Abstract
Viruses are obligate, intracellular pathogens that must manipulate and exploit host molecular mechanisms to prosper in the hostile cellular environment. Here we review the strategies used by viruses to evade the immunity controlled by 21- to 26-nt small RNAs. Viral suppressors of RNA silencing (VSRs) are encoded by genetically diverse viruses infecting plants, invertebrates, and vertebrates. VSRs target key steps in the small RNA pathways by inhibiting small RNA production,sequestering small RNAs,orpreventing short- and long-distance spread of RNA silencing. However, although VSRs are required for infection, explicit data demonstrating a role of silencing suppression in virus infection are available only for a few VSRs. A subset of VSRs bind double-stranded RNA, but a distinct protein fold is revealed for each of the four VSRs examined. We propose that VSR families are evolved independently as a viral adaptation to immunity. Unresolved issues on the role of RNA silencing in virus-host interactions are highlighted.
Keywords: RNAi, antiviral silencing, viral suppressors, viral adaptation, virus-host interactions
INTRODUCTION
RNA silencing was first discovered in transgenic plants as cosuppression between an introduced transgene and its homologous endogenous gene (85, 92). Similar homology-dependent gene-silencing phenomena have been reported in a wide range of eukaryotic organisms, including fungi, worms, insects, and vertebrates. Among the terms used in various organisms such as posttranscriptional gene silencing (PTGS), quelling, and RNA interference (RNAi), RNA silencing has emerged as the generic term referring to all gene-silencing mechanisms regulated by small RNAs 21 to 30 nucleotides (nt) in length. These small RNAs include short interfering RNAs (siRNAs) and microRNAs (miRNAs) and provide specificity to guide various activities of RNA silencing, including RNA cleavage, translational repression, and methylation of DNA or chromatin.
The first biological function established for RNA silencing was as an antiviral mechanism in plants (3, 13, 46, 56, 73, 104). Diverse roles have since been demonstrated for RNA silencing in many organisms. These include genome defense against mobile DNA elements, establishing heterochromatin, developmentally regulated DNA elimination in Tetrahymena, control of plant and animal development, and downregulation of gene expression by specific cleavage and translational repression of target mRNAs that contain complementary sites to miRNAs or siRNAs. For example, hundreds of miRNAs have been identified in humans and each miRNA has the potential to target up to 200 genes. This has led to the suggestion that well over one third of human genes are under the control of miRNAs (9).
In 1998 researchers demonstrated that essential virulence factors of plant viruses are suppressors of RNA silencing, providing the strongest experimental support for a role of RNA silencing in host immunity against viruses (3, 13, 56, 67). Numerous viral suppressors of RNA silencing (VSRs) encoded by genetically diverse viruses infecting plants, insects, fish, and humans have been reported since the discovery of the first potyviral and cucumoviral VSRs in 1998. In this review, we summarize current knowledge about VSRs. We first briefly discuss the key steps and effector mechanisms of the small RNA pathways and the experimental evidence establishing induction of antiviral silencing in plants and animals. We focus on general properties, diverse mechanisms, and unresolved issues of VSRs, as well as assays used for VSR identification in plants and animals. For other aspects of antiviral silencing, readers are referred to several recent reviews (66, 132, 133).
KEY STEPS IN RNA SILENCING
Production of Small RNAs
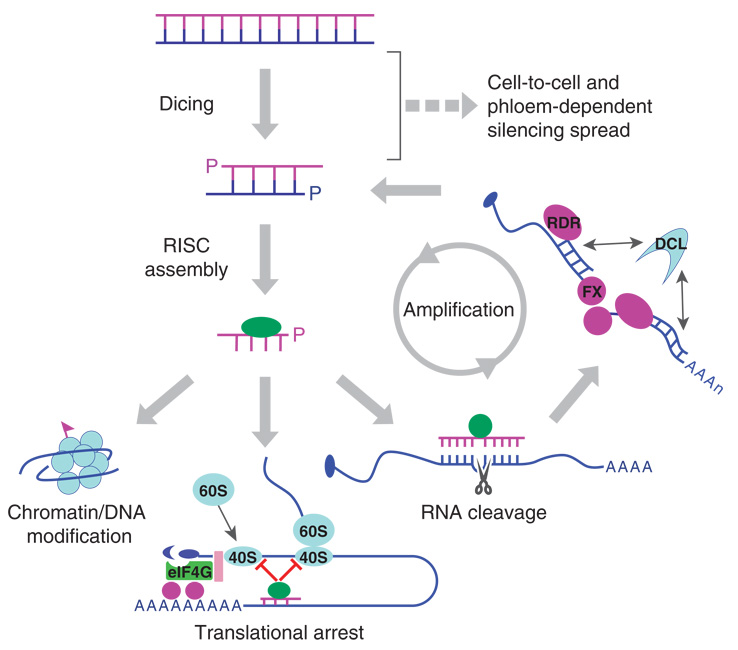
RNA silencing begins with recognition of an RNA trigger by a type III endonuclease Dicer (10) (Figure 1), leading to the production of small duplex RNAs 21 to 30 nt in length with the characteristic 2-nt overhang at the 3′ ends. Subsequently, the small RNAs are loaded into an effector complex to guide specific RNA silencing (124).
Figure 1.
Key steps in RNA silencing. RNA silencing starts with dicing of dsRNA or pre-miRNAs. The resulting small RNAs are incorporated into distinct RISC complexes to direct chromatin modification, translational arrest, and mRNA cleavage. In organisms with RDR, secondary siRNA is synthesized from cleavage products. FX represents host factor implicated in stabilizing cleavage products similar to SGS3 (149). Dashed arrow represents cell-to-cell movement of 21 siRNAs and the potential long-distance movement of 24-nt siRNAs.
Two types of RNA molecules have the potential to serve as a trigger of RNA silencing. Double-stranded RNAs (dsRNAs) are precursors of siRNAs, whereas single-stranded RNAs with step-loop structures serve as precursors of miRNAs (pre-miRNAs). Both siRNAs and miRNAs are products of the single Dicer protein encoded in worms and humans. In Drosophila, however, pre-miRNAs and dsRNAs are processed by two distinct Dicers, Dicer-1 and Dicer-2, respectively. Arabidopsis encodes four Dicer proteins designated Dicer-like (DCL) 1–4, all of which recognize dsRNA triggers, although the primary role of DCL1 is to process pre-miRNAs. Two classes of siRNAs of sizes 21 to 22 and 24 nt, respectively, are produced in Arabidopsis plants, and it is likely that the 21-nt, 22-nt, and 24-nt siRNAs are produced by DCL4, DCL2, and DCL3, respectively (34, 43, 45, 100, 141). Tetrahymena produces the longest siRNAs, which are 27 to 30 nt in length. Arabidopsis miRNAs and siRNAs are methylated at the 3′ end, which is essential for protection of the small RNAs from degradation mediated by 3′-uridylation activity (68, 150).
Assembly of Effector Complexes
Three types of effector complexes of RNA silencing have been described (Figure 1). These include RNA-induced transcriptional silencing complex (RITS), which directs methylation of chromatin, and siRNA- and miRNA-dependent RNA-induced-silencing complex (siRISC and miRISC), which guide cleavages and translational arrest, respectively, of target mRNAs. All of these complexes contain one (guide) strand of the duplex small RNAs as the specificity determinants and a member of the Argonaute protein (AGO) family. The PIWI domain of AGOs has structural similarities to RNaseH, and the ability to cleave the target RNA base-paired with the guide strand siRNA, called slicing, has been demonstrated for Drosophila AGO1 and AGO2, human AGO2, and Arabidopsis AGO1 (6, 76, 87, 115). It is likely that assembly of these effector complexes may follow a pathway similar to that described for siRISC in Drosophila. siRISC assembly begins with binding of siRNA duplexes by the heterodimer of Dicer-2 and R2D2, a dsRNA binding protein with tandem dsRNA binding motifs, in the RISC loading complex (RLC) (123). Thus, Dicer-2 is also required in RISC assembly downstream of siRNA production. Next, RLC delivers the siRNA duplex into AGO2, which subsequently cleaves the passenger strand siRNA, triggering its dissociation from the complex and activation of RISC that contains only the siRNA guide strand (84, 103).
Amplification and Transitive Silencing
Fungi, plants, and worms encode eukaryotic RNA-dependent RNA polymerases (RDR) that generate new sources of dsRNA for dicing, leading to further silencing amplification (Figure 1).In both plants and Caenorhabditis elegans, RDR amplification results in the spread of silencing along the target gene beyond the region initially targeted for silencing, referred to as transitive RNAi (111, 126). RDR genes essential for RNA silencing in the germline (ego-1) and somatic tissues (rrf-1) in C. elegans have been identified (111, 113). Arabidopsis encodes six RDRs designated RDR1–6, which, together with individual DCLs, control specific small RNA biogenesis pathways. For example, RDR2 is required for the production of 24-nt siRNAs by DCL3, which are involved in guiding chromatin modification (18, 48, 55, 93, 143, 152). In contrast, a genetic requirement of RDR6 for the production of distinct classes of siRNAs by DCL1, DCL2, or DCL4 has been demonstrated (12, 34, 43, 149).
Noncell Autonomous Silencing
In both plants and worms, the effects of RNA silencing can spread beyond the sites of silencing initiation via a putative specific silencing signal (133) (Figure 1). Systemic silencing in worms requires SID-1,a transmembrane protein that efficiently transports dsRNA longer than 100 nt (40, 140). Two distinct steps have been observed in the spread of RNA silencing in plants. Current data suggest a role for 21-nt siRNAs in the short-distance spread and 24-nt siRNAs in the phloem-dependent long-distance transport (45, 49, 94). Although RDR amplification is not required for the cell-to-cell spread, extensive short-distance spread beyond 10 to 15 cells in plants requires the RDR6/DCL4 pathway and its product, the 21-nt siRNAs (34, 49, 110). In contrast, a predicted role for the 24-nt siRNAs (or their longer precursor dsRNA) produced by the RDR2/DCL3/AGO4 pathway in the longdistance silencing spread remains to be rigorously examined. However, both classes of siRNAs are found in the phloem, indicating their potential to mediate silencing spread in plants (148). Similarly, it is also not clear if DNA methylation associated with the maintenance or persistent silencing of transgenes plays a specific role in noncell autonomous silencing.
VIRUS INFECTION INDUCES RNA SILENCING
RNA Silencing is an Antiviral Mechanism in Plants
Early evidence that indicated an antiviral role for RNA silencing came from molecular analyses of transgenic plants following infection with a potyvirus from which the transgene was derived (73). The infected plants displayed symptoms initially but later recovered and became resistant to subsequent infection with the homologous virus. Recovery and establishment of the virus-resistant state were correlated with a posttranscriptional breakdown of the transgene mRNA. It was thus concluded that virus infection induces PTGS of the homologous transgene,which then targets the viral RNAs for silencing to confer virus resistance (73). A number of important studies subsequently published support this model. For example, plants carrying a silencing GUS transgene were resistant to infection of the GUS-expressing recombinant viruses but not to the wild-type (wt) viruses, indicating that viruses are targets of PTGS (39). That viruses are inducers of PTGS is not specific to transgenic plants, because virus-induced gene silencing occurs in wt plants when the infecting virus with either an RNA or DNA genome is engineered to express an endogenous gene (59, 62, 108). Furthermore, viral RNAs are targeted for silencing (26, 104), and virus-specific siRNAs of both positive and negative polarities accumulate in wt plants infected with wt viruses (46, 88, 148), demonstrating that viruses are both inducers and targets of RNA silencing in plants.
The idea that RNA silencing is an antiviral mechanism in plants is further supported by two additional lines of evidence. First, genetic studies indicate that the RNA-silencing mechanism protects host plants against virus infection. For example, Arabidopsis mutants carrying loss-of-function mutations in essential silencing pathway genes such as RDR6, AGO1, and DCL2 show enhanced disease susceptibility (EDS) to virus infection (28, 89, 90, 143). EDS was also observed in transgenic tobacco plants with reduced expression of either the RDR1 or RDR6 homolog (110, 142). Second, RNA silencing as an antiviral mechanism in plants is strongly supported by the demonstration that essential virulence factors of many plant RNA and DNA viruses are VSRs, which is discussed in detail below.
RNA Silencing Controls Innate Immunity Against Viruses in Invertebrates
It has not been clear what mechanism controls innate immunity against viruses in Drosophila melanogaster (51). Innate immunity against bacterial and fungal pathogens is mediated by the Toll and Imd (immunodeficiency) pathways (50). However, a global microarray analysis has revealed induction of a new set of genes by virus infection that does not include the well-characterized antimicrobial peptide genes (33).
The first indication for a role of RNA silencing in the response of D. melanogaster to virus infection came from the observation that the B2 protein of Flock house virus (FHV) exhibited activity to suppress RNA silencing in plants (65). FHV is a member of the family Nodaviridae, which contains a positivestrand RNA genome and includes pathogens of insects (Alphanodavirus) and fishes (Betanodavirus), although the type alphanodavirus, Nodamura virus (NoV), can lethally infect not only insects but also mammals.
Indeed, FHV infection of cultured Drosophila cells triggers production of FHV-specific siRNAs, and B2 expression is essential to establish infection. Accumulation of a FHV mutant not expressing B2 was detected in Drosophila cells only after depletion of AGO2 by RNAi, indicating that FHV infection triggers antiviral silencing in an AGO2-dependent manner (65). Induction and suppression of the AGO2-dependent antiviral silencing by NoV have also been demonstrated in cultured D. melanogaster and Anopheles gambiae cells (70). Using genetic loss-of-function mutants, we have recently shown that the RNA-silencing pathway controls innate immunity against two distinct viruses in adult D. melanogaster (138).
Two recent studies have shown that viral RNA replication also triggers the RNA-silencing immunity in C. elegans (80, 139), which encodes one Dicer as do fission yeasts and humans. Thus, although both plants and insects encode multiple Dicers, hosts that contain a single Dicer also have the potential to mount the RNAi-mediated antiviral response.
RNA Silencing Plays a Role in Mammalian Virus-Host Interactions
Three lines of evidence indicate that mammalian viruses interact directly with the RNA-silencing pathway in their mammalian hosts (66). First, infection of several mammalian DNA viruses in cell culture induces miRNA silencing, which includes (a) recognition of virus transcripts by the RNAi machinery as precursors of miRNAs, (b) production of viral miRNAs, and (c) cleavages of viral mR-NAs as shown for the SV40 miRNAs (99, 117). However, the role of most of the approximately 40 viral miRNAs that have been cloned and/or characterized is currently not understood (66). Second, two cellular miR-NAs specifically interact with mammalian viruses, leading to either down- or up-regulation of viral RNA replication (54, 64). Third, diverse mammalian viruses encode the activity to suppress RNA silencing (66), suggesting a role in virus infection of mammalian hosts for the suppression of RNA silencing, possibly mediated by small RNAs of either a host or virus origin.
IDENTIFICATION OF VIRAL SUPPRESSORS OF RNA SILENCING
Coinfiltration
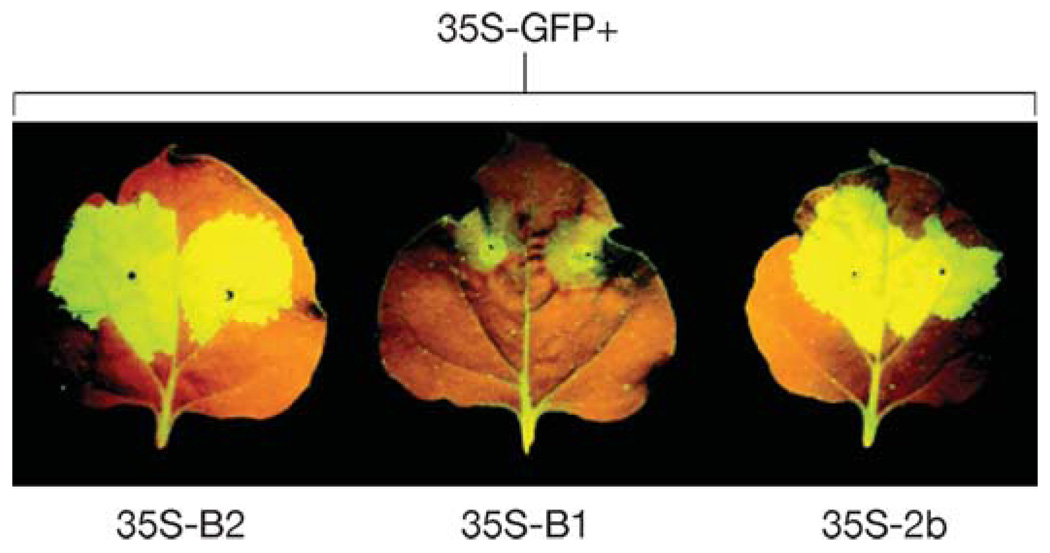
A number of assays have been established to identify VSRs. Two assays have been widely used in plants. The first is based on the transient, mixed expression of two transgenes in leaves coinfiltrated with two Agrobacterium tumefaciens strains (44, 53, 134) (Figure 2). One strain induces RNA silencing of a reporter gene such as green fluorescent protein (GFP) in the infiltrated leaf (local silencing) (Figure 2, middle leaf) and subsequent spread of silencing into upper noninfiltrated tissues in transgenic plants that carry a homologous, integrated transgene (systemic silencing). The other strain directs high-level expression of the candidate viral protein in the coinfiltrated patches to test suppression of local silencing (Figure 2, left and right leaves) and/or systemic silencing. Coinfiltration is the most popular assay used in the identification of plant viral VSRs because it is simple and quick. However, this assay is not capable of identifying those VSRs, such as the coat protein of Citrus tristeza virus (CTV) (79), that suppress systemic silencing but not local silencing. This is because this type of VSR is expressed only at low levels in the infiltrated patches owing to local silencing against the viral suppressor transgene induced by Agroinfiltration.
Figure 2.
Coinfiltration assay. Two A. tumefaciens strains are mixed and infiltrated into the green fluorescent leaves of Nicotiana benthamiana 16c plants that stably express the 35S-GFP transgene. One strain carries 35S-GFP and directs a high level of GFP expression, which induces silencing of GFP, visualized as a bright red color zone surrounding the infiltrated patch caused by chlorophyll autofluorescence and loss of GFP expression (middle leaf). The second strain carries a binary plasmid that contains a VSR gene cloned under the control of 35S promoter. Coexpression of a VSR such as 2b of Tomato aspermy virus (right leaf) and B2 of Flock house virus (left leaf) suppressed the local GFP silencing, leading to high intensity of green fluorescence in the infiltrated patch and loss of the red zone. Silencing suppression is not visible after a mutation that abolishes the expression of B2 was introduced (middle leaf).
Grafting
The use of grafting experiments makes it possible to identify VSRs that are active against systemic silencing but not local silencing (Figure 3). In this assay, selected transgenic plants stably expressing a candidate VSR are genetically crossed with a transgenic plant line that carries an autonomously silencing reporter transgene such as 35S-GUS in tobacco line 6b5. Whether or not expression of the viral protein suppresses spread of the reporter transgene silencing in the resulting F1 progeny (6b5xVSR) can be determined by grafting reporter scions onto rootstocks made from the F1 plants (82, 94). The reporter scions are from another transgenic plant line such as T19 that expresses the reporter GUS transgene at high levels, which becomes silenced a few weeks after grafting onto 6b5 rootstocks owing to the import of a sequence-specific silencing signal from the silencing rootstock. Silencing does not occur in the scions if the VSR can inhibit either the synthesis of the mobile silencing signal in the F1 rootstocks or its export from rootstock to scion (44, 79). Moreover, analysis of expression of the reporter transgene in the F1 progeny can also reveal if the VSR suppresses local silencing, DNA methylation of the reporter transgene, or both.
Figure 3.
Grafting assay. Two transgenic tobacco lines carrying the 35S-GUS transgene were used: T19 plants are high expressors of the GUS transgene (blue), whereas the GUS transgene in line 6b5 is autonomously silenced (white). T19 plants are used as the reporter scions, and rootstocks are made from the F1 plants from crosses of 6b5 with either wt plants (6b5xWT) or plants preselected for high-level expression of a candidate VSR (6b5xVSR). Suppression of GUS silencing spread by a VSR will not reduce GUS expression in the new growth of the scions 4 to 6 weeks after grafting (a), in contrast to what occurs in the control grafts (b).
Distinct suppressor activities have been revealed in this experimental system (44, 79, 82). A VSR may suppress (a) DNA methylation and local and systemic silencing of the reporter transgene (such as cucumoviral 2b), (b) local silencing but not DNA methylation and systemic silencing (HC-Pro and p23 of CTV), (c) systemic silencing but not local silencing and DNA methylation (CP of CTV), or (d) local and systemic silencing but not DNA methylation (p20 of CTV). However, this approach is time-consuming and does not work if stable transgenic plants cannot be obtained owing to toxicity associated with constitutive expression of some VSRs such as 2b of Tomato aspermy virus (S.-W. Ding, unpublished data).
Cell-Based Assays for Identifying Animal VSRs
Several animal VSRs have been identified by assaying for suppression of RNA silencing induced by viral RNA replication in cultured Drosophila cells (70). The core component of this assay is pFR1gfp (Figure 4a), a derivative of pFR1 that contains the full-length cDNA to FHV RNA1. The viral cDNA is under the transcriptional control of a metal-inducible promoter, and a ribozyme located 3′ end to the cDNA is designed to remove the nonviral sequence from the transcripts (65). Thus, after transfection into Drosophila S2 cells, transcriptional induction produces RNA transcripts identical to FHV RNA1. The viral RNA-dependent RNA polymerase (RdRP), which shares no homology with RDRs, is translated directly from RNA1 to initiate replication of RNA1 and subsequent production of subgenomic RNA3, which serves as a mRNA to express B2.
Figure 4.
Cell-based assay for identifying animal VSRs. (a) Structures of the viral cDNA in plasmids pFR1 and pFR1gfp. (b) Expression of GFP from pFR1gfp is detected in pFR1gfp-transfected Drosophila cells together with either dsRNA to Drosophila AGO2 (middle panel) or a plasmid expressing B2 of NoV (right panel), but not dsRNA of LacZ (left panel).
In this system, B2 suppression of the RNA-silencing immunity triggered by viral RNA replication is essential for detectable accumulation of FHV RNA1 and RNA3 (65). In S2 cells transfected with pFR1gfp, B2 is not expressed owing to the insertion and translational fusion of the coding sequence for GFP after approximately the first 20 codons of B2. Thus, expression of GFP from the recombinant RNA3 occurs only in S2 cells in which RNA silencing is suppressed by either depletion of AGO2 (Figure 4b, middle panel) or expression of a VSR from a superinfected virus or cotransfected plasmid (Figure 4b, right panel). This assay can be easily adapted to mosquito cell culture for determining if mammalian viruses transmitted by mosquitoes encode VSRs (70).
The most commonly used trigger of RNA silencing in mammalian cell culture is 21-nt siRNAs that are chemically synthesized by commercial companies (38). However, many mammalian VSRs have been identified using short hairpin RNAs (shRNAs) (66, 116). shRNAs are analogous to pre-miRNAs, as they require de novo processing by Dicer to produce siRNAs. Thus, use of shRNAs as the inducer allows researchers to identify those VSRs that target early steps of the RNA-silencing pathway (see below).
DIVERSE VIRAL SUPPRESSORS OF RNA SILENCING
Multiple VSRs from Single Plant Viruses
Every plant virus that has been closely examined to date encodes a VSR, and these include viruses that contain positive-, negative-, or double-strand RNA genomes as well as geminiviruses with a single-stranded circular DNA genome (Table 1). However, suppression of RNA silencing by any of the known VSRs is partial and the VSR encoded by each virus often targets only one of the effector mechanisms of RNA silencing. Nevertheless, CTV and geminiviruses encode multiple VSRs, each of which appears to have a distinct mode of action (79, 129, 135). In contrast to the large RNA genome of CTV (∼20 kb), geminiviruses have a small genome, suggesting that other small viruses may also have the potential to develop distinct strategies for evading the viral immunity.
Table 1.
Plant and animal VSRs
| Virus genus | Virus name | VSR | Motif implicated in VSR activity | Reference(s) |
|---|---|---|---|---|
| Positive-strand RNA viruses in plants | ||||
| Aureusvirus | Pothos latent virus | P14 | dsRNA binding | 86 |
| Carmovirus | Turnip crinkle virus | CP | 101, 102 | |
| Closterovirus | Beet yellows virus | P21 | dsRNA binding | 20, 23, 79, 106 |
| Citrus tristeza virus | P20 | |||
| P23 | ||||
| CP | ||||
| Grapevine leafroll-associated virus-2 | P24 | |||
| Beet yellow stunt virus | P22 | |||
| Crinivirus | Sweet potato chlorotic stunt virus | P22 | 60 | |
| RNase3 | RNaseIII | |||
| Comovirus | Cowpea mosaic virus | Small CP | 78 | |
| Cucumovirus | Cucumber mosaic virus | 2b | dsRNA binding | 13, 67, 101 |
| Tomato aspermy virus | ||||
| Furovirus | Soil-borne wheat mosaic virus | 19K | Cysteine-rich protein | 122 |
| Hordeivirus | Barley stripe mosaic virus | γb | Cysteine-rich protein | 31, 147 |
| Pecluvirus | Peanut clump virus | P15 | Cysteine-rich protein | 35, 36 |
| Polerovirus | Beet western yellows virus | P0 | 97 | |
| Cucurbit aphid-born yellows virus | ||||
| Potexvirus | Potato virus X | P25 | 134 | |
| Potyvirus | Tobacco etch virus | Hc-Pro | 3, 13, 56, 57 | |
| Potato virus Y | ||||
| Turnip mosaic virus | ||||
| Sobemovirus | Rice yellow mottle virus | P1 | 135 | |
| Tobamovirus | Tobacco mosaic viruses | P130 | 61 | |
| Tomato mosaic viruses | ||||
| Tobravirus | Tobacco rattle virus | 16K | Cysteine-rich protein | 75 |
| Tombusvirus | Tomato bushy stunt virus | P19 | dsRNA bindinga | 101, 112, 135 |
| Cymbidium ringspot virus | ||||
| Tymovirus | Turnip yellow mosaic virus | P69 | 22 | |
| Vitiviruses | Grapevine virus A | P10 | 23 | |
| Negative-strand RNA viruses in plants | ||||
| Tenuivirus | Rice hoja blanca virus | NS3 | 15 | |
| Tospovirus | Tomato spotted wilt virus | NSs | 15, 120 | |
| Double-stranded RNA viruses in plants | ||||
| Phytoreovirus | Rice dwarf virus | Pns10 | 17 | |
| DNA viruses in plants | ||||
| Begomovirus | Tomato leaf curl virus | C2 | DNA binding, NLS |
21, 27, 125, 127, 129, 135, 136 |
| TYLCCNV-Y10 Y10β | βC1 | DNA binding, NLS | ||
| African cassava mosaic virus (KE) | AC2 | DNA binding, NLS, AD | ||
| EACMCV, ICMV, TGMV | ||||
| Mungbean yellow mosaic virus | ||||
| African cassava mosaic virus (CM) | AC4 | miRNA bindingb | ||
| Curtovirus | Beet curly top virus | L2 | Protein binding | 136 |
| Positive-strand RNA viruses in animals | ||||
| Nodavirus |
Flock house virus, nodamura virus, Striped jack nervous necrosis virus, Greasy grouper nervous necrosis virus |
B2 | dsRNA binding | 41, 52, 65, 70 |
| Negative-strand RNA viruses in animals | ||||
| Orthomyxovirus | Influenza virus A | NS1 | dsRNA binding | 70 |
| Orthobunyavirus | La Crosse virus | NSs | 114 | |
| Double-stranded RNA viruses in animals | ||||
| Orthoreovrivus | σ3 | dsRNA bindingc | 71, 151 | |
| Retroviruses in animals | ||||
| Lentivirus | HIV-1 | Ta t | 8 | |
| Spumavirus | PFV-1 | Tas | 64 | |
| DNA viruses in animals | ||||
| Adenovirus | Adenovirus | VA1 RNA | Dicer binding | 81 |
| Poxvirus | Vaccinia virus | E3L | dsRNA binding | 70 |
Prefer 19-nt RNA duplex.
Single-strand mature miRNA.
Prefer dsRNA longer than 30 nt.
Insect VSRs
We have recently shown that infection of Drosophila with Cricket paralysis virus (CrPV) induces antiviral silencing and that CrPV encodes a novel VSR (138). CrPV is a member of the picorna-like insect virus group that is only distantly related to the insect nodaviruses from which the first animal VSR was identified (65). Thus, active suppression of RNA silencing likely represents a conserved function of true insect viruses, which are pathogenic to insect hosts.
Vertebrate VSRs
Nine mammalian VSRs have been identified (66). These include NS1 of influenza A, B, and C viruses (70), E3L of vaccinia virus (70), B2 of NoV (70, 116), NSs of La Crosse virus (114), VA1 of adenovirus (4, 81), Tas of PFV-1 (64), and Tat of human immunodeficiency virus (8). The reovirus σ3 protein suppresses RNA silencing in plants (71), but its activity is yet to be verified in animal cells. In addition, B2 from two fish pathogenic nodaviruses were found to suppress RNA silencing in heterologous systems (Table 1). These vertebrate viruses are genetically diverse and the VSRs identified were previously known to play key roles in infection of their mammalian hosts. However, it should be pointed out that suppression of virus-induced RNA silencing has been demonstrated in cultured insect cells only for a few of the known VSRs. Thus, a role of silencing suppression in the infection of vertebrate hosts remains to be established.
Structured Viral RNAs as VSRs
In addition to viral proteins, the highly structured RNA of approximately 160 nt encoded by adenovirus, VA1, can inhibit RNA silencing induced by either shRNAs or human pre-miRNAs (4, 81). Takeda and colleagues (121) have recently demonstrated suppression of transgene RNA silencing by RNA replication of Red clover necrotic mosaic virus (RCNMV) but not by any of the RCNMV-encoded proteins, suggesting that either the viral replicative intermediate dsRNA or an amplified RNA structural element may be involved in silencing suppression.
VSR Families
VSRs encoded by viruses of different families often share no homology at the primary amino acid sequence level. However, homologs or counterparts (by location in the viral genome) of a known VSR from the same virus family always encode silencing suppression activity, even if they may share minimal sequence homology. These include 2b of the cucumoviruses (13, 67), NS1 from three of the four genera in the family Orthomyxoviridae (70), B2 from both genera of the family Nodaviridae (41, 52, 65, 70), P19/P14 from the genera of tombusvirus and aureusvirus in the Tombusviridae(86, 135),AC2 and its homologs from two of four genera in the family Geminiviridae (11), and the cysteine-rich proteins from furoviruses, hordeiviruses, pecluviruses, and tobraviruses (36, 105, 122).
Cross-Kingdom Suppression of RNA Silencing
The first animal VSR, B2 of FHV, was identified in an RNA-silencing assay established in plants (65). These broad-spectrum activities of VSRs have been demonstrated for NS1 of influenza A virus (14, 29, 70) and p19 of tombusviruses (70, 135). That these VSRs are all dsRNA binding proteins may explain why they are active in both the animal and plant kingdoms (see below).
Possible Evolutionary Origin of VSR Genes
Many known VSRs are encoded by out-of-frame overlapping genes (69) (Figure 5). These include cucumoviral 2b, tymoviral P69, tombusviral P19 and P14, poleroviral P0, geminiviral AC2 and AC4, nodaviral B2, retroviral Tas and Tat, and influenza NS1 (Figure 5). Overlapping genes are thought to be created by overprinting, in which an existing coding sequence is translated in a different reading frame (58). As a result, for each pair of overlapping genes, one is more ancient and widespread, whereas the other is novel and has a confined lineage in the phylogeny of viruses. Previous analyses indicated that 2b, B2, and P69 are all encoded by the novel overlapping gene (30, 58). Recent structural determination of P19 and B2 also has shown that neither shares structural similarities with NS1, although all are dsRNA binding proteins (see below). Thus, these VSR genes are each evolutionarily novel and may represent a recent viral adaptation to the RNA-silencing immunity of the hosts. That VSR genes in each virus family may arise independently explains the structural and functional diversity of VSRs identified so far.
Figure 5.
VSRs are encoded by overlapping genes. Sequences of Cucumber mosaic virus (CMV) RNA2 (NC_002035), Beet western yellows virus (BWYV) (NC_004756), Flock house virus (FHV) RNA1 (NC_004146), Tomato bushy stunt virus (TBSV) (NC_001554), and Turnip yellow mosaic virus (TYMV) (NC_004063) were analyzed by the DNA Strider program using universal codes. The maps show the location of open reading frames (ORFs) as well as start (short vertical lines) and stop codons (long vertical lines) in each of the three reading frames. >> and * refer to translational readthrough and −1 ribosomal frameshift, respectively.
Most of these VSR genes are overprinted on either the N-terminal (such as p69 and P0) or C-terminal (such as 2b and B2) region of the viral RdRP gene (Figure 5), enabling their translation from either the genomic RNA or a 3′-coterminalsubgenomicRNA(30,97). This unique coupling of the RNA replication and silencing suppression functions creates a viral genome block that is perhaps most effective as a gene module for viral evolution and adaptation, since productive viral RNA replication requires suppression of the RNA-silencing immunity as has been shown in plants and invertebrate animals. This strategy is similar to that of picorna-like plant and insect viruses in which the VSR and viral RdRP are translated as part of the long polyprotein.
DIVERSE MECHANISMS INVOLVED IN VIRAL SUPPRESSION OF RNA SILENCING
Distinct Protein Folds Evolved Independently in Viruses to Bind dsRNA
A role for RNA binding in the suppression of RNA silencing by the cucumoviral 2b protein had been proposed previously (44). It is now clear that a major class of VSRs are dsRNA-binding proteins, as revealed first for the tombusviral P19 (112). However, dsRNA binding is unusual for P19 among the dsRNA binding proteins known so far because it specifically selects its substrates on the basis of the length of the duplex region of the RNA P19 binds 21-nt duplex siRNAs with high affinity and independent of the 2-nt overhang at the 3′ end of siRNAs, but its affinity is much weaker for dsRNAs 22 nt or longer (130, 145). Such a size selection in dsRNA binding has not been observed for influenza NS1 (14, 70), nodaviral B2 (80), closteroviral P21 (20, 146), cucumoviral 2b (F. Li & S.-W. Ding, unpublished data), or aureusviral P14 (86), which is a P19 homolog of a different genus from the same family Tombusviridae. All these VSRs bind duplex siRNAs and long dsRNA, and B2 in fact exhibits higher affinity to long dsRNA than to siRNAs (80).
Strikingly, vaccinia E3L (70) is the only example among the known dsRNA binding VSRs that has sequence similarity to the canonical dsRNA binding motif (DSRM) found in many cellular proteins, such as Drosophila Staufen protein, PKR, Dicer, and R2D2 (16, 91). DSRM adopts a α1β1β2β3α2 fold, in which the two helices lie on one side and pack against a three-stranded antiparallel β sheet (Figure 6a). Three protein-RNA interaction regions include α2 across the RNA major groove, and α1 and the loop between β1 and β2 to contact the minor groove at either side (91, 109). By contrast, NS1, P19, B2, and P21 share no structural similarities with the canonical DSRM and each adopts a novel protein fold, which are discussed briefly below. This provides further support at the structural level for independent origins of VSRs encoded by the novel overlapping gene as indicated by our evolutionary analyses.
Figure 6.
Structures of dsRNA-binding motifs. (a) Canonical DSRM [reprinted, with permission, from Macmillan Publishers Ltd: EMBO J. (109), copyright 1998]. (b) NS1 of influenza A virus in complex with dsRNA [reprinted, with permission, from The American Chemical Society: Biochemistry (25), copyright 2004]. (c) P19 in complex with siRNA [reprinted, with permission, from Macmillan Publishers Ltd: Nature (145), copyright 2003]. (d) B2 in complex with dsRNA [reprinted, with permission, from Macmillan Publishers Ltd: Nat. Struct. Mol. Biol. (19), copyright 2005].
NS1
NS1 is approximately 230 amino acids (aa) in length (137). The N-terminal region of 73 aa contains complete dsRNA binding activity of the full-length protein and retains most of the VSR activity (24, 25, 77). Both the NMR and crystal structural analyses, reported in 1997, have revealed a novel, six-helical fold in a homodimer for the NS1 dsRNA binding domain (24, 25, 77) (Figure 6b). The RNA binding surface is constituted by the antiparallel α2 α2′, in which several basic residues form electrostatic interaction with the phosphor group of the RNA backbone (25). The protein sits over the minor groove of the A form duplex and there is no significant conformation change during the RNA-protein complex formation (25, 137). Possible protein-protein interaction between multiple NS1 dimers may account for higher affinity for longer dsRNA substrates (25, 137).
P19
P19 of two tombusviruses in complex with siRNA has been solved and both display a common protein fold, α1β1β2α2α3β3β4α4. P19 also binds dsRNA in a homodimer (130, 145), as has been found for NS1, and the protein-protein interaction is mediated by the antiparallel β4β4′ strands and α4α4′ helices (Figure 6c). The eight β strands (four from each monomer) form a saddle-like β sheet surface that covers the central minor groove and two adjacent partial major grooves of the siRNA duplex. The N-terminal α helix brackets at the ends of the siRNA duplex and poses a size constraint for the substrate. The remaining α helices are packed on the other side of the saddle.
B2
B2 of FHV is 106 aa long and also contains a dsRNA binding domain located at the N-terminal region. Both NMR and crystallization structural analyses have revealed an all-helix structure for the N-terminal 72 aa (19, 74) (Figure 6d). The first two helices (α1 and α2) fold into a helix-turn-helix hairpin structure, whereas the third, shorter α helix (α3) packs perpendicular to α1 and α2. B2 binds dsRNA as a homodimer, in which α1 and α1′, α2 and α2′ pack against each other and α3 and α3′ are located at the opposite ends. The antiparallel α2α2′ helices form an extended RNA binding surface that covers two minor grooves and the intervening major groove.
P21
P21 of the closterovirus Beet western yellows virus is folded into nine α helices (146), which can be divided into a N-terminal domain (NTD) of 93 aa and a C-terminal domain (CTD) of 83 aa. NTD is mainly a three-helix bundle (α1α2α3) arranged in an up-and-down fashion. CTD folds into a two-layer array: The first layer includes α4α5α9 and the second includes α6α7α8, producing an octamer-ring structure with two types of head-to-head and tail-to-tail arrangements.
The main secondary structures of NS1, B2, and P21 are all α helices, and NS1, P19, and B2 bind dsRNA as a homodimer. The canonical DSRM, P19, and B2 interact with the 2′ OH group of ribose on the backbone, which provides a structural basis for their substrate preference for RNA rather than DNA. The interaction between the phosphor group on the backbone and the side chains of amino acids in the dsRNA binding surface involves both electrostatic and hydrogen-bond interactions. The ribose and phosphor group recognition confer dsRNA binding in a sequence-independent manner, which may also apply to other dsRNA binding viral suppressors.
Suppression of siRNA Production
A result of dsRNA binding by VSRs is inhibition of viral siRNA production in infected cells, possibly by preventing Dicer from access to the viral RNA trigger(s). Inhibition of the dicing of input long dsRNA by B2 of FHV was first demonstrated in vitro using the Dicer extracts from Drosophila cells (19, 80). Reduced accumulation of siRNAs processed from hpRNA was also observed in mammalian cells expressing B2 of NoV (116). Notably, both dsRNA binding and dicing inhibition in vitro were abolished by the replacement of Arg by Gln at position 54 (R54Q) of FHV B2 (80), which was later shown by structural analyses to be in the center of the dsRNA binding surface (19, 74). An FHV mutant carrying the R54Q mutation in B2 was as defective as the FHV mutant not expressing B2 (FHVΔB2) in the infection of Drosophila S2 cells, but was rescued by RNAi depletion of AGO2 (H. W. Li & S.-W. Ding, unpublished data). This indicates a role for dicing inhibition in B2 suppression of the RNA-silencing immunity. We have recently found that similar levels of FHV replication produced much higher levels of viral siRNAs in S2 cells cotransfected with FHVΔB2 and AGO2 dsRNA than in cells transfected with wt FHV alone (H. W. Li & S.-W. Ding, unpublished data). These findings thus establish inhibition of siRNA production as a mechanism in B2 suppression of RNA silencing (Figure 7).
Figure 7.
VSRs block key steps in RNA silencing. The big pale green square represents an infected cell, with the white ellipse as the nucleus. The filled green rectangles represent the pathways for the short- and long-distance spread of RNA silencing. The amplification phase and possible nuclear processing of dsRNA (dashed arrows) are integrated with the initiation of antiviral silencing (solid arrows). For convenience we also use the initiation phase in this figure to describe antiviral silencing in animal cells where amplification may not occur. The possible steps targeted by different VSRs are red.
VA1 appears to inhibit the production of small RNAs by a mechanism distinct to B2 as it directly binds to Dicer and thus may act as a substrate to compete for Dicer binding (4, 81). A similar mechanism may be used by RCNMV (121). HC-Pro also inhibits Dicer processing (Figure 7), because HC-Pro expression in transgenic plants is associated with accumulation of unprocessed dsRNA (35,83). Interestingly, HC-Pro mainly inhibits the accumulation of the 21-nt siRNAs but does not inhibit, or has a less pronounced effect on, the accumulation of the 24-nt siRNAs (35, 83). This is probably because the biogenesis of the 24-nt siRNAs by the DCL3/RDR2/AGO4 pathway occurs in the nucleus that is insensitive to HC-Pro, a cytoplasmic protein.
Alternatively, HC-Pro expression may selectively increase the instability of the shorter class of siRNAs, since a recent study showed that HC-Pro expression caused a significantly more pronounced reduction in the 3′-terminal methylation of the 21- to 22-nt siRNA population than the 24-nt siRNA population (37). It may be informative therefore to determine if overexpression of rgsCaM, a cellular calmodulin-related protein that interacts with HC-Pro (2), suppresses either the 3′-terminal methylation or the processing of the 21- to 22-nt siRNAs by DCL2 and DCL4. In this regard, it may not be coincidental that expression of HC-Pro does not interfere with transgene DNA methylation and systemic silencing spread in the 6b5 tobacco plants (82) or silencing-mediated recovery of transgenic tobacco plants (73), as these processes are all possibly mediated by the 24-nt class of siRNAs, which is not inhibited by HC-Pro. In contrast to HC-Pro, expression of both P25 of Potato virus X (PVX) and P1 of Rice yellow mottle virus specifically inhibits the accumulation of the 24-nt siRNA but has a less pronounced effect on the accumulation of the 21-nt siRNA (45). Interestingly, it appears that only the shorter class of viral siRNAs accumulated in wt plants infected with PVX (110), suggesting that P25 expression may also inhibit the production of the longer class of viral siRNAs in infected plants.
Sequestration of siRNAs
Selective binding of the 21- to 22-nt class but not the 24-nt class of siRNAs by P19 suggests a unique mechanism of RNA-silencing suppression by sequestering siRNAs (63, 112, 130, 145). The role of siRNA sequestering by P19 has been examined in both in vitro Drosophila embryo extracts (63) and infected plants (47, 118). P19 inhibited siRNA-directed slicing of the target mRNA only when P19 and the siRNA duplex were added to the embryo extracts at the same time. P19 was inactive when P19 was added 20 min after the addition of the siRNA duplex and P19 did not inhibit slicing initiated by single-stranded siRNA, indicating that P19 binds duplex siRNA to prevent siRNA from being incorporated into siRISC (63). Inhibition of siRISC assembly by siRNA sequestering may account for the observed P19 suppression of the RNA-silencing immunity induced by FHV infection of cultured Drosophila cells (70).
However, expression of the Cymbidium ringspot virus (CRSV) P19 from its own genome had no detectable effect either on the accumulation levels of CRSV and CRSV-specific siRNAs in infected protoplasts and inoculated leaves, or on the spread of virus through the vasculature to reach the first systemically infected leaves (47, 112, 118). Thus, it is unlikely that P19 inhibition of siRISC assembly, as observed in the heterologous system, plays a role in these initially infected cells and tissues. Elegant in situ hybridization experiments have revealed that expression of P19 allowed the virus to exit the vascular bundles and invade the surrounding tissues and beyond in the systemically infected leaves (47). As the 21-nt viral siRNAs are found in the phloem (148) and have the potential to mediate the cell-to-cell spread of RNA silencing (34), siRNA sequestering by P19 may prevent the viral 21-nt siRNAs from entering the vasculature in the inoculated leaves and/or exiting the vasculature in the first systemically infected leaves (Figure 7). In contrast, abundant viral 21-nt siRNAs may enter and exit the vasculature to initiate antiviral silencing in the first leaves systemically infected with the CRSV mutant that does not express P19, leading to arrest of further virus spread and recovery from infection (118). An ultimate test of this model is to determine if P19 mutation in a tombusvirus can be rescued in a host mutant, such as dcl4 or dcl2/dcl4 double mutant, that is defective in the 21- and/or 22-nt siRNA biogenesis pathway (34, 43).
Several VSRs bind both siRNAs and longer dsRNA without a preference for the 21-nt siRNAs. These include NS1 (14, 70), B2 (19, 80), P21 (20, 146), 2b (F. Li & S.-W. Ding, unpublished data), and P14, the P19 homolog encoded by Pothos latent virus (86). In vivo binding of duplex siRNA and miRNA has been demonstrated in transgenic Arabidopsis expressing P21 (20). Unlike P19 and P14, however, P21 is required for efficient viral RNA amplification in single-cell infection (96). Thus, sequestering siRNAs by P21 may lead to inhibition of the assembly of siRISC in infected plants (Figure 7).
The geminiviral VSR AC4 does not bind duplex small RNAs, but it does bind the single-stranded siRNA or miRNA in vitro and possibly in vivo also because the AC4 protein-miRNA complex could be isolated from AC4-expressing cells by using a tethered 2′-O-methyloligonucleotide (21). Thus, AC4 may suppress RNA silencing by inhibiting the RISC activity after its maturation (Figure 7).
Inhibition of Systemic Silencing
Suppression of the phloem-dependent longdistance spread of RNA silencing represents another distinct viral strategy for evading the RNA-silencing immunity in plants. This was first demonstrated for P25, as systemic silencing of a transgene induced by a movement-defective PVX did not occur in Nicotiana benthamiana plants unless P25 was inactivated (134). The loss of meristem exclusion to virus invasion in transgenic plants expressing a P25 homolog may also be a result of suppression of systemic silencing (42). Interestingly, expression of either P25 or P1 was found to inhibit the accumulation of the longer class of siR-NAs in N. benthamiana (45), suggesting that these VSRs may act by either inhibiting the synthesis or promoting removal of the longer class of siRNAs. In PVX infection, deletion in the gene for P25 does not reduce PVX accumulation in inoculated protoplasts but abolishes spread of PVX out of the initially infected cells (5). A recent study has further shown that P25 suppression of RNA silencing is required for the cell-to-cell movement of PVX (7). Thus, the short-distance spread of antiviral silencing has the potential to inhibit virus cell-to-cell movement (7) and P25 may in fact block the function of the 21-nt siR-NAs in PVX-infected plants. However, P19 sequestering of 21-nt siRNAs is required only for CRSV unloading in the first systemically infected leaves (47), which argues against a role for the 21-nt siRNAs of CRSV in inhibiting virus cell-to-cell movement in the inoculated leaves.
Suppression of systemic silencing by the cucumoviral 2b may involve inactivation of the silencing signal (Figure 7), as indicated by a set of grafting experiments (44). Expression of 2b reduced transgene DNA methylation and prevented transgene silencing from spreading into reporter scions. Silencing spread into the scions also did not occur when 2b was expressed only in the intergraft between rootstock and scion, and the silencing signal imported into the 2b-expressing scion failed to initiate specific RNA silencing (44). Suppression of the phloem-dependent long-distance spread of silencing by 2b is consistent with previous studies that have demonstrated a role for 2b in facilitating the long-distance spread of Cucumber mosaic virus (CMV) (30). Our recent results further reveal a correlation between suppression of systemic silencing and 2b binding of dsRNA 25 nt or longer (F. Li & S.-W. Ding, unpublished data), suggesting that 2b may act by sequestering the longer class of siRNAs or their precursor dsRNA. It will be interesting to determine if 2b mutation can be rescued in a host mutant such as dcl3 that is defective in the 24-nt siRNA biogenesis pathway.
Silencing Suppression by DNA Viruses
VSRs encoded by the family Geminiviridae include AC2 and AC4 as well as βC1, which is encoded by a satellite DNA (27, 129, 135). In contrast to AC4, AC2 may suppress silencing by interacting with DNA, cellular proteins, or both (11). The AC2 family of proteins typically acts as a transcription factor and contains a zinc-finger domain, a nuclear localization signal, and a C-terminal acidic type of activation domain. It is likely that AC2 suppression of RNA silencing is transcription dependent as mutations in any of the three domains abolish the VSR activity of AC2 (11, 32,125,127). This is further supported by the findings that AC2 expression in protoplasts induced the expression of approximately 30 host genes, one of which may encode a negative regulator of RNA silencing (125).
In contrast, silencing suppression by the AC2 homologs of Tomato golden mosaic virus and Beet curly top virus does not require the activation domain but depends on inactivation of adenosine kinase (ADK) by a direct protein-protein interaction (136). ADK catalyzes the synthesis of 5′-AMP from adenosine and ATP and plays a key role in sustaining the methyl cycle as does S-adenosyl-homocysteine (SAH) hydrolase. Thus, inactivation of ADK by AC2 may interfere with a general methylation pathway in plants, as occurs in Arabidopsis hog1 mutants, which carry loss-of-function mutations in HOG1 coding for a SAH hydrolase and do not support DNA methylation-dependent gene silencing (107). This finding suggests that the viral genomic DNA may be targeted for silencing in a methylation-dependent manner, possibly triggered in the nucleus by the viral siRNAs (66).
Altering the Function of Host miRNAs: A Role in Viral Pathogenesis?
Stable expression of VSRs in transgenic plants also interferes with the function of host miRNAs, which may explain why these plants often exhibit developmental abnormalities (20–22, 35, 57). Enhanced accumulation of host miRNAs has been observed in plants expressing HC-Pro, P19, P21, or P69 of Turnip yellow mosaic virus (20, 22, 35). Nevertheless, miRNA-directed cleavages of target mRNAs were inhibited by HC-Pro, P19, P21, and P15 of Peanut clump virus (20, 35). Because DCL1, which produces miRNAs, is downregulated by miR162 (144), enhanced levels of miRNAs may be a result of enhanced miRNA processing due to inhibition of miR162-directed cleavage of DCL1 mRNA. However, this model does not explain why P15 inhibition of miRNA silencing does not enhance miRNA accumulation or why P69 enhances the accumulation of miRNAs despite the fact it does not inhibit the target cleavages by miRNAs.
Transgenic expression of HC-Pro, P21, or P19 in Arabidopsis plants allowed Northern blot detection of the normally labile passenger strand of the miRNA duplex, referred to as miRNA*. P19 and P21, but not HC-Pro, also interacted with the miRNA/miRNA* duplexes in vivo. Thus, P19 and P21 may employ a similar mechanism to suppress miRNA-and siRNA-directed silencing by sequestration of small RNA duplexes. In contrast, it appears that distinct mechanisms are involved in HC-Prosuppression of miRNA and siRNA silencing. Notably, these observations have provided an attractive model to explain viral pathogenesis as a result of viral suppression of shared steps in siRNA silencing as an antiviral defense and miRNA silencing required for development. However, note that all these studies were based on constitutive expression of VSRs in a much broader range of cell and tissue types than in natural plant infections.
FUTURE DIRECTIONS
Genetic Control of the RNA-Silencing Immunity
Although a role for RNA silencing in the viral immunity has been established in plants and invertebrates, many key questions on the genetic control of antiviral silencing remain to be addressed. For example, it is not clear how viruses are recognized by the small RNA pathway(s) in host cells. Plants and insects contain four and two Dicer proteins, respectively. Our observation that antiviral silencing against FHVisAGO2-dependent implies that Dicer-2 and the siRNA pathway play a role in the RNA-silencing immunity in Drosophila. However, it will be necessary to determine if the production of viral siRNAs requires Dicer-2 and if the siRNA pathway also mediates Drosophila immunity against viruses distinct to FHV.
The Arabidopsis genome encodes four DCLs that are involved in the production of distinct classes of small RNAs. In comparison to wt plants, reduced siRNA accumulation and EDS were observed in dcl2 plants, but only in the early stages of infection with Turnip crinkle virus, a carmovirus. However, this transient effectofDCL2 was not observed in Arabidopsis response to a cucumovirus and a potyvirus. This observation raises several critical questions regarding the genetic control of antiviral silencing in plants. For example, is there redundancy among DCLs in the initiation of antiviral silencing? Are viruses from distinct families recognized by different DCLs? Does cellular compartmentation of small RNA biogenesis and virus replication play a role? Thus, it will be important to determine if infection with viruses from different families produces distinct classes of viral siRNAs and if these siRNAs have the potential to activate and guide distinct effector mechanisms of RNA silencing against virus infection. For example, detection of a virus by DCL3 probably will produce the 24-nt siRNA, which may potentially facilitate the phloem-dependent long-distance spread of antiviral silencing and/or methylation of DNA/chromatin in the nucleus.
Two of the six plant RDRs have been implicated in defense against viruses and available data also suggest specific interactions between RDRs and different viruses. There are several interesting questions regarding the role of host RDR in the RNA-silencing immunity. Is host RDR involved in the production of viral siRNAs (referred to as secondary siRNAs)? Do viral secondary siRNAs have function(s) distinct from those of primary siRNAs? Is there a RISC-independent antiviral silencing that involves only synthesis and dicing by host RDRs and DCLs? Do RNA virus-encoded RdRPs amplify the endogenous RNA silencing in plant and animal hosts?
Another important question to be addressed is what serves as the pathogen trigger(s) of the RNA-silencing immunity in plants and animals. Detection and/or cloning of viral siRNAs in both positive and negative polarities in infected plants (88, 128, 148) implicate as the trigger dsRNA produced during replication of RNA viruses or through convergent transcription from opposing promoters in DNA viruses. This hypothesis is supported by the observation that antiviral silencing in Drosophila requires the dsRNA-siRNA pathway initiated by Dicer-2 (138), which does not process the intramolecularly base-paired stem-loop structures found in pre-miRNAs. In contrast, 80% of the viral siRNAs cloned from plants infected with a tombusvirus correspond to the viral positive-strand RNA and 85% are derived from several short regions in the viral genome (88), leading to the hypothesis that stem-loop hairpin structures present in the single-stranded virion RNAs may trigger antiviral silencing via the DCL1-dependent miRNA pathway in plants. However, this hypothesis is not consistent with the observation that reduced processing of miRNAs in dcl1 mutant plants resulted in enhanced virus resistance rather than EDS (121).
Viral Evasion of the RNA-Silencing Immunity
Most of the reported assays in plants cannot reliably identify VSRs that interfere with the spread of RNA silencing, particularly those that are inactive against intracellular silencing (79). Inactivation of this antiviral effector mechanism may be more important in those hosts that encode the RDR system, which is known to amplify the silencing signal involved in both cell-to-cell and phloem-dependent silencing spread (34, 110). Thus, this type of VSR may be more widespread and more plant viruses may encode multiple VSRs than is currently thought. However, the grafting assay capable of identifying this type of VSR is time-consuming, and thus it is necessary to develop an assay that is as simple and rapid as the coin-filtration assay.
Most of the known VSRs were not identified and characterized in assays in which RNA silencing is induced by virus replication to destroy virus and homologous RNAs, including mRNA of VSR. Although previously known to play key roles in virus infection and pathogenesis, a specific role for the suppression of RNA silencing in virus infection remains to be established for many of the known VSRs. Informative experimental evidence along this line includes rescue of defects in host infection by heterologous VSRs and correlation between the activities of a VSR in silencing suppression and host infection. However, an ultimate test is to demonstrate that expression of a VSR becomes redundant in virus infection of a host mutant that is defective in a small RNA pathway. This approach will also provide insight into the mechanism involved in viral suppression because it identifies the genetic pathway as the antiviral effector mechanism targeted by the VSR.
Many questions concerning the mechanisms of VSRs remain to be addressed. Because VSRs are known to up- or downregulate the accumulation of host miRNAs, it is expected that they also interfere with the accumulation and function of endogenous siRNAs of hosts such as the trans-acting siRNAs (1, 34, 43, 95, 131, 141, 149) and natural antisense siRNAs (12). Does viral interference of the endogenous siRNA pathways play a role in the development of disease symptoms? More importantly, does expression of VSRs differentially influence the accumulation and function of the different classes of viral siRNAs? For those VSRs suchasP25 essential for virus cell-to-cell movement, this question may have to be addressed by comparing the profiles of viral siRNAs isolated from protoplasts infected with either wt or the VSR deletion mutant viruses.
Finally, what is the role of mammalian VSRs? Is their activity in silencing suppression required for virus infection? Do mammalian VSRs interfere with the biogenesis and function of miRNAs of either a host or virus origin? Influenza viruses, unlike many mammalian viruses, replicate in the nucleus. Are influenza viral RNAs targets of the host miRNA pathway, as found for mammalian DNA viruses that replicate in the nucleus? Suppression of RNA silencing by the nuclear-localized cucumoviral 2b is associated with reduced DNA methylation of the target transgene in the nucleus. Do nuclear-localized mammalian VSRs such as NS1 interfere with nuclear silencing?
Viral Exploitation of the RNA-Silencing Pathway
RNA silencing has almost always been portrayed in the literature as a host antiviral mechanism. However, several recent studies suggest that viruses also exploit this gene regulatory mechanism guided by small RNAs for their own benefit. For example, cleavages of the early SV40 mRNAs by its own miRNAs led to reduced expression of viral T antigens and reduced sensitivity to lysis by cytotoxic T cells without reducing the yield of infectious virus (117). Whether the herpesviral miRNAs play a similar role remains to be examined. Furthermore, a liver-specific miRNA was found to upregulate the expression of the Hepatitis C virus genome in cell culture by binding to the 5′ end of the viral genome (54), and Arabidopsis mutant dcl1 showed reduced susceptibility to RCNMV infection (121). These findings raise several important questions. Does virus infection require production and function of small RNAs of either a host or virus origin? Do plant and invertebrate viruses produce miRNAs? Do plantand insect-virus-derived small RNAs direct RNA-silencing processes against host mR-NAs, DNA or both? What is the role of host RNA silencing directed by viral small RNAs?
SUMMARY POINTS
Standard assays for the identification of VSRs have been developed in both plant and animal systems.
Numerous viruses encode VSRs. These include viruses that are pathogens of plants, insects, fish, and humans, and contain single- and double-stranded RNA or DNA genomes. Viruses may encode either single or multiple VSRs.
Many VSRs are dsRNA binding proteins without a preference for siRNAs. Protein structure has been solved for four such VSRs, and notably all adopt a novel fold distinct from each other and from the canonical dsRNA binding motif.
Several of the key steps in the RNA-silencing pathway are targeted by VSRs. Some VSRs sequester siRNAs or inhibit production of siRNAs, whereas others prevent short- and long-distance spread of RNA silencing.
All VSRs were previously known to play key roles in virus infection and pathogenesis. A specific requirement for silencing suppression in virus infection has been demonstrated by the genetic complementation of loss-of-function mutations in a VSR gene and a host gene essential for RNA silencing.
Several plant VSRs interfere with the function of host miRNAs and their stable expression in transgenic plants caused developmental abnormalities. Thus, viral pathogenesis may represent a consequence of viral suppression of shared steps in siRNA silencing as an antiviral defense and miRNA silencing required for development.
Many VSRs are encoded by out-of-frame overlapping genes. Thus, independent creation of VSR genes by overprinting may represent an evolutionary adaptation of viruses to the RNA-silencing immunity of hosts and explain the structural and functional diversities of VSRs.
ACKNOWLEDGMENTS
The research projects on antiviral silencing in the authors’ lab are supported by grants from NIH (R01 AI052447) and USDA National Research Initiative (2005-35319-15331 and 2005-34399-16077). The authors wish to thank members of the Ding laboratory and Olivier Voinnet for stimulating discussion.
Glossary
- PTGS
posttranscriptional gene silencing
- siRNA
short interfering RNA
- Viral suppressor of RNA silencing (VSR)
a virus-encoded protein or RNA element that incompletely blocks silencing of viral nucleic acid sequences guided by viral siRNAs or miRNAs
- DCL
Dicer-like protein
- RITS
RNA-induced transcriptional silencing complex
- RISC
RNA-induced silencing complex
- RDR
host RNA-dependent RNA polymerase
- EDS
enhanced disease susceptibility
- FHV
Flock house virus
- RNA-silencing immunity
an innate immunity mechanism against viruses that is mediated by the RNA-silencing pathway
- Viral miRNAs
virus-derived miRNAs of single polarity processed from stem-loop hairpin structures present in the single-stranded RNA transcripts of DNA viruses
- RdRP
viral RNA-dependent RNA polymerase
- DSRM
dsRNA binding motif
- Viral siRNAs
virus-derived siRNAs of two polarities processed from viral dsRNA produced during viral RNA replication or through convergent transcription from opposing promoters in DNA viruses
- CMV
Cucumber mosaic virus
- Coinfiltration assay
identification of VSRs by mixed injection of two Agrobacterium strains into leaves where induction of transgene silencing occurs in the presence of expression of a candidate VSR
LITERATURE CITED
- 1.Allen E, Xie Z, Gustafson AM, Carrington JC. microRNA-directed phasing during transacting siRNA biogenesis in plants. Cell. 2005;121:207–221. doi: 10.1016/j.cell.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Anandalakshmi R, Marathe R, Ge X, Herr JM, Jr, Mau C, et al. A calmodulin-related protein that suppresses posttranscriptional gene silencing in plants. Science. 2000;290:142–144. doi: 10.1126/science.290.5489.142. [DOI] [PubMed] [Google Scholar]
- 3.Anandalakshmi R, Pruss GJ, Ge X, Marathe R, Mallory AC, et al. A viral suppressor of gene silencing in plants. Proc. Natl. Acad. Sci. USA. 1998;95:13079–13084. doi: 10.1073/pnas.95.22.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson MG, Haasnoot PC, Xu N, Berenjian S, Berkhout B, Akusjarvi G. Suppression of RNA interference by adenovirus virus-associated RNA. J. Virol. 2005;79:9556–9565. doi: 10.1128/JVI.79.15.9556-9565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angell SM, Davies C, Baulcombe DC. Cell-to-cell movement of potato virus X is associated with a change in the size-exclusion limit of plasmodesmata in trichome cells of Nicotiana clevelandii. Virology. 1996;216:197–201. doi: 10.1006/viro.1996.0046. [DOI] [PubMed] [Google Scholar]
- 6.Baumberger N, Baulcombe DC. Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc. Natl. Acad. Sci. USA. 2005;102:11928–11933. doi: 10.1073/pnas.0505461102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayne EH, Rakitina DV, Morozov SY, Baulcombe DC. Cell-to-cell movement of Potato Potexvirus X is dependent on suppression of RNA silencing. Plant J. 2005;44:471–482. doi: 10.1111/j.1365-313X.2005.02539.x. [DOI] [PubMed] [Google Scholar]
- 8.Bennasser Y, Le SY, Benkirane M, Jeang KT. Evidence that HIV-1 encodes an siRNA and a suppressor of RNA silencing. Immunity. 2005;22:607–619. doi: 10.1016/j.immuni.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Bentwich I. Prediction and validation of microRNAs and their targets. FEBS Lett. 2005;579:5904–5910. doi: 10.1016/j.febslet.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 11.Bisaro DM. Silencing suppression by geminivirus proteins. Virology. 2006;344:158–168. doi: 10.1016/j.virol.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Borsani O, Zhu J, Verslues PE, Sunkar R, Zhu JK. Endogenous siRNAs derived from a pair of natural cisantisense transcripts regulate salt tolerance in Arabidopsis. Cell. 2005;123:1279–1291. doi: 10.1016/j.cell.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brigneti G, Voinnet O, Li WX, Ji LH, Ding SW, Baulcombe DC. Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J. 1998;17:6739–6746. doi: 10.1093/emboj/17.22.6739. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Bucher E, Hemmes H, de Haan P, Goldbach R, Prins M. The influenza A virus NS1 protein binds small interfering RNAs and suppresses RNA silencing in plants. J. Gen. Virol. 2004;85:983–991. doi: 10.1099/vir.0.19734-0. [DOI] [PubMed] [Google Scholar]
- 15.Bucher E, Sijen T, De Haan P, Goldbach R, Prins M. Negative-strand tospoviruses and tenuiviruses carry a gene for a suppressor of gene silencing at analogous genomic positions. J. Virol. 2003;77:1329–1336. doi: 10.1128/JVI.77.2.1329-1336.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bycroft M, Grunert S, Murzin AG, Proctor M, St Johnston D. NMR solution structure of a dsRNA binding domain from Drosophila staufen protein reveals homology to the N-terminal domain of ribosomal protein S5. EMBO J. 1995;14:3563–3571. doi: 10.1002/j.1460-2075.1995.tb07362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao X, Zhou P, Zhang X, Zhu S, Zhong X, et al. Identification of an RNA silencing suppressor from a plant double-stranded RNA virus. J. Virol. 2005;79:13018–13027. doi: 10.1128/JVI.79.20.13018-13027.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan SW, Zilberman D, Xie Z, Johansen LK, Carrington JC, Jacobsen SE. RNA silencing genes control de novo DNA methylation. Science. 2004;303:1336. doi: 10.1126/science.1095989. [DOI] [PubMed] [Google Scholar]
- 19.Chao JA, Lee JH, Chapados BR, Debler EW, Schneemann A, Williamson JR. Dual modes of RNA-silencing suppression by Flock House virus protein B2. Nat. Struct. Mol. Biol. 2005;12:952–957. doi: 10.1038/nsmb1005. [DOI] [PubMed] [Google Scholar]
- 20.Chapman EJ, Prokhnevsky AI, Gopinath K, Dolja VV, Carrington JC. Viral RNA silencing suppressors inhibit the microRNA pathway at an intermediate step. Genes Dev. 2004;18:1179–1186. doi: 10.1101/gad.1201204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chellappan P, Vanitharani R, Fauquet CM. MicroRNA-binding viral protein interferes with Arabidopsis development. Proc. Natl. Acad. Sci. USA. 2005;102:10381–10386. doi: 10.1073/pnas.0504439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Li WX, Xie D, Peng JR, Ding SW. Viral virulence protein suppresses RNA silencing-mediated defense but upregulates the role of microRNA in host gene expression. Plant Cell. 2004;16:1302–1313. doi: 10.1105/tpc.018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiba M, Reed JC, Prokhnevsky AI, Chapman EJ, Mawassi M, et al. Diverse suppressors of RNA silencing enhance agroinfection by a viral replicon. Virology. 2006;346:7–14. doi: 10.1016/j.virol.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 24.Chien CY, Tejero R, Huang Y, Zimmerman DE, Rios CB, et al. A novel RNA-binding motif in influenza A virus non-structural protein 1. Nat. Struct. Biol. 1997;4:891–895. doi: 10.1038/nsb1197-891. [DOI] [PubMed] [Google Scholar]
- 25.Chien CY, Xu Y, Xiao R, Aramini JM, Sahasrabudhe PV, et al. Biophysical characterization of the complex between double-stranded RNA and the N-terminal domain of the NS1 protein from influenza A virus: evidence for a novel RNA-binding mode. Biochemistry. 2004;43:1950–1962. doi: 10.1021/bi030176o. [DOI] [PubMed] [Google Scholar]
- 26.Covery S, Al-Kaff NS, Langara A Turner DS. Plants combat infection by gene silencing. Nature. 1997;385:781–782. [Google Scholar]
- 27.Cui X, Li G, Wang D, Hu D, Zhou X. A Begomovirus DNAbeta-encoded protein binds DNA, functions as a suppressor of RNA silencing, and targets the cell nucleus. J. Virol. 2005;79:10764–10775. doi: 10.1128/JVI.79.16.10764-10775.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalmay T, Horsefield R, Braunstein TH, Baulcombe DC. SDE3 encodes an RNA helicase required for post-transcriptional gene silencing in Arabidopsis. EMBO J. 2001;20:2069–2078. doi: 10.1093/emboj/20.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delgadillo MO, Saenz P, Salvador B, Garcia JA, Simon-Mateo C. Human influenza virus NS1 protein enhances viral pathogenicity and acts as an RNA silencing suppressor in plants. J. Gen. Virol. 2004;85:993–999. doi: 10.1099/vir.0.19735-0. [DOI] [PubMed] [Google Scholar]
- 30.Ding SW, Li WX, Symons RH. A novel naturally-occurring hybrid gene encoded by a plant RNA virus facilitates long-distance virus movement. EMBO J. 1995;14:5762–5772. doi: 10.1002/j.1460-2075.1995.tb00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donald RG, Jackson AO. RNA-binding activities of barley stripe mosaic virus gamma b fusion proteins. J. Gen. Virol. 1996;77(Pt 5):879–888. doi: 10.1099/0022-1317-77-5-879. [DOI] [PubMed] [Google Scholar]
- 32.Dong X, van Wezel R, Stanley J, Hong Y. Functional characterization of the nuclear localization signal for a suppressor of posttranscriptional gene silencing. J. Virol. 2003;77:7026–7033. doi: 10.1128/JVI.77.12.7026-7033.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, et al. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat. Immunol. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- 34.Dunoyer P, Himber C, Voinnet O. DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat. Genet. 2005;37:1356–1360. doi: 10.1038/ng1675. [DOI] [PubMed] [Google Scholar]
- 35.Dunoyer P, Lecellier CH, Parizotto EA, Himber C, Voinnet O. Probing the microRNA and small interfering RNA pathways with virus-encoded suppressors of RNA silencing. Plant Cell. 2004;16:1235–1250. doi: 10.1105/tpc.020719. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Dunoyer P, Pfeffer S, Fritsch C, Hemmer O, Voinnet O, Richards KE. Identification, subcellular localization and some properties of a cysteine-rich suppressor of gene silencing encoded by peanut clump virus. Plant J. 2002;29:555–567. doi: 10.1046/j.0960-7412.2001.01242.x. [DOI] [PubMed] [Google Scholar]
- 37.Ebhardt HA, Thi EP, Wang MB, Unrau PJ. Extensive 3′ modification of plant small RNAs is modulated by helper component-proteinase expression. Proc. Natl. Acad. Sci. USA. 2005;102:13398–13403. doi: 10.1073/pnas.0506597102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 39.English JJ, Mueller E, Baulcombe DC. Suppression of virus accumulation in transgenic plants exhibiting silencing of nuclear genes. Plant Cell. 1996;8:179–188. doi: 10.1105/tpc.8.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feinberg EH, Hunter CP. Transport of dsRNA into cells by the transmembrane protein SID-1. Science. 2003;301:1545–1547. doi: 10.1126/science.1087117. [DOI] [PubMed] [Google Scholar]
- 41.Fenner BJ, Thiagarajan R, Chua HK, Kwang J. Betanodavirus b2 is an RNA interference antagonist that facilitates intracellular viral RNA accumulation. J. Virol. 2006;80:85–94. doi: 10.1128/JVI.80.1.85-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foster TM, Lough TJ, Emerson SJ, Lee RH, Bowman JL, et al. A surveillance system regulates selective entry of RNA into the shoot apex. Plant Cell. 2002;14:1497–1508. doi: 10.1105/tpc.001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasciolli V, Mallory AC, Bartel DP, Vaucheret H. Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing transacting siRNAs. Curr. Biol. 2005;15:1494–1500. doi: 10.1016/j.cub.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Guo HS, Ding SW. A viral protein inhibits the long range signaling activity of the gene silencing signal. EMBO J. 2002;21:398–407. doi: 10.1093/emboj/21.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton A, Voinnet O, Chappell L, Baulcombe D. Two classes of short interfering RNA in RNA silencing. EMBO J. 2002;21:4671–4679. doi: 10.1093/emboj/cdf464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- 47.Havelda Z, Hornyik C, Crescenzi A, Burgyan J. In situ characterization of Cymbidium Ringspot Tombusvirus infection-induced posttranscriptional gene silencing in Nicotiana benthamiana. J. Virol. 2003;77:6082–6086. doi: 10.1128/JVI.77.10.6082-6086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herr AJ, Jensen MB, Dalmay T, Baulcombe DC. RNA polymerase IV directs silencing of endogenous DNA. Science. 2005;308:118–120. doi: 10.1126/science.1106910. [DOI] [PubMed] [Google Scholar]
- 49.Himber C, Dunoyer P, Moissiard G, Ritzenthaler C, Voinnet O. Transitivity-dependent and -independent cell-to-cell movement of RNA silencing. EMBO J. 2003;22:4523–4533. doi: 10.1093/emboj/cdg431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- 51.Ip YT. Drosophila innate immunity goes viral. Nat. Immunol. 2005;6:863–864. doi: 10.1038/ni0905-863. [DOI] [PubMed] [Google Scholar]
- 52.Iwamoto T, Mise K, Takeda A, Okinaka Y, Mori K, et al. Characterization of Striped jack nervous necrosis virus subgenomic RNA3 and biological activities of its encoded protein B2. J. Gen. Virol. 2005;86:2807–2816. doi: 10.1099/vir.0.80902-0. [DOI] [PubMed] [Google Scholar]
- 53.Johansen LK, Carrington JC. Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiol. 2001;126:930–938. doi: 10.1104/pp.126.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 55.Kanno T, Huettel B, Mette MF, Aufsatz W, Jaligot E, et al. Atypical RNA polymerase subunits required for RNA-directed DNA methylation. Nat. Genet. 2005;37:761–765. doi: 10.1038/ng1580. [DOI] [PubMed] [Google Scholar]
- 56.Kasschau KD, Carrington JC. A counterdefensive strategy of plant viruses: suppression of posttranscriptional gene silencing. Cell. 1998;95:461–470. doi: 10.1016/s0092-8674(00)81614-1. [DOI] [PubMed] [Google Scholar]
- 57.Kasschau KD, Xie Z, Allen E, Llave C, Chapman EJ, et al. P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA function. Dev. Cell. 2003;4:205–217. doi: 10.1016/s1534-5807(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 58.Keese PK, Gibbs A. Origins of genes: “big bang” or continuous creation? Proc. Natl. Acad. Sci. USA. 1992;89:9489–9493. doi: 10.1073/pnas.89.20.9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kjemtrup S, Sampson KS, Peele CG, Nguyen LV, Conkling MA, et al. Gene silencing from plant DNA carried by a geminivirus. Plant J. 1998;14:91–100. doi: 10.1046/j.1365-313X.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- 60.Kreuze JF, Savenkov EI, Cuellar W, Li X, Valkonen JP. Viral class 1 RNase III involved in suppression of RNA silencing. J. Virol. 2005;79:7227–7238. doi: 10.1128/JVI.79.11.7227-7238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubota K, Tsuda S, Tamai A, Meshi T. Tomato mosaic virus replication protein suppresses virus-targeted posttranscriptional gene silencing. J. Virol. 2003;77:11016–11026. doi: 10.1128/JVI.77.20.11016-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumagai MH, Donson J, della-Cioppa G, Harvey D, Hanley K, Grill LK. Cytoplasmic inhibition of carotenoid biosynthesis with virus-derived RNA. Proc. Natl. Acad. Sci. USA. 1995;92:1679–1683. doi: 10.1073/pnas.92.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lakatos L, Szittya G, Silhavy D, Burgyan J. Molecular mechanism of RNA silencing suppression mediated by p19 protein of tombusviruses. EMBO J. 2004;23:876–884. doi: 10.1038/sj.emboj.7600096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lecellier CH, Dunoyer P, Arar K, Lehmann-Che J, Eyquem S, et al. A cellular microRNA mediates antiviral defense in human cells. Science. 2005;308:557–560. doi: 10.1126/science.1108784. [DOI] [PubMed] [Google Scholar]
- 65.Li H, Li WX, Ding SW. Induction and suppression of RNA silencing by an animal virus. Science. 2002;296:1319–1321. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- 66.Li HW, Ding SW. Antiviral silencing in animals. FEBS Lett. 2005;579:5965–5973. doi: 10.1016/j.febslet.2005.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li HW, Lucy AP, Guo HS, Li WX, Ji LH, et al. Strong host resistance targeted against a viral suppressor of the plant gene silencing defence mechanism. EMBO J. 1999;18:2683–2691. doi: 10.1093/emboj/18.10.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Yang Z, Yu B, Liu J, Chen X. Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr. Biol. 2005;15:1501–1507. doi: 10.1016/j.cub.2005.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li WX, Ding SW. Viral suppressors of RNA silencing. Curr. Opin. Biotechnol. 2001;12:150–154. doi: 10.1016/s0958-1669(00)00190-7. [DOI] [PubMed] [Google Scholar]
- 70.Li WX, Li H, Lu R, Li F, Dus M, et al. Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing. Proc. Natl. Acad. Sci. USA. 2004;101:1350–1355. doi: 10.1073/pnas.0308308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lichner Z, Silhavy D, Burgyan J. Double-stranded RNA-binding proteins could suppress RNA interference-mediated antiviral defences. J. Gen. Virol. 2003;84:975–980. doi: 10.1099/vir.0.18987-0. [DOI] [PubMed] [Google Scholar]
- 72.Deleted in proof
- 73.Lindbo JA, Silva-Rosales L, Proebsting WM, Dougherty WG. Induction of a highly specific antiviral state in transgenic plants: implications for regulation of gene expression and virus resistance. Plant Cell. 1993;5:1749–1759. doi: 10.1105/tpc.5.12.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lingel A, Simon B, Izaurralde E, Sattler M. The structure of the Flock house virus B2 protein, a viral suppressor of RNA interference, shows a novel mode of double-stranded RNA recognition. EMBO Rep. 2005;6:1149–1155. doi: 10.1038/sj.embor.7400583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu H, Reavy B, Swanson M, MacFarlane SA. Functional replacement of the tobacco rattle virus cysteine-rich protein by pathogenicity proteins from unrelated plant viruses. Virology. 2002;298:232–239. doi: 10.1006/viro.2002.1421. [DOI] [PubMed] [Google Scholar]
- 76.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 77.Liu J, Lynch PA, Chien CY, Montelione GT, Krug RM, Berman HM. Crystal structure of the unique RNA-binding domain of the influenza virus NS1 protein. Nat. Struct. Biol. 1997;4:896–899. doi: 10.1038/nsb1197-896. [DOI] [PubMed] [Google Scholar]
- 78.Liu L, Grainger J, Canizares MC, Angell SM, Lomonossoff GP. Cowpea mosaic virus RNA-1 acts as an amplicon whose effects can be counteracted by a RNA-2-encoded suppressor of silencing. Virology. 2004;323:37–48. doi: 10.1016/j.virol.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 79.Lu R, Folimonov A, Shintaku M, Li WX, Falk BW, et al. Three distinct suppressors of RNA silencing encoded by a 20-kb viral RNA genome. Proc. Natl. Acad. Sci. USA. 2004;101:15742–15747. doi: 10.1073/pnas.0404940101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu R, Maduro M, Li F, Li HW, Broitman-Maduro G, et al. Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature. 2005;436:1040–1043. doi: 10.1038/nature03870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu S, Cullen BR. Adenovirus VA1 noncoding RNA can inhibit small interfering RNA and microRNA biogenesis. J. Virol. 2004;78:12868–12876. doi: 10.1128/JVI.78.23.12868-12876.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mallory AC, Ely L, Smith TH, Marathe R, Anandalakshmi R, et al. HC-Pro suppression of transgene silencing eliminates the small RNAs but not transgene methylation or the mobile signal. Plant Cell. 2001;13:571–583. doi: 10.1105/tpc.13.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mallory AC, Reinhart BJ, Bartel D, Vance VB, Bowman LH. A viral suppressor of RNA silencing differentially regulates the accumulation of short interfering RNAs and micro-RNAs in tobacco. Proc. Natl. Acad. Sci. USA. 2002;99:15228–15233. doi: 10.1073/pnas.232434999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–620. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 85.Matzke MA, Primig M, Trnovsky J, Matzke AJM. Reversible methylation and inactivation of marker genes in sequentially transformed tobacco plants. EMBO J. 1989;8:643–649. doi: 10.1002/j.1460-2075.1989.tb03421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Merai Z, Kerenyi Z, Molnar A, Barta E, Valoczi A, et al. Aureusvirus P14 is an efficient RNA silencing suppressor that binds double-stranded RNAs without size specificity. J. Virol. 2005;79:7217–7226. doi: 10.1128/JVI.79.11.7217-7226.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miyoshi K, Tsukumo H, Nagami T, Siomi H, Siomi MC. Slicer function of Drosophila Argonautes and its involvement in RISC formation. Genes Dev. 2005;19:2837–2848. doi: 10.1101/gad.1370605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Molnar A, Csorba T, Lakatos L, Varallyay E, Lacomme C, Burgyan J. Plant virus-derived small interfering RNAs originate predominantly from highly structured single-stranded viral RNAs. J. Virol. 2005;79:7812–7818. doi: 10.1128/JVI.79.12.7812-7818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morel JB, Godon C, Mourrain P, Beclin C, Boutet S, et al. Fertile hypomorphic ARGONAUTE (ago1) mutants impaired in post-transcriptional gene silencing and virus resistance. Plant Cell. 2002;14:629–639. doi: 10.1105/tpc.010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mourrain P, Beclin C, Elmayan T, Feuerbach F, Godon C, et al. Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell. 2000;101:533–542. doi: 10.1016/s0092-8674(00)80863-6. [DOI] [PubMed] [Google Scholar]
- 91.Nanduri S, Carpick BW, Yang Y, Williams BR, Qin J. Structure of the double-stranded RNA-binding domain of the protein kinase PKR reveals the molecular basis of its dsRNA-mediated activation. EMBO J. 1998;17:5458–5465. doi: 10.1093/emboj/17.18.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Napoli C, Lemieux C, Jorgensen R. Introduction of a chimeric chalcone synthase gene into Petunia results in reversible co-suppression of homologous genes in trans. Plant Cell. 1990;2:279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Onodera Y, Haag JR, Ream T, Nunes PC, Pontes O, Pikaard CS. Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell. 2005;120:613–622. doi: 10.1016/j.cell.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 94.Palauqui JC, Elmayan T, Pollien JM, Vaucheret H. Systemic acquired silencing: transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J. 1997;16:4738–4745. doi: 10.1093/emboj/16.15.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peragine A, Yoshikawa M, Wu G, Albrecht HL, Poethig RS. SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of transacting siRNAs in Arabidopsis. Genes Dev. 2004;18:2368–2379. doi: 10.1101/gad.1231804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peremyslov VV, Hagiwara Y, Dolja VV. Genes required for replication of the 15.5-kilobase RNA genome of a plant closterovirus. J. Virol. 1998;72:5870–5876. doi: 10.1128/jvi.72.7.5870-5876.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pfeffer S, Dunoyer P, Heim F, Richards KE, Jonard G, Ziegler-Graff V. P0 of beet Western yellows virus is a suppressor of posttranscriptional gene silencing. J. Virol. 2002;76:6815–6824. doi: 10.1128/JVI.76.13.6815-6824.2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Deleted in proof
- 99.Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, et al. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- 100.Qi Y, Denli AM, Hannon GJ. Biochemical specialization within Arabidopsis RNA silencing pathways. Mol. Cell. 2005;19:421–428. doi: 10.1016/j.molcel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 101.Qi Y, Zhong X, Itaya A, Ding B. Dissecting RNA silencing in protoplasts uncovers novel effects of viral suppressors on the silencing pathway at the cellular level. Nucleic Acids Res. 2004;32:e179. doi: 10.1093/nar/gnh180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qu F, Ren T, Morris TJ. The coat protein of turnip crinkle virus suppresses posttranscriptional gene silencing at an early initiation step. J. Virol. 2003;77:511–522. doi: 10.1128/JVI.77.1.511-522.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rand TA, Petersen S, Du F, Wang X. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell. 2005;123:621–629. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 104.Ratcliff F, Bryan D, Baulcombe D. A similarity between viral defense and gene silencing in plants. Science. 1997;276:1558–1560. doi: 10.1126/science.276.5318.1558. [DOI] [PubMed] [Google Scholar]
- 105.Reavy B, Dawson S, Canto T, MacFarlane SA. Heterologous expression of plant virus genes that suppress post-transcriptional gene silencing results in suppression of RNA interference in Drosophila cells. BMC Biotechnol. 2004;4:18. doi: 10.1186/1472-6750-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reed JC, Kasschau KD, Prokhnevsky AI, Gopinath K, Pogue GP, et al. Suppressor of RNA silencing encoded by Beet yellows virus. Virology. 2003;306:203–209. doi: 10.1016/s0042-6822(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 107.Rocha PS, Sheikh M, Melchiorre R, Fagard M, Boutet S, et al. The Arabidopsis HOMOLOGY-DEPENDENT GENE SILENCING1 gene codes for an S-adenosyl-L-homocysteine hydrolase required for DNA methylation-dependent gene silencing. Plant Cell. 2005;17:404–417. doi: 10.1105/tpc.104.028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruiz MT, Voinnet O, Baulcombe DC. Initiation and maintenance of virus-induced gene silencing. Plant Cell. 1998;10:937–946. doi: 10.1105/tpc.10.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ryter JM, Schultz SC. Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J. 1998;17:7505–7513. doi: 10.1093/emboj/17.24.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schwach F, Vaistij FE, Jones L, Baulcombe DC. An RNA-dependent RNA polymerase prevents meristem invasion by potato virus X and is required for the activity but not the production of a systemic silencing signal. Plant Physiol. 2005;138:1842–1852. doi: 10.1104/pp.105.063537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, et al. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- 112.Silhavy D, Molnar A, Lucioli A, Szittya G, Hornyik C, et al. A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J. 2002;21:3070–3080. doi: 10.1093/emboj/cdf312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smardon A, Spoerke JM, Stacey SC, Klein ME, Mackin N, Maine EM. EGO-1 is related to RNA-directed RNA polymerase and functions in germ-line development and RNA interference in C. elegans. Curr. Biol. 2000;10:169–178. doi: 10.1016/s0960-9822(00)00323-7. [DOI] [PubMed] [Google Scholar]
- 114.Soldan SS, Plassmeyer ML, Matukonis MK, Gonzalez-Scarano F. La Crosse virus nonstructural protein NSs counteracts the effects of short interfering RNA. J. Virol. 2005;79:234–244. doi: 10.1128/JVI.79.1.234-244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 116.Sullivan CS, Ganem D. A virus-encoded inhibitor that blocks RNA interference in mammalian cells. J. Virol. 2005;79:7371–7379. doi: 10.1128/JVI.79.12.7371-7379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sullivan CS, Grundhoff AT, Tevethia S, Pipas JM, Ganem D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature. 2005;435:682–686. doi: 10.1038/nature03576. [DOI] [PubMed] [Google Scholar]
- 118.Szittya G, Molnar A, Silhavy D, Hornyik C, Burgyan J. Short defective interfering RNAs of tombusviruses are not targeted but trigger post-transcriptional gene silencing against their helper virus. Plant Cell. 2002;14:359–372. doi: 10.1105/tpc.010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deleted in proof
- 120.Takeda A, Sugiyama K, Nagano H, Mori M, Kaido M, et al. Identification of a novel RNA silencing suppressor, NSs protein of Tomato spotted wilt virus. FEBS Lett. 2002;532:75–79. doi: 10.1016/s0014-5793(02)03632-3. [DOI] [PubMed] [Google Scholar]
- 121.Takeda A, Tsukuda M, Mizumoto H, Okamoto K, Kaido M, et al. A plant RNA virus suppresses RNA silencing through viral RNA replication. EMBO J. 2005;24:3147–357. doi: 10.1038/sj.emboj.7600776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Te J, Melcher U, Howard A, Verchot-Lubicz J. Soilborne wheat mosaic virus (SBWMV) 19K protein belongs to a class of cysteine rich proteins that suppress RNA silencing. Virol J. 2005;2:18. doi: 10.1186/1743-422X-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tomari Y, Du T, Haley B, Schwarz DS, Bennett R, et al. RISC assembly defects in the Drosophila RNAi mutant armitage. Cell. 2004;116:831–841. doi: 10.1016/s0092-8674(04)00218-1. [DOI] [PubMed] [Google Scholar]
- 124.Tomari Y, Zamore PD. Perspective: machines for RNAi. Genes Dev. 2005;19:517–529. doi: 10.1101/gad.1284105. [DOI] [PubMed] [Google Scholar]
- 125.Trinks D, Rajeswaran R, Shivaprasad PV, Akbergenov R, Oakeley EJ, et al. Suppression of RNA silencing by a geminivirus nuclear protein, AC2, correlates with transactivation of host genes. J. Virol. 2005;79:2517–2527. doi: 10.1128/JVI.79.4.2517-2527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vaistij FE, Jones L, Baulcombe DC. Spreading of RNA targeting and DNA methylation in RNA silencing requires transcription of the target gene and a putative RNA-dependent RNA polymerase. Plant Cell. 2002;14:857–867. doi: 10.1105/tpc.010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van WR, Dong X, Liu H, Tien P, Stanley J, Hong Y. Mutation of three cysteine residues in Tomato yellow leaf curl virus-China C2 protein causes dysfunction in pathogenesis and posttranscriptional gene-silencing suppression. Mol. Plant Microbe Interact. 2002;15:203–208. doi: 10.1094/MPMI.2002.15.3.203. [DOI] [PubMed] [Google Scholar]
- 128.Vanitharani R, Chellappan P, Fauquet CM. Short interfering RNA-mediated interference of gene expression and viral DNA accumulation in cultured plant cells. Proc. Natl. Acad. Sci. USA. 2003;100:9632–9636. doi: 10.1073/pnas.1733874100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vanitharani R, Chellappan P, Pita JS, Fauquet CM. Differential roles of AC2 and AC4 of cassava geminiviruses in mediating synergism and suppression of posttranscriptional gene silencing. J. Virol. 2004;78:9487–9498. doi: 10.1128/JVI.78.17.9487-9498.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vargason JM, Szittya G, Burgyan J, Tanaka Hall TM. Size selective recognition of siRNA by an RNA silencing suppressor. Cell. 2003;115:799–811. doi: 10.1016/s0092-8674(03)00984-x. [DOI] [PubMed] [Google Scholar]
- 131.Vazquez F, Vaucheret H, Rajagopalan R, Lepers C, Gasciolli V, et al. Endogenous transacting siRNAs regulate the accumulation of Arabidopsis mRNAs. Mol. Cell. 2004;16:69–79. doi: 10.1016/j.molcel.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 132.Voinnet O. Induction and suppression of RNA silencing: insights from viral infections. Nat. Rev. Genet. 2005;6:206–220. doi: 10.1038/nrg1555. [DOI] [PubMed] [Google Scholar]
- 133.Voinnet O. Non-cell autonomous RNA silencing. FEBS Lett. 2005;579:5858–5871. doi: 10.1016/j.febslet.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 134.Voinnet O, Lederer C, Baulcombe DC. A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell. 2000;103:157–167. doi: 10.1016/s0092-8674(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 135.Voinnet O, Pinto YM, Baulcombe DC. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc. Natl. Acad. Sci. USA. 1999;96:14147–14152. doi: 10.1073/pnas.96.24.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang H, Buckley KJ, Yang X, Buchmann RC, Bisaro DM. Adenosine kinase inhibition and suppression of RNA silencing by geminivirus AL2 and L2 proteins. J. Virol. 2005;79:7410–7418. doi: 10.1128/JVI.79.12.7410-7418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang W,RiedelK, LynchP,Chien CY, Montelione GT, Krug RM. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA. 1999;5:195–205. doi: 10.1017/s1355838299981621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang XH, Aliyari R, Li WX, Li HW, Kim K, et al. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wilkins C, Dishongh R, Moore SC, Whitt MA, Chow M, Machaca K. RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature. 2005;436:1044–1047. doi: 10.1038/nature03957. [DOI] [PubMed] [Google Scholar]
- 140.Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002;295:2456–2459. doi: 10.1126/science.1068836. [DOI] [PubMed] [Google Scholar]
- 141.Xie Z, Allen E, Wilken A, Carrington JC. DICER-LIKE4functions in transacting small interfering RNA biogenesis and vegetative phase change in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2005;102:12984–12989. doi: 10.1073/pnas.0506426102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xie Z, Fan B, Chen C, Chen Z. An important role of an inducible RNA-dependent RNA polymerase in plant antiviral defense. Proc. Natl. Acad. Sci. USA. 2001;98:6516–6521. doi: 10.1073/pnas.111440998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, et al. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004;2:E104. doi: 10.1371/journal.pbio.0020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xie Z, Kasschau KD, Carrington JC. Negative feedback regulation of Dicer-Like1 in Arabidopsis by microRNA-guided mRNA degradation. Curr. Biol. 2003;13:784–789. doi: 10.1016/s0960-9822(03)00281-1. [DOI] [PubMed] [Google Scholar]
- 145.Ye K, Malinina L, Patel DJ. Recognition of small interfering RNA by a viral suppressor of RNA silencing. Nature. 2003;426:874–878. doi: 10.1038/nature02213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ye K, Patel DJ. RNA silencing suppressor p21 of Beet yellows virus forms an RNA binding octameric ring structure. Structure. 2005;13:1375–1384. doi: 10.1016/j.str.2005.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yelina NE, Savenkov EI, Solovyev AG, Morozov SY, Valkonen JP. Long-distance movement, virulence, and RNA silencing suppression controlled by a single protein in hordei- and potyviruses: complementary functions between virus families. J. Virol. 2002;76:12981–12991. doi: 10.1128/JVI.76.24.12981-12991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yoo BC, Kragler F, Varkonyi-Gasic E, Haywood V, Archer-Evans S, et al. A systemic small RNA signaling system in plants. Plant Cell. 2004;16:1979–2000. doi: 10.1105/tpc.104.023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yoshikawa M, Peragine A, Park MY, Poethig RS. A pathway for the biogenesis of transacting siRNAs in Arabidopsis. Genes Dev. 2005;19:2164–2175. doi: 10.1101/gad.1352605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu B, Yang Z, Li J, Minakhina S, Yang M, et al. Methylation as a crucial step in plant microRNA biogenesis. Science. 2005;307:932–935. doi: 10.1126/science.1107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yue Z, Shatkin AJ. Double-stranded RNA-dependent protein kinase (PKR) is regulated by reovirus structural proteins. Virology. 1997;234:364–371. doi: 10.1006/viro.1997.8664. [DOI] [PubMed] [Google Scholar]
- 152.Zilberman D, Cao X, Jacobsen SE. ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science. 2003;299:716–719. doi: 10.1126/science.1079695. [DOI] [PubMed] [Google Scholar]