (Fig. 3 of Deshpande et al., 2001). The authors conclude that these results suggest a direct attraction of germ cells to regions with ectopic hh expression. However, hh is normally expressed at high levels at the posterior of the embryo (Fig. 1A,B), thus one may expect germ cells to move there even without misexpression.
Furthermore, pan-mesodermal expression using twi-Gal4 causes broad defects in mesodermal segmentation (Azpiazu et al., 1996) and thus it seems unlikely that this pattern of hh misexpression would evoke attraction of germ cells to a specific site. Indeed, after global hmgcr expression in the mesoderm we observed broad germ cell migration defects, but no attraction to specific cell groups (Van Doren et al., 1998a).
Mutant analysis of the Hh pathway reveals no direct effect on germ cell migration
hh mutant embryos show patterning abnormalities which preclude the direct analysis of Hh as a germ cell attractant in these embryos (Moore et al., 1998b). To determine whether activation of the Hh signaling pathway in germ cells is required for normal germ cell migration, we generated germ line clones for an allele of smo. Since zygotic transcription cannot be detected in germ cells until shortly before germ cell migration is initiated (Van Doren et al., 1998b; Zalokar, 1976), the rationale is that the Hh signaling pathway would have to be provided maternally to the germ cells. Thus, embryos derived from homozygous germ line clones, which receive a wild-type smo+ gene copy from the father, should have sufficient wild-type Smo activity to pattern the soma, but should lack Smo in the germ cells. We recently used this type of experiment to demonstrate a role for DE-cadherin in germ cell migration (Kunwar et al. 2008). For smoX43 germline clones, within the same staining reaction we observed half of the embryos develop into normally segmented larva with properly formed gonads. These embryos had presumably received a smo+ allele from the father (Fig. 3A, smo M-Z+) and showed normal germ cell migration. Half of the embryos, which presumably received the mutant smo- allele from the father, developed abnormally (Fig. 3B, smo M-Z-) and in cuticle preparations showed the “lawn” cuticle phenotype typical for hh or smo mutants (data not shown, van den Heuvel and Ingham, 1996).
Figure 3.
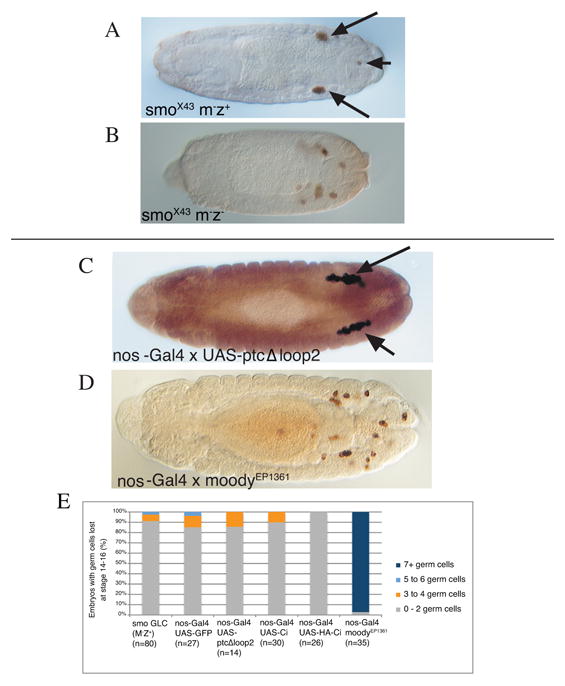
Blocking hh-signaling does not affect germ cell migration.
(A-D) Dorsal views of embryos stained for Vasa to mark germ cells (brown). (A) Stage 15 smoX43 M-Z+ embryo showing wild-type germ cell migration with only few germ cells outside (arrowhead) of the embryonic gonads (arrows). Germ cells are transcriptionally quiescent and would be expected to require maternal smo and therefore mismigrate in smo M- embryos if they used hedgehog as a chemoattractant.
(B) Stage 15 smoX43 M-Z- embryo with germ cells outside of the embryonic gonads due to patterning defects. As a consequence of poor patterning these embryos are visibly shorter than a wild-type embryo. (C,D) Stage 14 embryos laid by females carrying the germ cell driver nos-Gal4 mated to males carrying UAS-ptcΔloop2, a mutant version of the Hh receptor that constitutively blocks Hh signaling (C) and males carrying EP1631, a UAS containing P-element upstream of the GPCR moody (D).
(E) Quantification of phenotypes when Hh signaling is disrupted in germ cells. Percentage of stage 14-16 embryos with indicated number of germ cells outside of the embryonic gonads as assayed by staining germ cells with an anti-Vasa antibody. Females homozygous for the germ cell driver, nos-Gal4 were crossed with males homozygous for the UAS transgene. GFP was used as a negative control, Ci is the transcription factor downstream of Hh, EP1631 results in over-expression of the GPCR moody.
Since germ cell migration was wild type in our smo M-Z+ embryos, we do not support the idea that Hh signal transduction is required in germ cells for normal germ cell migration. Whilst we cannot rule out the possibility that smo is zygotically expressed in germ cells, smo was not detected in germ cells in late embryos by Shigenobu et al. (2006). These data are in contrast to findings in Deshpande et al. 2001 who made germline clones with the same smo allele (referred to as smo2 in Deshpande et al. 2001) and reported that germ cells fail to associate with SGPs and scatter in the posterior in smo M-Z+ embryos.
We were unable to analyze the progeny of germ line clones mutant for other components of the Hh signal transduction pathway, such as the receptor ptc (using the null allele ptcIIW) and the effector DCO which encodes the catalytic subunit of PKA (using the null allele DCOH2), since no eggs were produced over many days of collection. These finding are supported by a previous study where King et al. (2001) showed that germ line clones for ptcIIW fail to develop past stage 9 of oogenesis. Thus females, which lack ptc activity in the germ line, produce no embryos. Lane and Kalderon (1994) showed that homozygous mutant PKA germ line clones (using the null alleles DCOB3 and DCOH2) are defective in the microtubule organization of the oocyte and fail to complete oogenesis. Curiously using the same ptc and PKA alleles (ptcIIW and DCOH2 respectively), Deshpande et al. were able to demonstrate germ cell migration defects in the progeny of mutant germ line clones. Furthermore, they report an increase in the number of germ cells in embryos from PKA mutant germ line clones. These results seem surprising given findings by Lane and Kalderon (1994), who showed that in homozygous mutant PKA germ line clones oskar RNA, the germ cell determinant, is localized to the middle of the oocyte and eggs are not produced. Thus, if PKA mutant germ line clones could produce progeny, these embryos would be expected to have no or reduced Oskar protein levels and thus would be expected to be defective for germ cell formation (Lane and Kalderon, 1994).
Expression of Hh signaling components in germ cells does not lead to migration defects
To address the role of the Hh signaling pathway in germ cells more directly, we used a Gal4 driver to specifically drive components of the signaling pathway in germ cells (Van Doren et al., 1998b). As described previously, the nos-Gal4 driver contains the transcriptional regulator region and RNA localization and translation elements (5′ and 3′UTR) of the nanos gene while the nanos coding region was replaced by Gal4-VP16 (Van Doren et al., 1998b). Consequently, Gal4 is maternally synthesized, localized to and translated at the posterior pole of the embryo. Gal4 activity persists during embryogenesis in the germ cells and we (Kunwar et al., 2003; Renault et al., 2004; Starz-Gaiano et al., 2001) and others (Hanyu-Nakamura et al., 2004; Hanyu-Nakamura et al., 2008) have used this construct successfully to drive gene expression in germ cells. We used the following UAS lines in this experiment: 1) UAS-ptcΔloop2 which constitutively blocks Hh signaling (Briscoe et al., 2001), 2) UAS-Ci76, a dominant negative truncated form of Ci (Porter et al., 1996), 3) UAS-HACi(m1-4) a dominant active version of Ci (Chen et al., 1999) and 4) moodyEP1631, a UAS containing P-element inserted upstream of the moody GPCR, which leads to germ cell migration defects when ectopically expressed in germ cells (Kunwar et al., 2003).
As illustrated in Fig. 3C-E, expression of Hh signaling components in germ cells did not affect their migration whilst expression of the moody GPCR caused significant migration defects (Kunwar et al., 2003). Thus, while expression of particular genes in germ cells can affect germ cell migration, none of the components of the Hh signaling pathway that we tested had an effect on germ cell migration or survival. This is in contrast to findings in (Deshpande et al., 2007) who found that germ cell expression of UAS-ptcΔloop2 did lead to germ cell migration defects in a proportion of embryos.
Ectopic expression of hmgcr in hh negative cells results in germ cell migration defects
It has been proposed that Hmgcr functions in germ cell migration due to its role in transmission or release of the germ cell attractant Hh (Deshpande and Schedl, 2005). Hmgcr is essential for the production of farnesyl pyrophosphate and geranylgeranyl pyrophsphate and these lipids are used to prenylate several proteins including the Rab subfamily of small GTPases, which are known regulators of secretion. Potentially the secretion of many molecules could be up-regulated upon Hmgcr over-expression. To test whether Hmgcr acts through Hh we tested whether Hmgcr over-expression in hh non-expressing cells can disrupt germ cell migration. If Hh were the germ cell attractant we would not expect any ectopic germ cell migration when Hmgcr is expressed in Hh non-expressing cells. We used hh-Gal4 for expression in Hh expressing cells and ptc-Gal4 for expression in Hh non-expressing/receiving cells (Deshpande et al. 2005). We find that Hmgcr mis-expression in both Hh expressing and non-expressing cells causes germ cells to migrate ectopically at equivalent levels (Fig. 4). As a positive control we used hairy-Gal4 and as a negative control we used expression of lacZ with these drivers. These data strongly argue against a role for Hh as a mediator of Hmgcr-induced germ cell attraction.
Figure 4.
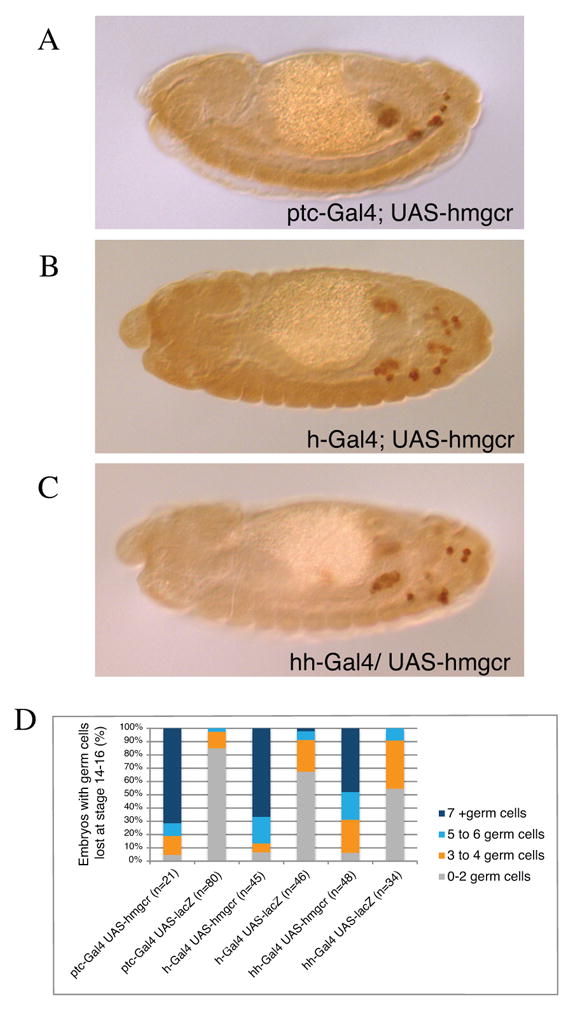
Expression of hmgcr in cells not expressing Hh leads to germ cell migration defects.
(A-C) Lateral views of stage 14 embryos stained for Vasa to mark germ cells (brown) mis-expressing hmgcr in Hh receving cells with the ptc-Gal4 (A) in the epidermis with hairy-Gal4 (B) and in Hh expressing cells with the hh-Gal4 (C). In each case ectopic hmgcr expression leads to germ cells outside of the gonads.
(D) Quantification of hmgcr misexpression phenotypes. Percentage of stage 14-16 embryos with indicated number of germ cells outside of the embryonic gonads as assayed by staining germ cells with an anti-Vasa antibody. lacZ was used as a negative control.
ttv does not affect germ cell migration
Heparan sulphate proteoglycans (HSPGs) are required for the distribution of extracellular signaling molecules such as Hh. ttv encodes a subunit of heparan sulphate polymerase and regulates Hh distribution through its role in HSPG biosynthesis (The et al., 1999). Deshpande et al. (2007) reports finding ttv in a zygotic second chromosome deficiency screen looking for deficiencies that result in germ cell migration defects. The authors report that the relatively mild defects observed in their zygotic deficiency could be due to high maternal contribution, but M-Z-ttv embryos display segment polarity defects precluding an assessment of germ cell migration. Therefore they examined the phenotype of an unnamed allele of ttv in M-Z+ embryos and claim that 27% of embryos have 7 or more germ cells lost.
We used the ttv null allele ttvl(2)00681 and verified that it is correct by performing cuticle preparations of ttv M-Z- embryos, which displayed segment polarity defects identical to those reported in (The et al., 1999) (data not shown). We then examined germ cell migration in embryos that either lacked maternal function of ttv, zygotic function, or both maternal and zygotic function. We found that M-Z+ embryos from ttvl(2)00681 germ line clones are perfectly patterned and germ cell migration is completely wild type (Fig. 5A). Only 1 embryo (of 27 examined) had 5 or more germ cells outside the gonad. We then asked whether ttv M+Z- embryos have a germ cell migration phenotype and found that zygotic embryos, which again are normally patterned, have no germ cell migration phenotype (Fig. 5B). We then assessed germ cell migration in ttv M-Z- embryos. Such embryos had segment polarity defects and germ cell migration was highly perturbed most likely as a consequence of patterning defects. However, we noted that in many embryos germ cells were found in bisymmetrical clusters at the expected positions of the embryonic gonads (Fig. 5C). Thus in spite of the poor patterning of these embryos many germ cells still find and associate with the SGPs. We have also performed a genetic screen on the right arm of the second chromosome (described in (Barbosa et al., 2007), where ttv is located, searching for mutants affecting germ cell migration. Although we obtained a number of mutants that result in clear germ cell migration defects including fpps (A.D.R., H.S., P.S.K. and R.L., unpublished) and shg (Kunwar et al., 2008) we did not recover any alleles of ttv. Taken together our results suggest that ttv loss of function has no effect on germ cell migration.
Figure 5.
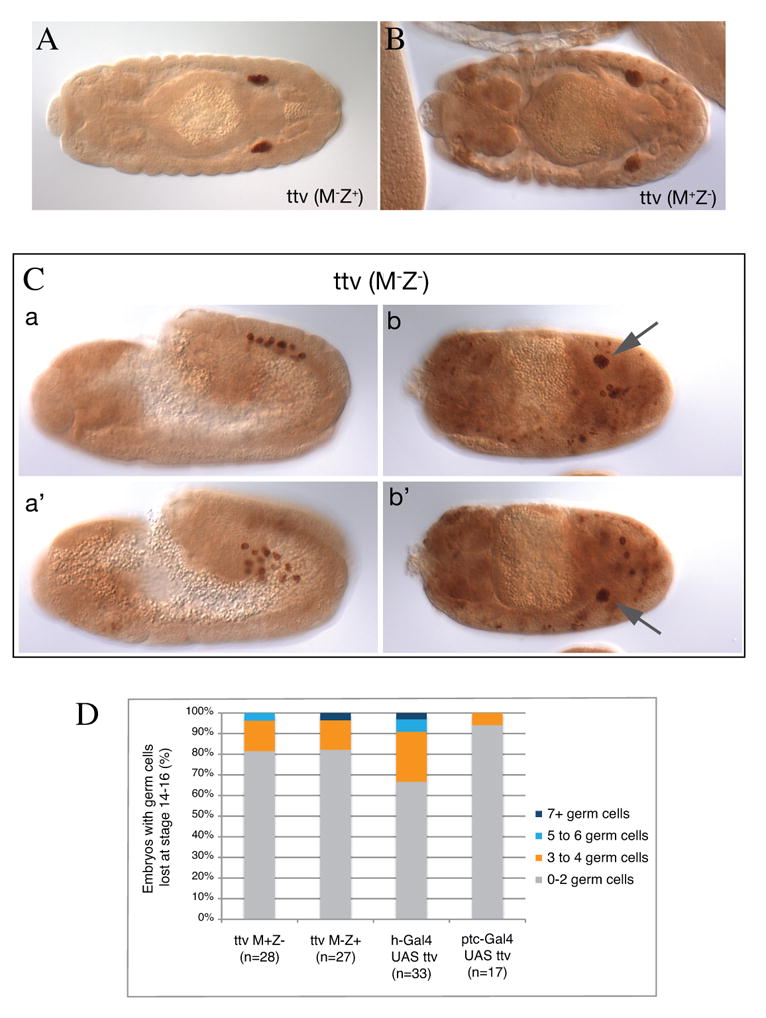
ttv does not affect germ cell migration.
(A-B) Dorsal views of stage 14 embryos stained for Vasa to mark germ cells (brown). (A) Embryo laid by ttv germline clone females mated to wild-type males (ttv M-Z+ embryo) showing wild type germ cell migration. (B) a ttv zygotic mutant (ttv M+Z-) embryo showing wild type germ cell migration. (C) Embryos laid by ttv germline clone females mated to mutant males (ttv M-Z- embryos) showing that in spite of patterning defects germ cells are able to separate into bilateral groups (a, a', stage 11 embryo lateral view represented in 2 focal planes) and often cluster bisymmetrically at the presumed sites of the embryonic gonads (arrows) (b, b″, stage 14 embryo dorsal view represented in 2 focal planes). Note that the non-germ cell staining in B and C b and b' comes from the lacZ containing P-element of the ttv l(2)00681 allele and the anti-lacZ antibody used to distinguish between balancer and mutant embryos.
(D) Quantification of ttv loss and gain of function phenotypes. M-Z- mutant embryos were not quantified due to patterning defects. Positive and negative controls for ptc and hairy (h) Gal4 drivers are included in Figure 4D.
We also examined the effects of ttv over-expression on germ cell migration. We used hairy-Gal4 and ptc-Gal4 to over-express ttv in the epidermis and in Hh-receiving cells respectively. As positive and negative controls we used UAS-hmgcr and UAS-lacZ respectively. We find that ttv over-expression with these drivers does not lead to significant differences in the fidelity of germ cell migration compared to the negative control (Fig. 4D and Fig. 5D). In contrast, and as described above, Hmgcr over-expression with these drivers caused a significant number of germ cells to remain outside of the gonads (Fig. 4D). Therefore we find no evidence that over-expression of ttv leads to defects in germ cell migration. These data are again in contrast to findings in (Deshpande et al., 2007) who found that ttv over-expression with hairy-Gal4 led to germ cell migration defects in the majority of embryos.
In summary, we have used a mixture of mutant and over-expression analysis of components of the Hh pathway, including ptc, smo, ci and ttv, to probe the role of Hh in germ cell migration and have found no evidence to support a role for any of these molecules in germ cell migration. In addition we find that the ability of hmgcr to attract germ cells does not act through Hh.
Mich et al. (2008), in the accompanying paper, have examined the role of Hh in germ cell migration in Zebrafish. The authors made Smo M-Z- embryos using the germline transplantation technique using two Smo alleles, smuhi1640 and smub577, that lack detectable Smo message and are probably null (Chen et al., 2001). In both cases, although the embryos displayed phenotypes consistent with disruption of Hh signaling, germ cell migration was wild-type. The authors conclude that Smo and Smo-dependent processes such as Hh signaling are not required for germ cell migration in zebrafish (Mich et al., 2008).
Sonic hedgehog has been demonstrated to be a chemoattractant for commissural axons in mouse (Charron et al., 2003) acting through a receptor other than Ptc, namely Boc (Okada et al., 2006). Whilst we cannot rule out the possibility that germ cells utilize a completely different and as yet undiscovered pathway for Hh signaling as compared to somatic cells, we have strong evidence indicating that Hh is not a direct attractant for germ cell migration in flies. Hh does however play a very important role in the cell fate specification of the mesoderm including the gonadal mesoderm (Azpiazu et al., 1996; Moore et al., 1998a; Moore et al., 1998b; Riechmann et al., 1998).
Supplementary Material
Acknowledgments
We thank many colleagues who have contributed strains and thought to this project, in particular we would like to thank Jessica Treisman, Gary Struhl, Daniel Kalderon and Haifan Lin for providing us with tester lines. We would like to thank Meng-Chi Lin for generation of smo germ line clones. This work was supported by the NIH. R. L. is an HHMI investigator and a member of the Kimmel Center for Biology and Medicine. A.D.R. was a Charles H. Revson Senior Fellow in Biomedical Science.
Footnotes
Note added in proof: As this work was being revised Desphande et al (2009) published a study in which they report that Gγ1 is required for directed germ cell migration in Drosophila. In a parallel study we have addressed this question using the same fly stock as Desphande et al. (2009) and have found no evidence of a somatic requirement for Gγ1 in germ cell migration (Ricardo and Lehmann, Science in press).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aza-Blanc P, Ramirez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–53. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- Azpiazu N, Lawrence PA, Vincent JP, Frasch M. Segmentation and specification of the Drosophila mesoderm. Genes Dev. 1996;10:3183–94. doi: 10.1101/gad.10.24.3183. [DOI] [PubMed] [Google Scholar]
- Barbosa V, Kimm N, Lehmann R. A maternal screen for genes regulating Drosophila oocyte polarity uncovers new steps in meiotic progression. Genetics. 2007;176:1967–77. doi: 10.1534/genetics.106.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–14. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Bellaiche Y, The I, Perrimon N. Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature. 1998;394:85–8. doi: 10.1038/27932. [DOI] [PubMed] [Google Scholar]
- Boyle M, Bonini N, DiNardo S. Expression and function of clift in the development of somatic gonadal precursors within the Drosophila mesoderm. Development. 1997;124:971–82. doi: 10.1242/dev.124.5.971. [DOI] [PubMed] [Google Scholar]
- Boyle M, DiNardo S. Specification, migration and assembly of the somatic cells of the Drosophila gonad. Development. 1995;121:1815–25. doi: 10.1242/dev.121.6.1815. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Chen Y, Jessell TM, Struhl G. A hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Mol Cell. 2001;7:1279–91. doi: 10.1016/s1097-2765(01)00271-4. [DOI] [PubMed] [Google Scholar]
- Broihier HT, Moore LA, Van Doren M, Newman S, Lehmann R. zfh-1 is required for germ cell migration and gonadal mesoderm development in Drosophila. Development. 1998;125:655–66. doi: 10.1242/dev.125.4.655. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Chen W, Burgess S, Hopkins N. Analysis of the zebrafish smoothened mutant reveals conserved and divergent functions of hedgehog activity. Development. 2001;128:2385–96. doi: 10.1242/dev.128.12.2385. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cardinaux JR, Goodman RH, Smolik SM. Mutants of cubitus interruptus that are independent of PKA regulation are independent of hedgehog signaling. Development. 1999;126:3607–16. doi: 10.1242/dev.126.16.3607. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Schedl P. HMGCoA reductase potentiates hedgehog signaling in Drosophila melanogaster. Dev Cell. 2005;9:629–38. doi: 10.1016/j.devcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Sethi N, Schedl P. toutvelu, a regulator of heparan sulfate proteoglycan biosynthesis, controls guidance cues for germ-cell migration. Genetics. 2007;176:905–12. doi: 10.1534/genetics.107.071415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Swanhart L, Chiang P, Schedl P. Hedgehog signaling in germ cell migration. Cell. 2001;106:759–69. doi: 10.1016/s0092-8674(01)00488-3. [DOI] [PubMed] [Google Scholar]
- Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, Esguerra CV, Leung T, Raz E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–59. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- Dormann D, Weijer CJ. Chemotactic cell movement during development. Curr Opin Genet Dev. 2003;13:358–64. doi: 10.1016/s0959-437x(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Greig S, Akam M. The role of homeotic genes in the specification of the Drosophila gonad. Curr Biol. 1995;5:1057–62. doi: 10.1016/s0960-9822(95)00210-7. [DOI] [PubMed] [Google Scholar]
- Hanyu-Nakamura K, Kobayashi S, Nakamura A. Germ cell-autonomous Wunen2 is required for germline development in Drosophila embryos. Development. 2004;131:4545–53. doi: 10.1242/dev.01321. [DOI] [PubMed] [Google Scholar]
- Hanyu-Nakamura K, Sonobe-Nojima H, Tanigawa A, Lasko P, Nakamura A. Drosophila Pgc protein inhibits P-TEFb recruitment to chromatin in primordial germ cells. Nature. 2008;451:730–3. doi: 10.1038/nature06498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk J, DiNardo S. Drosophila hedgehog acts as a morphogen in cellular patterning. Cell. 1994;76:449–60. doi: 10.1016/0092-8674(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Heitzler P, Haenlin M, Ramain P, Calleja M, Simpson P. A genetic analysis of pannier, a gene necessary for viability of dorsal tissues and bristle positioning in Drosophila. Genetics. 1996;143:1271–86. doi: 10.1093/genetics/143.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jacob L, Lum L. Deconstructing the hedgehog pathway in development and disease. Science. 2007;318:66–8. doi: 10.1126/science.1147314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King FJ, Szakmary A, Cox DN, Lin H. Yb modulates the divisions of both germline and somatic stem cells through piwi- and hh-mediated mechanisms in the Drosophila ovary. Mol Cell. 2001;7:497–508. doi: 10.1016/s1097-2765(01)00197-6. [DOI] [PubMed] [Google Scholar]
- Knaut H, Werz C, Geisler R, Nusslein-Volhard C. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature. 2003;421:279–82. doi: 10.1038/nature01338. [DOI] [PubMed] [Google Scholar]
- Kunwar PS, Sano H, Renault AD, Barbosa V, Fuse N, Lehmann R. Tre1 GPCR initiates germ cell transepithelial migration by regulating Drosophila E-cadherin. J Cell Biol. 2008 doi: 10.1083/jcb.200807049. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar PS, Siekhaus DE, Lehmann R. In vivo migration: a germ cell perspective. Annu Rev Cell Dev Biol. 2006;22:237–65. doi: 10.1146/annurev.cellbio.22.010305.103337. [DOI] [PubMed] [Google Scholar]
- Kunwar PS, Starz-Gaiano M, Bainton RJ, Heberlein U, Lehmann R. Tre1, a G Protein-Coupled Receptor, Directs Transepithelial Migration of Drosophila Germ Cells. PLoS Biol. 2003;1:372–384. doi: 10.1371/journal.pbio.0000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane ME, Kalderon D. RNA localization along the anteroposterior axis of the Drosophila oocyte requires PKA-mediated signal transduction to direct normal microtubule organization. Genes Dev. 1994;8:2986–95. doi: 10.1101/gad.8.24.2986. [DOI] [PubMed] [Google Scholar]
- Lehmann R, Tautz D. In situ hybridization to RNA. Methods Cell Biol. 1994;44:575–98. doi: 10.1016/s0091-679x(08)60933-4. [DOI] [PubMed] [Google Scholar]
- Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–9. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- Mich JK, Blaser H, Thomas N, Yelon D, Raz E, Chen JK. Germ cell migration in zebrafish is cyclopamine-sensitive but Smoothened-independent. Dev Biol. 2008 doi: 10.1016/j.ydbio.2009.01.036. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler J, Vani K. Molecular organization and embryonic expression of the hedgehog gene involved in cell-cell communication in segmental patterning of Drosophila. Development. 1992;115:957–71. doi: 10.1242/dev.115.4.957. [DOI] [PubMed] [Google Scholar]
- Moore LA, Broihier HT, Van Doren M, Lehmann R. Gonadal mesoderm and fat body initially follow a common developmental path in Drosophila. Development. 1998a;125:837–44. doi: 10.1242/dev.125.5.837. [DOI] [PubMed] [Google Scholar]
- Moore LA, Broihier HT, Van Doren M, Lunsford LB, Lehmann R. Identification of genes controlling germ cell migration and embryonic gonad formation in Drosophila. Development. 1998b;125:667–78. doi: 10.1242/dev.125.4.667. [DOI] [PubMed] [Google Scholar]
- Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444:369–73. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–9. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- Renault AD, Sigal YJ, Morris AJ, Lehmann R. Soma-germ line competition for lipid phosphate uptake regulates germ cell migration and survival. Science. 2004;305:1963–6. doi: 10.1126/science.1102421. [DOI] [PubMed] [Google Scholar]
- Riechmann V, Rehorn KP, Reuter R, Leptin M. The genetic control of the distinction between fat body and gonadal mesoderm in Drosophila. Development. 1998;125:713–23. doi: 10.1242/dev.125.4.713. [DOI] [PubMed] [Google Scholar]
- Santos AC, Lehmann R. Germ Cell Specification and Migration in Drosophila and beyond. Curr Biol. 2004a;14:R578–R589. doi: 10.1016/j.cub.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Santos AC, Lehmann R. Isoprenoids control germ cell migration downstream of HMGCoA reductase. Dev Cell. 2004b;6:283–93. doi: 10.1016/s1534-5807(04)00023-1. [DOI] [PubMed] [Google Scholar]
- Shigenobu S, Kitadate Y, Noda C, Kobayashi S. Molecular characterization of embryonic gonads by gene expression profiling in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2006;103:13728–33. doi: 10.1073/pnas.0603767103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starz-Gaiano M, Cho NK, Forbes A, Lehmann R. Spatially restricted activity of a Drosophila lipid phosphatase guides migrating germ cells. Development. 2001;128:983–91. doi: 10.1242/dev.128.6.983. [DOI] [PubMed] [Google Scholar]
- Struhl G, Barbash DA, Lawrence PA. Hedgehog organises the pattern and polarity of epidermal cells in the Drosophila abdomen. Development. 1997;124:2143–54. doi: 10.1242/dev.124.11.2143. [DOI] [PubMed] [Google Scholar]
- The I, Bellaiche Y, Perrimon N. Hedgehog movement is regulated through tout velu-dependent synthesis of a heparan sulfate proteoglycan. Mol Cell. 1999;4:633–9. doi: 10.1016/s1097-2765(00)80214-2. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Ingham PW. smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature. 1996;382:547–51. doi: 10.1038/382547a0. [DOI] [PubMed] [Google Scholar]
- Van Doren M, Broihier HT, Moore LA, Lehmann R. HMG-CoA reductase guides migrating primordial germ cells. Nature. 1998a;396:466–9. doi: 10.1038/24871. [DOI] [PubMed] [Google Scholar]
- Van Doren M, Williamson AL, Lehmann R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol. 1998b;8:243–6. doi: 10.1016/s0960-9822(98)70091-0. [DOI] [PubMed] [Google Scholar]
- Zalokar M. Autoradiographic study of protein and RNA formation during early development of Drosophila eggs. Dev Biol. 1976;49:425–37. doi: 10.1016/0012-1606(76)90185-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


