Abstract
Exposure to polychlorinated biphenyls impairs cognition and behavior in ‘children. Two environmental PCBs 2,2′3,3′4,4′5-heptachlorobiphenyl (PCB170) and 2,2′3,5′6-pentachlorobiphenyl (PCB95) were examined in vitro for influences on synaptic transmission in rat hippocampal slices. Field excitatory postsynaptic potentials (fEPSPs) were recorded in the CA1 region using a multi-electrode array. Perfusion with PCB170 (10 nM) had no effect on fEPSP slope relative to baseline period, whereas (100 nM) initially enhanced then depressed fEPSP slope. Perfusion of PCB95 (10 or 100 nM) persistently enhanced fEPSP slope >200%, an effect that could be inhibited by dantrolene, a drug that attenuates ryanodine receptor signaling. Perfusion with picrotoxin (PTX) to block GABA neurotransmission resulted in a modest increase in fEPSP slope, whereas PTX+PCB170 (1–100 nM) persistently enhanced fEPSP slope in a dose dependent manner. fEPSP slope reached >250% of baseline period in the presence of PTX+100 nM PCB170, conditions that evoked marked epileptiform after-potential discharges. PCB95 and PCB170 were found to differentially influence the Ca2+-dependence of [3H]ryanodine-binding to hippocampal ryanodine receptors. Non-coplanar PCB congeners can differentially alter neurotransmission in a manner suggesting they can elicit imbalances between inhibitory and excitatory circuits within the hippocampus. Differential sensitization of ryanodine receptors by Ca2+ appears to mediate, at least in part, hippocampal excitotoxicity by non-coplanar PCBs.
Keywords: non-coplanar PCBs, hippocampus, ryanodine receptor, neurotransmission
Introduction
Following extensive industrial use and chemical stability in the environment, PCBs have tended to bio-accumulate in biota. Despite their ban over 30 years ago, PCBs have been identified in nearly one third of the sites listed in National Priorities List (NPL), a list associated with Superfund Sites (US Environmental Protection Agency, 2007). Results from epidemiological studies performed on populations in the United States and several European countries have indicated that PCBs continue to threaten human health. Such studies have shown that levels of exposure to PCBs are positively correlated with a decreased IQ scores, impaired learning and memory, decreased neuromuscular function, and lower reading comprehension (Chen et al., 1992; Jacobson and Jacobson, 1996; Koopman-Esseboom et al., 1996; Despres et al., 2005; Carpenter, 2006; Roegge and Schantz, 2006). A recent report also identified an association between perinatal exposure to PCBs and impaired immune responses to childhood vaccinations (Heilmann et al., 2006).
Although environmental levels of PCBs have steadily declined since they were banned in most industrialized countries, ortho-rich PCBs (congeners with 2 to 4 ortho chlorine atoms) remain prevalent in environmental reservoirs and in human samples (Frame et al., 1996; Hansen, 1998; Frame, 1999; Humphrey et al., 2000). The relative abundance of ortho-rich PCBs in air has been documented due to their greater volatility (Hornbuckle, 1995; Cousins et al., 1996; Miller et al., 2001; Wethington and Hornbuckle, 2005). Fish from contaminated waters are enriched in ortho-PCBs due to differential metabolism (Porte and Albaiges, 1994). Elevated levels of ortho-substituted PCB congeners are found in both the tissues of Great Lakes fish and serum of humans consuming these fish (Gerstenberger et al., 1997; Gerstenberger et al., 2000; Gerstenberger and Dellinger, 2002). Anaerobic meta/para dechlorination of highly chlorinated PCB congeners in sediments has been suggested to contribute to the propagation of ortho-rich PCB congeners along the food chain, and enriching their levels in human serum (Gerstenberger et al., 2000; Bedard et al., 2005).
Although ortho-substituted PCBs possess little or no activity toward the aryl hydrocarbon hydroxylase receptor (AhR), their neurotoxic properties have been well documented in a number of in vivo and in vitro studies (Maier et al., 1994; Schantz et al., 1997; Tilson et al., 1998; Kodavanti and Tilson, 2000). Non-coplanar PCB’s can decrease catecholamine levels in certain brain regions (Seegal et al., 1990; Seegal et al., 1991a; Seegal et al., 1991b). The ability of non-coplanar PCBs to reduce dopamine levels has also been measured in cultured rat pheochromocytoma cells (PC12 cells) (Shain et al., 1991). Perinatal exposure to PCB95 (2,2′,3,5′6-pentahlorobiphenyl) and the commercial mixture Aroclor 1254, in which non-coplanar congeners are more abundant, were shown to persistently alter excitability and LTP in rat hippocampal slices in vitro (Wong et al., 1997b; Gilbert and Crofton, 1999; Altmann et al., 2001; Gilbert, 2003; Ozcan et al., 2004). The mechanisms underlying the neurotoxic effects of non-coplanar PCBs are only partially understood. However, three principal mechanisms currently under study include (1) disruption of thyroid hormone and other endocrine functions due to their structural similarity with thyroid hormone (Zoeller and Crofton, 2000; Zoeller, 2005), (2) direct interference with dopamine transport (Mariussen and Fonnum, 2001), and (3) altered Ca2+ signaling in neurons and other cells types (Wong et al., 2001; Knerr and Schrenk, 2006; Mariussen and Fonnum, 2006). Molecular targets of non-coplanar PCBs include the family of microsomal Ca2+ release channels termed ryanodine receptors (RyRs) and Inositol 1,4,5-trisphosphate receptors (IP3Rs) that are expressed in most types of cells including neurons (Pessah and Wong 2001; Inglefield et al., 2001). Neurons depend on Ca2+ signals for early differentiation and migration (Spitzer, 1994; Komuro and Kumada, 2005), growth and elaboration of dendritic arbors (Lohmann and Wong, 2005), axonal guidance (Gomez and Zheng, 2006), synaptic plasticity (Brose et al., 1992; Zucker, 1999; Zucker and Regehr, 2002), maintenance of the cytoskeleton (Trifaro and Vitale, 1993; Gomez and Zheng, 2006), and rates of gene expression (Trifaro and Vitale, 1993; Santella and Carafoli, 1997; Gomez and Zheng, 2006). The contribution of RyRs and IP3Rs towards shaping the localized and global Ca2+ signals necessary for orchestrating these processes has been recently reviewed (Berridge, 2006). Therefore, abnormalities in the spatial and temporal properties of Ca2+ signals can lead to persistent developmental neurotoxicity, neurodegeneration and epileptogenesis (Arundine and Tymianski, 2003; DeLorenzo et al., 2005).
Of particular relevance for understanding PCB neurotoxicity is the fact that RyRs are present in the soma as well as pre- and post-synaptic terminals of neurons (Llano et al., 2000; Shimuta et al., 2001; Martin and Buno, 2003). The three RyR isoforms exist differentially between brain regions, among neuronal cell types and within neuronal processes (De Crescenzo et al., 2004; Berridge, 2006). RyR-mediated signals influence neuronal excitability and regulate synaptic plasticity by regulating Ca2+ signals that induce and maintain LTP and LTD (Shimuta et al., 2001; Conti et al., 2004; Collin et al., 2005; Raymond and Redman, 2006). Specifically, it is likely that the activation of RyR might induce an imbalance in synaptic transmission based on these studies. A recent microdialysis study demonstrated that local application of ryanodine to rat hippocampus in situ altered the balance between glutamatergic and GABAergic transmission (Mori et al., 2005), and developmental exposure of PCB95 which is the most potent PCB congener affecting RyRs function (Pessah et al., 2006) significantly disrupted the excitation and inhibition ratio in developing rat auditory cortex (Kenet et al., 2007). This imbalance could contribute importantly to PCB neurotoxicity.
The possible contributions of low levels of PCBs known to sensitize RyRs, and to the balance of inhibitory and excitatory synaptic activity have not been explored. In the present study we measured fEPSPs from the CA1 region of hippocampal slices perfused with nanomolar levels of PCB170 or PCB95. The new findings in this study are that: (1) PCBs congeners can produce distinct temporal changes in the pattern of hippocampal excitability, (2) PCB170 and PCB95 differentially altered the regulation of hippocampal RyRs by Ca2+, (3) PCB95 enhancement of fEPSP slopes was completely inhibited by dantrolene, an inhibitor of RyR signaling, and (4) GABAA receptor block with picrotoxin (PTX) unmasks pure excitatory actions of PCB170. These results show that specific non-coplanar PCB congeners can differentially alter the excitability of hippocampus in vitro and in a manner that can be synergized by an imbalance of inhibitory and excitatory neurotransmission. Thus, RyR dysfunction appears to contribute to the excitotoxicity of non-coplaner PCBs.
Material and methods
Chemicals
PCB95, 2,2′3,5′6-pentachlorobiphenyl and PCB170, 2,2′3,3′4,4′5-heptachlorobiphenyl, (Fig. 1A) (AccuStandard Inc., New Haven, CT) were dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 50~100 μM. Aliquots from these stock solutions were dissolved in artificial cerebrospinal fluid (aCSF) for perfusion of hippocampal slices. Picrotoxin (PTX) and dantrolene were purchased from Sigma-Aldrich (St. Louis, MO). PTX was dissolved in aCSF and dantrolene was dissolved in water (w/v) and diluted in aCSF as described below for use in hippocampal slices. [3H]Ryanodine ([3H]Ry; 50 or 56 Ci/mmol) was purchased from Perkin-Elmer, Wilmington, DE and stored in 50% ethanol at −20°C until needed. All other chemicals were obtained at the highest purity commercially available.
Figure 1. Hippocampal slice recordings on Med-64 multielectrode array.
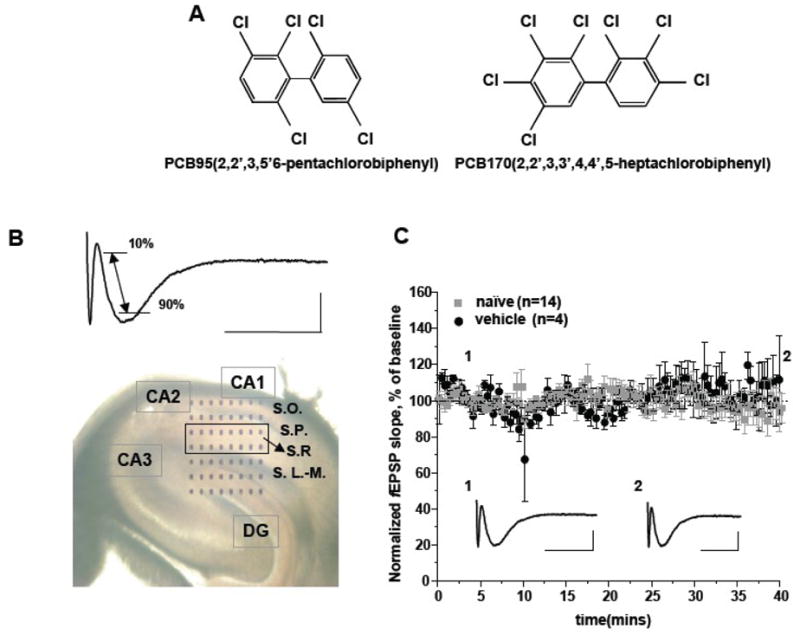
A. The structures of the ortho-substituted PCB95 and PCB170 used in this study. B. Med-64 multielectrode array positioned on a hippocampal slice (upper panel) and the lower panel depicts a typical waveform indicating the location where fEPSP slope was measured (see text). Abbreviation: CA, Cornu Ammon; DG, Dentate Gyrus; S.O., Stratum Oriens; S.T., Stratum Pyramidale; S.R., Stratum Radiatum (the region indicated by the box) ; S. L.-M.; Stratum Lucidum-Moleculare. C. Normalized fEPSP slopes recorded in stratum radiatum (S.R.) in hippocampal slices isolated from mice in naïve (n=14) and vehicle treated (DMSO, n=4) groups. Representative traces and slope of fEPSP are shown below their respective normalized data at the times indicated by the numbers (1&2). Scale: 200 μV, 10 ms.
Hippocampal slice preparation
Male Spraque-Dawley (SD) rats (150 ~200 g, Charles River Laboratories, Inc. Wilmington, MA) were decapitated by guillotine under an ALAAC approved animal protocol. Whole brain was rapidly removed from the cranium and placed for 30 sec in aCSF at 5°C containing (in mM) NaCl (124), KCl (3), NaH2PO4 (1.25), MgCl2 (1.3), NaHCO3 (26), glucose (10), CaCl2 (2.5) and bubbled with 95 % O2/5 % CO2. The brain was trimmed to expose the hippocampus, affixed to the stage of a vibrating blade microtome (Model VT1000S; Leica, Bannockburn, IL) and cut horizontally at 350 μm and maintained in cold aCSF. Three to four slices from the medial hippocampus were transferred to a recovery chamber supplied with humidified 95 % O2/5 % CO2 aCSF for 1 hr. Individual hippocampal slices were then positioned on a MED64 (8×8, 150 μm inter-electrode spacing) microelectrode array (Alpha MED Science, Berkeley, CA) with the aid of an inverted microscope (Nikon, Melville, NY) equipped with a 4X phase lens. The recording of evoked fEPSPs was performed in temperature-controlled incubator set to maintain a constant 35 °C at the slice-aCSF interface. During the electrophysiological recording, the slice was perfused with oxygenated 35 °C aCSF at a rate of 2~3 ml/min using microinjection pump (Harvard apparatus, Holliston, MA). Test compounds (PCB, PTX, dantrolene) or solvent controls were added directly into the aCSF at the time indicated in individual figures. The volumes of vehicle or solutions of test compounds added to the aCSF never exceeded 0.05 %.
Hippocampal slice electrophysiology
The relative position of the MED64 electrodes to CA1 was used as a reference to identify the stimulus electrode for each slice (see Fig. 1B upper panel). Although the MED64 system permits recording of extracellular field potentials from any region immediately above the electrode array, we measured fEPSPs using one of the 16 electrodes within the stratum radiatum (Fig. 1B; boxed region labeled S.R.) of CA1 of the hippocampal slice. The stimulating electrode was chosen based on the magnitude of the fEPSPs response detected by the electrode immediately adjacent to the stimulus electrode along the Schaffer-Collateral/Commissural pathway. Single-pulse, biphasic stimuli (10~80 μA, 0.1 ms) were delivered to Schaffer-Collateral/Commissural pathway at 0.05 Hz (i.e., once every 20 sec). The evoked field excitatory postsynaptic potentials (fEPSPs) were sampled at a 20 kHz using a MED 64 multi-channel amplifier, digitized and graphically displayed using Performer® software (Tensor biosciences, Irvine, CA). Baseline fEPSPs were experimentally set between 50~60 % of maximum of amplitude for each slice and the slope of each fEPSP was calculated and displayed by Performer® software. The fEPSP slope was measured using 7 data points between 10% and the 90% of maximum amplitude of the fEPSP waveform (Fig. 1B, lower panel) using the equation:
Where SN=slope (μV/msec)), R=reponse (μV), t=time(msec)
Slices that exceeded more than 20% fluctuation during the stabilization period (at least 30 min) were discarded. Once the slice was stabilized, fEPSPs were collected for an additional 10 min period in the absence of test compound to establish a baseline control (i.e., 100% baseline). Subsequent fEPSPs collected after exposure to test compounds were normalized to this baseline period for analysis and presentation of data. Perfusion with vehicle or test compound dissolved in aCSF was initiated at the completion of baseline period and the effects on fEPSP slope were recorded for a minimum of 30~40 additional min depending on experimental design. For each experimental condition, 4 to 14 slices were recorded and the mean change fEPSP slope (normalized to each slice’s baseline period) calculated and statistically analyzed as described below.
Rat hippocampus membrane preparation
To study how PCB95 and PCB170 differentially influenced the sensitivity of RyRs to activation by Ca2+, a whole particulate membrane fraction was isolated from hippocampi dissected from naïve (untreated) male rats (~200 g). Hippocampi were dissected and homogenized in 10-fold (w/v) ice-cold buffer containing (in mM) sucrose (300), EGTA (2), phenylmethylsulfonyl fluoride (0.1), HEPES (20) pH 7.4, and 10 μg/ml leupeptin. Tissue was homogenized with 3 × 30 sec bursts from a Powergen 700 D homogenizer (Fisher Scientific, Pittsburgh, PA) set at 20,000 rpm. Homogenates were transferred to polycarbonate tubes, placed in a Ti 50 rotor and centrifuged in a Beckman L7-55 ultracentrifuge (Beckman Instrument, Inc. Fullerton, CA) at 110, 000 × g for 1 hr. The membrane pellets were collected and re-suspended using a glass dounce homogenizer (2~4 strokes) in 300 mM sucrose, 20 mM HEPES, pH 7.4 at a protein concentration of 4–9 mg/ml determined by the BCA protein assay (Bio-Rad, Hercules, CA). The whole membrane homogenates were rapidly frozen with liquid N2 and stored at −80 °C until assayed.
Hippocampal [3H]Ry-binding sites
The binding of 5 nM [3H]Ry to high affinity sites on hippocampal membrane protein (200 μg) was measured in 500 μl buffer consisting of 250 mM KCl, 15 mM NaCl, 10% sucrose, 20 mM HEPES pH 7.4. Under these conditions [3H]Ry binds to the open state of the channel and is therefore a measure of the relative degree of receptor activation resulting from physiological and non-physiological ligands added to the assay buffer (Pessah et al., 1987; Zimanyi and Pessah, 1991). In order to measure the influence of PCB95 and PCB170 on the Ca2+-dependence of [3H]Ry-binding to hippocampal RyR, free Ca2+ was adjusted in the assay buffer to 100 nM, 10 μM, or 100 mM using EGTA/CaCl2 in a ratio calculated by the software Bound And Determined (Brooks and Storey, 1992). Nonspecific binding was measured in the presence of 1000-fold excess unlabelled ryanodine and averaged 42–50 % of total binding across the experimental conditions use in this study. All binding reactions were incubated for 16 hr at 25 °C. Bound radioligand was separated from free by rapid filtration through Whatman GF/B glass fiber filters using a Brandell cell harvester (Model M-24, Brandell. Corp., Gaithersburg, MD). Filters were rinsed twice with 4 ml ice-cold harvest buffer containing (in mM) NaCl (15), KCl (140), CaCl2 (0.1), HEPES (20) pH 7.4, placed into vials and 5 ml of scintillation cocktail added (Ready Safe; Beckman Instrument, Inc., Fullerton, CA). After overnight incubation at room temperature, tritium emissions were counted by liquid scintillation with a Beckman LS 6500 liquid scintillation counter. Specific binding was calculated by subtracting total binding from non-specific binding, and was converted into pmol/mg [3H]Ry bound.
Statistical analysis
Electrophysiological results from the hippocampal slice experiments were represented as the mean changes in the normalized slope of fEPSP±standard error (S.E.) calculated from n=4~14 slices per condition, each prepared from a different animals. Statistical differences between groups were tested by one-way ANOVA with Bonferroni post hoc test (GraphPad Prism; version 5.0) at p≤0.05 or p≤ 0.01. For the binding analysis with [3H]Ry, differences in mean specific binding levels among assay conditions were tested for statistical significance using a one-way ANOVA.
Results
PCB95 and PCB170 elicit different temporal patterns of synaptic excitability
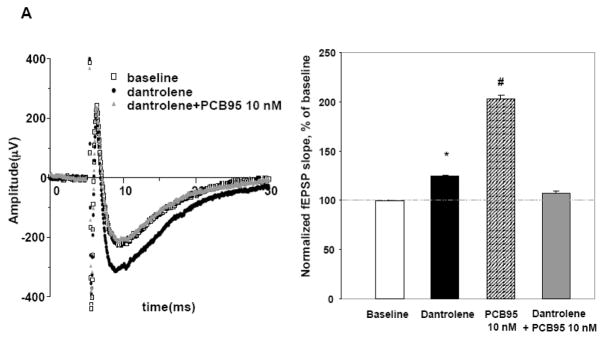
PCB170 and PCB95 (Fig. 1A), two non-coplanar PCB congeners detected in human tissues, were tested for their ability to elicit changes in synaptic transmission in the CA1 region of rat hippocampal slices. Single pulse stimuli were applied to Schaffer-Collateral/Commissural pathway in CA1 region, and the slope of fEPSP was measured near the striatum radiatum (Fig. 1B). After a 10 min recording to establish a baseline period, the slice was perfused for 30 min (test period) with vehicle (DMSO, 0.025% v/v) or test compound (PCB170 or PCB95) dissolved in aCSF. Perfusion of the slice with vehicle did not significantly alter fEPSP slope (n=4 slices) compared to that measured in hippocampal slices isolated from naïve rats (Fig. 1C). In contrast, perfusion with 100 nM of PCB170 altered synaptic transmission in a time-dependent manner during the treatment period (Fig. 2A). As shown in Figure 2B, 100 nM of PCB170 first significantly enhanced excitability by 145±14 % (averaged fEPSP slope between 18~20 min, p<0.05), followed by a statistically significant depression of fEPSP slope to 82±9 % (averaged fEPSP slope between 35~40 min, p<0.05) compared to baseline period. Perfusion of a ten-fold lower concentration (10 nM) of PCB170 in aCSF neither enhanced nor depressed the fEPSP slope significantly during test period (Fig. 2C). We then tested PCB95 which, based on a recent structure-activity study, was predicted to be significantly more potent than PCB170 in sensitizing RyRs to activation (Pessah et al., 2006). PCB95 (10 nM) facilitated synaptic transmission 207±16 % (averaged fEPSP slope between 45~50 min, p<0.01), and unlike PCB170 this facilitation persisted for the duration of test period (Fig. 2D). PCB95, 100nM in aCSF, also facilitated synaptic transmission to a level similar to that measured in the presence of 10 nM (Supplemental Fig. 1). We next tested the possibility that the synaptic facilitation produced by PCB95 in CA1 was due to activity at the ryanodine receptor (RyR) by first introducing dantrolene to the slice to attenuate RyR-mediated signaling in hippocampus (Lin et al., 2007). This was done by recording baseline synaptic activity (baseline period), followed by an additional 30 min recording in the presence of dantrolene (30 μM) in the aCSF. Subsequently 10 nM PCB95 was perfused with the aCSF in the presence of dantrolene for an additional 30 min to determine if dantrolene blocked PCB95-induced facilitation of the fEPSP. We used a 30 μM concentration of dantrolene because it has been shown to protect cells from kainate-induced apoptosis in vivo and in vitro (Popescu et al., 2002). Interestingly, dantrolene produced a small (125±3 %) but significant (p<0.05) increase in fEPSP slope compared to the slope measured during the respective baseline period (Fig. 3A). These effects of dantrolene were previously reported, even in the presence of PTX, and were associated with neuronal depolarization (Krnjevic and Xu, 1996). The addition of 10 nM PCB95 in the presence of dantrolene did not result in any further enhancement of the fEPSP slope. This is in contrast to the large enhancement of the fEPSP in the presence of PCB95 alone seen in Figure 2C. In fact, dantrolene pretreatment in the presence of PCB95 resulted in a gradual restoration of the fEPSP slope to that measured in baseline control period. Figure 3B presents summarized data normalized to the maximum increase in the mean fEPSP slopes measured during baseline period, in the presence of dantrolene alone, 10 nM of PCB95, and in the presence of dantrolene+PCB95. Collectively these results indicate that ortho-substituted PCB congeners confer different temporal patterns of enhancing and/or depressing synaptic transmission depending on the degree and position of chlorination. We therefore examined the possible contribution of PCB-induced changes in RyR activity that could contribute to the differential patterns of effects on CA1 excitability.
Figure 2. Normalized fEPSP slope reveals differential time-dependent alternations in hippocampus CA1 excitability by PCB170 and PCB95.
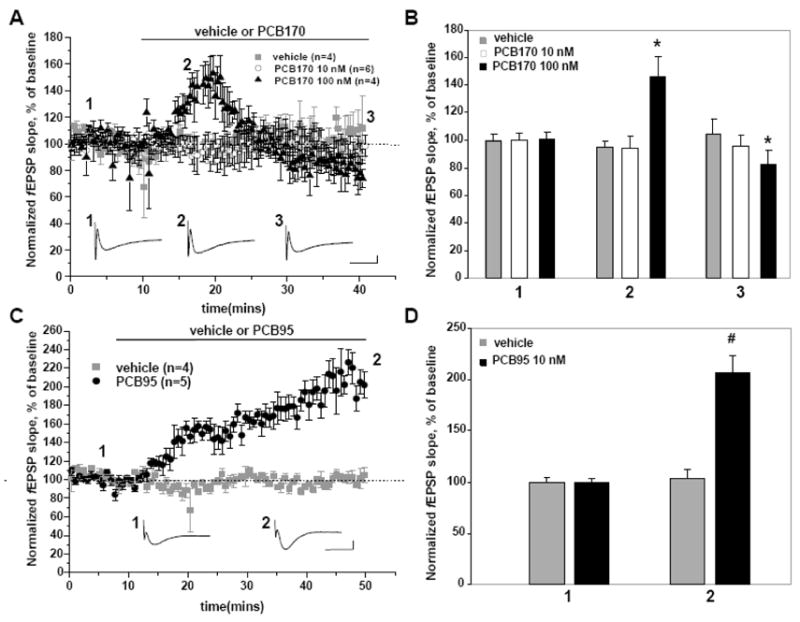
A. Temporal changes in normalized fEPSP slope when PCB170 is applied to aCSF at a final concentration of 100 nM (n=4) or 10 nM (n=6). Compared to vehicle control (DMSO), 100 nM of PCB 170 alters synaptic transmission in a time-dependent manner; first enhancing excitability (145±14%; *p<0.05 baseline period vs. test period measured between 18~20 min) followed by subsequent depression (82±9%; *p<0.05 baseline period vs. test period measured between 35–40min) of fEPSP slope. Horizontal arrow represents introduction of PCB170 and vehicle. B. Bar graph was shown that the mean + S.E. of averaged fEPSP slope, % of baseline with vehicle control and PCB170 treatment during the specific time frame (1: baseline period, 2: 18~20 min, 3: 35~40 min) C. PCB95 (n=5) enhanced synaptic excitability (202±16%, #p<0.01). D. Bar graph was shown that the mean+S.E. was clearly indicated that PCB95 (10 nM) enhanced the fEPSP slope for the duration of the experiment (1: baseline period, 2: 45~50 min). Representative traces of fEPSP before and after perfusion with 100 nM PCB170 or 10 nM PCB95 are shown below their respective averaged responses and their corresponding location indicated by numbers (1,2&3 for panel A, B; 1&2 for panel C, D). Scale: 200 μV, 10 ms.
Figure 3. Dantrolene prevents synaptic facilitation produced by 10 nM PCB95.
A. Sample traces of fEPSPs for vehicle control slices, after perfusion of dantrolene (30 μM) alone, and after dantrolene and 10 nM PCB95. B. Summarized data presented as the mean+S.E. of normalized fEPSP slopes for baseline, dantrolene alone, 10 nM of PCB95 and dantrolene+PCB95 in combination. Dantrolene alone caused a modest yet significant enhanced fEPSP slope compared control (*p<0.05), but prevented the synaptic excitation of PCB95 seen in experiments in the absence of dantrolene (e.g. Figure 2C). Dantrolene in the presence of PCB95 also restored fEPSP slope to the same level (p=0.3) as that measured during baseline period.
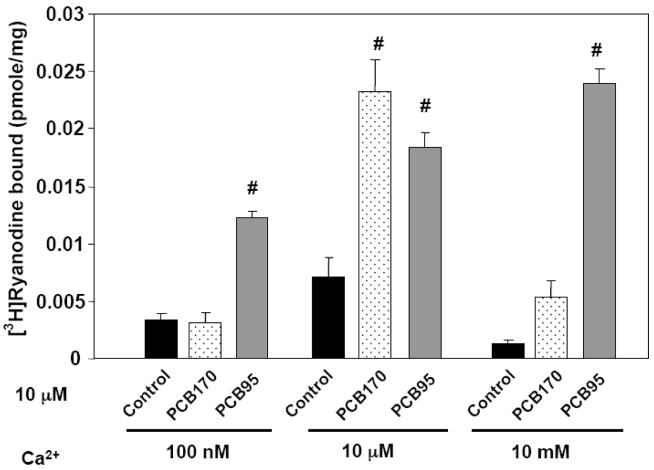
PCB95 and PCB170 differentially modulate the Ca2+-dependence of hippocampal RyRs
Ca2+ itself is an important physiological regulator of RyR activity, either enhancing or inhibiting activity depending on its concentration near the cytoplasmic domains of the channel complex (Pessah et al., 1987; Fill and Copello, 2002; Meissner, 2002; Bouchard et al., 2003). Generally, Ca2+ concentrations between 0.1 and ~100 μM enhance the activity of RyR channels and increase specific binding of [3H]Ry, whereas Ca2+ concentrations above 300 μM can inhibit RyRs. In order to test whether PCB95 and PCB170 differently alter the Ca2+-dependence of RyRs present in membrane preparations from rat hippocampus, we examined [3H]Ry-binding in the presence of the two congeners. Results summarized in Figure 4 showed that PCB95 and PCB170 differed in their ability to enhance specific [3H]Ry-binding to hippocampal RyRs when assayed at three Ca2+ concentrations; suboptimal (Ca2+ = 100 nM), optimal (Ca2+ = 10 μM), or inhibitory (Ca2+ = 10 mM). In Figure 4 specific [3H]Ry-binding was found optimal in the presence of 10 μM Ca2+ and significantly lower at 100 nM and 10 mM Ca2+. The presence of PCB95 at a maximal concentration (10 μM) for enhanced [3H]Ry-binding under all three Ca2+ conditions (suboptimal, optimal and inhibitory). By contrast PCB170 (10 μM) significantly enhanced channel activity only under the optimal Ca2+ assay condition (i.e., 10 μM Ca2+; Zimanyi and Pessah, 1991), whereas it had no measurable effect at sensitizing the channel at 100 nM Ca2+. PCB170 was also significantly less effective than PCB95 at negating the inhibition of the channel by excess (10 mM) Ca2+ compared to the vehicle control (Fig. 4).
Figure 4. PCB170 and PCB95 differentially alter the Ca2+ dependence of [3H]Ry binding top hippocampal RyRs.
The effect of a saturating concentration (10 μM) PCB170 or PCB95 on the Ca2+-dependence of high-affinity binding of [3H]Ry in a membrane fraction isolated from rat hippocampi. PCB95 increased binding under all free Ca2+ concentrations tested, whereas PCB170 only increased binding in the presence of 10 μM Ca2+. #p<0.01
The net effect of PCB170 on CA1 excitability depends on inhibitory inputs
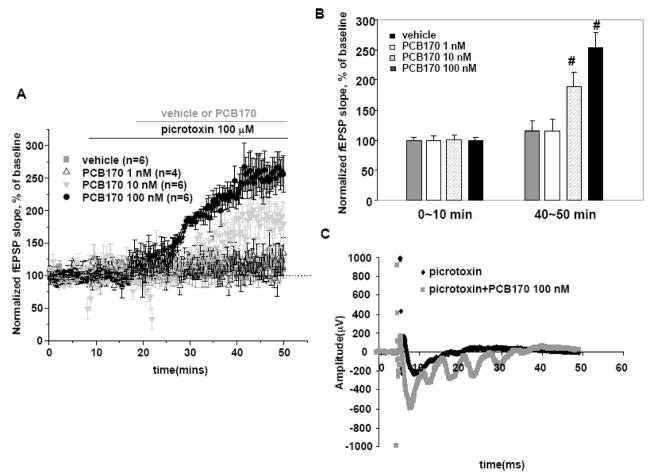
The strength of synaptic transmission within the stratum radiatum of CA1 is the result of summation from excitatory and inhibitory inputs (Andersen, 1990), and RyRs are known to functionally contribute to both inhibitory and excitatory synaptic inputs (Llano et al., 2000; Emptage et al., 2001; Liang et al., 2002). Therefore we tested the hypothesis that the transient nature of CA1 synaptic facilitation produced by PCB170 in the slice preparation (Fig. 2A) may, at least in part be the result of enhanced synaptic inhibition. Following a baseline recording period, we examined the effects of pretreatment with the GABAA receptor blocker picrotoxin (PTX; 100 μM) on effects of 1, 10 or 100 nM PCB 170 on synaptic transmission in CA1. PTX was perfused in aCSF following the baseline recording period. The initial slope of the fEPSP with single pulse stimuli at CA1 hippocampal slice was largely unaffected by blocking inhibitory synaptic activity with PTX (Wigstrom and Gustafsson, 1985; Pananceau et al., 1997). Figure 5A shows that in our single stimulus pulse protocol, PTX alone slightly enhanced the mean fEPSP slope measured between 45 and 50 min of recording (115±15 % of baseline period; p<0.05). Inclusion of 100 nM PCB170 in the aCSF perfusion containing PTX produced a significant and persistent increase in fEPSP slope (p<0.01) reaching 253±25 % at maximum facilitation (Fig. 5A and B). Importantly the block of GABAA transmission in CA1 with PTX also amplified the effects of PCB170 resulting in measurable synaptic facilitation in the range of 1~100 nM. For example 10 nM PCB170 enhanced fEPSP slope to 188±23 % of baseline period (p<0.01) after 45~50 minutes. The fEPSP waveforms measured in the presence of PCB170 and picrotoxin exhibited more prominent after-discharges (epileptic waveforms) than waveforms measured in the presence of PTX alone (Fig. 5C). Epileptic waveforms possessing prominent after discharges were also seen after slices were exposed to PTX + 10 nM PCB95 (data are not shown), although the presence of PTX did not further increase fEPSP slope. These data indicate that the net patterns with which non-coplanar PCB congeners alter synaptic strength in vitro depends on their structure and may reflect their relative influence on both excitatory and inhibitory synaptic activity.
Figure 5. Picrotoxin (PTX) unmasks excitatory actions of PCB 170.
A. PTX (100 μM) was used to block GABAA receptors resulting in modest enhancement of fEPSP using a single pulse protocol (115±15% of baseline period; P<0.05). Inclusion of 100 nM PCB170 in the aCSF perfusate resulted in a 253±25% enhancement in excitability relative to baseline period. Black arrow at top indicates period of PTX application and gray arrow indicates the period of PCBC170 application. B. Bar graph summarizes the mean fEPSP slope measured between specific time frame (1: baseline period, 2: 45~50 min) after adding vehicle (n=6) or PCB170 (1 nM, n=4; 10 nM, n=6; 100 nM, n=4) to the perfusate. PCB170 at 10 and 100 nM significantly enhanced the fEPSP slope compared to vehicle control (#p<0.01). C. Representative traces showing fEPSPs measured with PTX alone or with PTX and PCB170 (100 nM) perfused in combination 50 min after initiating recording. PTX+PCB170 induced a more pronounced epileptiform after-discharge in the compared to PTX alone.
Discussion
Most of the animal research on the developmental neurotoxicity of PCBs has been performed with complex mixtures that were originally manufactured such as Aroclor 1254. Developmental A1254 exposure altered the dendritic morphology of cerebellar Purkinje cells and neocortical pyramidal neurons, promoting dendritic growth in untrained animals but attenuating or reversing experience-dependent dendritic growth in maze-trained littermates. These structural changes coincided with altered patterns of RyR expression (Yang et al., 2009). Less is known about the possible potential for, or cellular mechanisms of developmental neurotoxicity of specific PCB congeners. In the present study we explored how acute exposure to very low concentrations (1–100 nM) of the non-coplanar PCB95 and PCB170 alters synaptic strength in the rat hippocampal slice preparation. The major conclusions from the data are: (1) Individual PCB congeners can induce different temporal patterns of synaptic excitability in CA1. (2) These changes correlate with differences in the ability of PCB’s to alter the Ca2+ dependence for activating and inhibiting RyRs. (3) The patterns of PCB activity in CA1 appear to be the sum of their ability to modulate excitation and inhibition within hippocampal circuits. Specifically, block of GABAA receptors with PTX unmasked the excitatory actions of PCB170, greatly amplifying its excitotoxicity, and generating epileptiform after-potentials in the fEPSP.
The results of the present study in combination with previous research indicate that non-coplanar PCBs mediate acute toxicity through their ability to alter the fidelity of intracellular Ca2+ signaling in a broad range of cell types. Specifically non-coplanar PCBs have been shown to potently alter the function of ryanodine receptors (RyRs) and inositol 1,4,5-triposphate receptors (IP3Rs) that regulate the release of Ca2+ from endoplasmic reticulum (ER)/junctional ER stores (Wong and Pessah, 1996; Wong et al., 1997a; Inglefield et al., 2001; Crofton and Zoeller, 2005). Non-coplanar PCBs sensitize RyRs by stabilizing a conformation that promotes the high-affinity binding of [3H]Ry, and by sensitizing Ca2+ release from isolated brain microsomes (Wong et al., 1997a). The sensitizing effect of non-coplanar PCBs requires an intact immunophilin FK506 binding protein 12-ryanodine receptor complex (FKBP12-RyR complex) which is critical for normal functioning of the Ca2+ release channel (Wong et al., 2001). Structure-activity studies indicate that the ortho- and meta-chlorine substitutions (2,3,6-Cl) on the biphenyl are the most important determinants to sensitize the activity of the FKBP12-RyR channel complex (Pessah et al., 2006).
In the present study two ortho-substituted PCBs (PCB95 and PCB170) were used to test our hypothesis that in addition to non-coplanarity, additional structural factors contribute to the complex patterns of synaptic facilitation or inhibition in vitro that appear to be at least partially mediated by RyR function. The physicochemical properties of these PCBs differ. PCB170 is significantly more lipophilic than PCB95 (log P = 8.27 vs. 6.55). It is unlikely that the differential activities of PCB95 and PCB170 are simply related to their ability to partition in the lipid phase of the slices. Moreover differences in partitioning cannot explain the fact that PCB170 displays significantly enhanced activity at 10nM in the presence of PTX. A more likely explanation for the patterns of excitability observed is that PCB170 and PCB95 differentially alter the balance of excitation and inhibition within the hippocampal circuitry in a manner dependent on how they influence Ca2+ regulation of RyRs within these circuits.
In support of our interpretation, the activity ofRyRs has been shown to affect several aspects of neuronal excitability and synaptic plasticity (Llano et al., 2000; Narita et al., 2000; Shimuta et al., 2001; Bardo et al., 2002; Vigh and Lasater, 2003; De Crescenzo et al., 2004; Duguid and Smart, 2004; Kravchenko et al., 2004; Collin et al., 2005). RyRs are expressed in most brain regions, and specific RyR isoforms appear to have distinct distributions (Sharp et al., 1993) where they are believed contribute essential aspects of presynaptic and postsynaptic neurotransmission of both excitatory and inhibitory circuits (Nishiyama et al., 2000; Collin et al., 2005). For example, Ca2+ release from ryanodine-sensitive stores within hippocampal circuitry contributes to both NMDAR-LTD and mGluR-LTD. At γ-amino butyric acid (GABA) synapses of the rat hippocampus, long-term depression (LTD) depends on activation of both presynaptic and postsynaptic calcium stores (Caillard et al 2000). An NMDA-dependent form of LTD triggered by prolonged low frequency stimuli is dependent on presynaptic RyRs. In fact the calcium signal provided by RyRs appears to be indispensable for LTD, and RyRs could be viewed as action potential integrators (Collin et al., 2005). Postsynaptic RyR activation also plays a critical role in the priming of LTP by Group 1 mGluRs (Mellentin et al., 2007). RyRs expressed within dendritic spines and dendrites of hippocampus also contribute to their morphology and plasticity (Yuste and Denk, 1995; Segal et al., 2000; Balkowiec and Katz, 2002; Raymond and Redman, 2006).
The observations from our study with PCB95 and PCB170 further define the role of RyRs in the neurotoxicity of PCBs. Of particular relevance to interpreting results with PCB95 and PCB170 is that pharmacological interventions known to enhance RyR activity were shown to increase both GABA and glutamate release in a biphasic and dose-dependent manner within rat hippocampus (Mori et al., 2005). In fact, modulation of RyRs with ryanodine produced an imbalance between GABAergic and glutamatergic neurotransmission that mirrored the relative concentrations of GABA and glutamate within the hippocampus measured using microdialysis. Therefore, the different temporal patterns of excitability observed with PCB95 and PCB170 in our electrophysiological experiments may be due to their differential action on RyRs that mirrors their net influence on evoked release of excitatory and inhibitory neurotransmitters and sensitization of their respective signaling pathways.
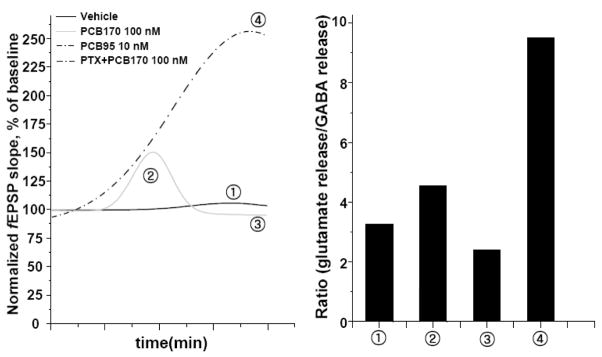
Figure 6 is designed to model the overall pattern of our electrophysiological results with PCB95 and PCB170 in Figures 2 and 5. The solid grey line shows baseline activity ➀, followed by the biphasic excitatory ➁ and inhibitory ➂ activity produced by PCB170 (100 nM) alone. This biphasic pattern converts to monophasic excitatory activity, ➁ and ➂ (black dashed line) when GABAergic transmission is blocked by PTX. In contrast, PCB95 (10 or 100 nM) shows only monophasic excitatory activity ➁ & ➂ (grey dashed line). This pattern of results suggests that the balance of inhibitory/excitatory activity due to PCB170 eventually favors inhibition, and that block of GABAergic inhibition unmasks its excitotoxic activity that parallels that seen with PCB95. These results may relate to the microdialysis results of Mori and coworkers who examined the effects of ryanodine on K+- stimulated GABA and glutamate release in hippocampal brain slices. They found that the peak ryanodine concentration that enhanced GABA release (10 nM) was lower than that for glutamate release (100 nM), with higher ryanodine concentrations inhibiting GABA and glutamate release (Mori et al., 2005). Their results indicate that ryanodine activation of RyR’s in the hippocampus stimulates both GABA (inhibitory) and glutamate (excitatory) release, with the balance determined by ryanodine concentration. Relating their results to the present results, it is likely that the biphasic effects of PCB170 were due to an initial imbalance between inhibitory (e.g., GABAergic) and excitatory (e.g., glutamatergic) activity, initially favoring excitation followed later by inhibition. Blocking the GABAergic component would then shift the balance to excitation, as seen in our results with the combination of PCB170 and PTX. In contrast, the monophasic excitatory activity seen with 10 nM PCB95 indicates that the balance of excitatory (e.g., glutamatergic) to inhibitory (GABAergic) activity is towards excitation compared to PCB170. The fact that PCB95 and PCB170 appear to have distinctly different impacts on the Ca2+ dependence of RyR activity are also likely to contribute to their differential patterns on CA1 excitability.
Figure 6. Possible relationship between patterns of changes in normalized fEPSP slope induced by PCB95 and PCB170 and respective changes in the ratio of electrically evoked excitation and inhibition in the hippocampal slice.
The proposed model to interpret our findings is based on the results of Mori and colleagues who directly the how microperfusion of ryanodine into the rat hippocampus influenced the relative levels of glutamate and GABA using microdialysis (Mori et al., 2005). See text for detailed discussion of the model.
Our laboratory previously reported that perfusion of PCB95 depressed synaptic transmission in rat hippocampal slices (Wong et al. 1997b), whereas enhanced excitability by PCB95 was observed in the present study. Several important experimental differences are likely to explain the different results between the studies. Most important is the difference in PCB95 concentrations used (10 μM vs. 10 nM) in the respective studies. Other important differences include the Mg2+ concentration (2 mM vs. 1.3 mM) in the aCSF, and the type of stimulus protocol used (paired vs. single pulse) between studies. Previous results have demonstrated that PCB95 significantly amplifies NMDA and AMPA-induced Ca2+ signals in cultured granule neurons from cerebellum (Gafni et al., 2004). Therefore, the relatively lower Mg2+ concentrations used in the present study might have enhanced NMDA signaling and therefore selectively promoted PCB95 activity at excitatory synapses.
The nanomolar activities of PCB170 and PCB95 reported in the current study, especially in the presence of a blocker of fast GABAA mediated inhibition, and are closely related with PCB mediated toxicity described in several studies. Developmental exposure to PCB95 has previously been shown to persistently alter patterns of specific [3H]Ry binding sites in hippocampus that were associated with locomotor and spatial learning deficits (Schantz et al., 1997). The same perinatal PCB95 exposure procedures were recently found to disrupt the topographic organization of the primary auditory cortex (A1) in rats without measurable hearing loss (Kenet et al., 2007). The ratio of neuronal inhibition to excitation (i.e., IPSC vs. EPSC) for A1 was reduced, consistent with our current electrophysiological findings with PCB95 in the hippocampal slice. PCB170 is among the most abundant PCB congeners found in human tissue (Hany et al., 1999; DeCaprio et al., 2005; Jaraczewska et al., 2006; Jursa et al., 2006). Historically, the presence of PCB95 was not detected or reported in human and environmental samples. However with improved analytical capabilities recent studies have detected this congener in several human tissues (Covaci et al., 2002; Chu et al., 2003; DeCaprio et al., 2005; Jursa et al., 2006). This is important because ongoing environmental exposure to PCB95 may also be more significant than previously thought as it represents 3–4% of the total PCB burden in from San Francisco Bay sediments (Hwang et al., 2006), and has been detected in indoor air, top soil, grass, diets and human feces (Robson and Harrad, 2004; Harrad et al., 2006). Collectively PCBs including PCB95 and PCB170, with the highest activity towards RyRs represent a major proportion (40–50%) of total PCBs currently found in environmental and biotic samples and their net effects are likely to be additive (Pessah et al., 2006).
In conclusion, PCB95 and PCB170 differentially regulate the synaptic activity of hippocampal CA1 and alter [Ca2+]i homeostasis through activity at the RyR. Should our results extend to other non-coplanar PCBs, this raises the intriguing question as to whether individuals with heritable deficits in GABAergic signaling might represent especially susceptible populations to PCB exposure. For example, some patients afflicted with childhood “absence seizure” and “febrile seizure” possess a mutation of GABAA beta2 receptor subunit (Wallace et al., 2001; Sperk et al., 2004; Audenaert et al., 2006). Intersetingly, our current data also indicate that PCB and PTX in combination produced epileptogenic fEPSP waveforms with pronounced after potentials in CA1. Additional research will be needed to clarify the relationship between developmental exposure to non-coplanar PCBs and seizure susceptibility through RyRs activation. Another example can be drawn from autistic children, who have been hypothesized to possess an increased ratio of excitatory/inhibitory neurotransmission that stems from a complex combination of genetic and environmental factors (Rubenstein and Merzenich, 2003). This hypothesis was recently supported by reports of a significant deficiency in the expression of the GABAA beta3 receptor subunit (Samaco et al., 2005) and several GABAA receptor polymorphisms in autism (Buxbaum et al., 2002; Tuchman and Rapin, 2002; Ma et al., 2005; Collins et al., 2006; Kim et al., 2007). The current results suggest that populations with heritable imbalances in neurotransmitter systems that regulate the ratio of inhibition and excitation in the brain may be especially susceptible to the toxicity of non-coplanar PCBs.
Supplementary Material
No significant difference in fEPSP slope was observed with perfusion of PCB95 at 10 nM (n=5) or 100nM (n=6) for 45~60 min.
Acknowledgments
Supported by NIEHS 1P01 ES11269 and 1R01 ES014901, the U.S. Environmental Protection Agency (U.S. EPA) through the Science to Achieve Results (STAR) program (Grant R829388), the UC Davis M.I.N.D. Institute, and the Superfund Basic Research Program (P42 ES04699).
Abbreviation used
- PCBs
Polychlorinated biphenyls
- fEPSPs
Field excitatory postsynaptic potentials
- CA
Cornu Ammon
- S.R
Striatum radiatum
- aCSF
artificial cerebral spinal fluid
- RyR
Ryanodine receptor
- PTX
Picrotoxin
- GD
Gestation day
- PND
Postnatal day
- AhR
Aryl hydrocarbon hydroxylase receptor
- IP3Rs
Inositol 1,4,5-trisphosphate receptors
- [3H]Ry
[3H]Ryanodine
- DMSO
Dimethyl Sulfoxide
- SD
Spraque-Dawley
- FKBP12
12 KDa FK506-binding protein
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NMDA
N-methyl-D-aspartic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann L, Mundy WR, Ward TR, Fastabend A, Lilienthal H. Developmental exposure of rats to a reconstituted PCB mixture or aroclor 1254: effects on long-term potentiation and [3H]MK-801 binding in occipital cortex and hippocampus. Toxicol Sci. 2001;61:321–330. doi: 10.1093/toxsci/61.2.321. [DOI] [PubMed] [Google Scholar]
- Andersen P. Synaptic integration in hippocampal CA1 pyramids. Prog Brain Res. 1990;83:215–222. doi: 10.1016/s0079-6123(08)61251-0. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Audenaert D, Van Broeckhoven C, De Jonghe P. Genes and loci involved in febrile seizures and related epilepsy syndromes. Hum Mutat. 2006;27:391–401. doi: 10.1002/humu.20279. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo S, Robertson B, Stephens GJ. Presynaptic internal Ca2+ stores contribute to inhibitory neurotransmitter release onto mouse cerebellar Purkinje cells. Br J Pharmacol. 2002;137:529–537. doi: 10.1038/sj.bjp.0704901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard DL, Pohl EA, Bailey JJ, Murphy A. Characterization of the PCB substrate range of microbial dechlorination process LP. Environ Sci Technol. 2005;39:6831–6838. doi: 10.1021/es050255i. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Bouchard R, Pattarini R, Geiger JD. Presence and functional significance of presynaptic ryanodine receptors. Prog Neurobiol. 2003;69:391–418. doi: 10.1016/s0301-0082(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Brooks SP, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal Biochem. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- Brose N, Petrenko AG, Sudhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, Cook EH, Jr, Fang Y, Song CY, Vitale R. Association between a GABRB3 polymorphism and autism. Mol Psychiatry. 2002;7:311–316. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- Caillard O, Ben-Ari Y, Gaïarsa JL. Activation of presynaptic and postsynaptic ryanodine-sensitive calcium stores is required for the induction of long-term depression at GABAergic synapses in the neonatal rat hippocampus. J Neurosci. 2000;20(RC94) doi: 10.1523/JNEUROSCI.20-17-j0002.2000. 1of 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 2006;21:1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- Chen YC, Guo YL, Hsu CC. Cognitive development of children prenatally exposed to polychlorinated biphenyls (Yu-Cheng children) and their siblings. J Formos Med Assoc. 1992;91:704–707. [PubMed] [Google Scholar]
- Chu S, Covaci A, Schepens P. Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ Res. 2003;93:167–176. doi: 10.1016/s0013-9351(03)00016-1. [DOI] [PubMed] [Google Scholar]
- Collin T, Marty A, Llano I. Presynaptic calcium stores and synaptic transmission. Curr Opin Neurobiol. 2005;15:275–281. doi: 10.1016/j.conb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Collins AL, Ma D, Whitehead PL, Martin ER, Wright HH, Abramson RK, Hussman JP, Haines JL, Cuccaro ML, Gilbert JR, Pericak-Vance MA. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics. 2006;7:167–174. doi: 10.1007/s10048-006-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti R, Tan YP, Llano I. Action potential-evoked and ryanodine-sensitive spontaneous Ca2+ transients at the presynaptic terminal of a developing CNS inhibitory synapse. J Neurosci. 2004;24:6946–6957. doi: 10.1523/JNEUROSCI.1397-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins IT, Hartlieb N, Teichmann C, Jones KC. Volatilization of polychlorinated biphenyls from sludge-amended soils. Organohal Comp. 1996;28:58–63. [Google Scholar]
- Covaci A, de Boer J, Ryan JJ, Voorspoels S, Schepens P. Distribution of organobrominated and organochlorinated contaminants in Belgian human adipose tissue. Environ Res. 2002;88:210–218. doi: 10.1006/enrs.2002.4334. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Zoeller RT. Mode of action: neurotoxicity induced by thyroid hormone disruption during development--hearing loss resulting from exposure to PHAHs. Crit Rev Toxicol. 2005;35:757–769. doi: 10.1080/10408440591007304. [DOI] [PubMed] [Google Scholar]
- De Crescenzo V, ZhuGe R, Velazquez-Marrero C, Lifshitz LM, Custer E, Carmichael J, Lai FA, Tuft RA, Fogarty KE, Lemos JR, Walsh JV., Jr Ca2+ syntillas, miniature Ca2+ release events in terminals of hypothalamic neurons, are increased in frequency by depolarization in the absence of Ca2+ influx. J Neurosci. 2004;24:1226–1235. doi: 10.1523/JNEUROSCI.4286-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio AP, Johnson GW, Tarbell AM, Carpenter DO, Chiarenzelli JR, Morse GS, Santiago-Rivera AL, Schymura MJ. Polychlorinated biphenyl (PCB) exposure assessment by multivariate statistical analysis of serum congener profiles in an adult Native American population. Environ Res. 2005;98:284–302. doi: 10.1016/j.envres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: The calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol Ther. 2005;105:229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres C, Beuter A, Richer F, Poitras K, Veilleux A, Ayotte P, Dewailly E, Saint-Amour D, Muckle G. Neuromotor functions in Inuit preschool children exposed to Pb, PCBs, and Hg. Neurotoxicol Teratol. 2005;27:245–257. doi: 10.1016/j.ntt.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Duguid IC, Smart TG. Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron-Purkinje cell synapses. Nat Neurosci. 2004;7:525–533. doi: 10.1038/nn1227. [DOI] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine Receptor Calcium Release Channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Frame GM. Improved procedure for single DB-XLB column GC-MS-SIM quantitation of polychlorinated biphenyl congeners distributions and characterization of two different preparations sold as Aroclor 1254. J High Resolute Chromatogr. 1999;22:533–540. [Google Scholar]
- Frame GM, Cochran JW, Bowadt SS. Complete PCB congener distributions for 17 aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. J High Resolute Chromatogr. 1996;19:657–668. [Google Scholar]
- Gafni J, Wong PW, Pessah IN. Non-coplanar 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) amplifies ionotropic glutamate receptor signaling in embryonic cerebellar granule neurons by a mechanism involving ryanodine receptors. Toxicol Sci. 2004;77:72–82. doi: 10.1093/toxsci/kfh004. [DOI] [PubMed] [Google Scholar]
- Gerstenberger SL, Dellinger JA. PCBs, mercury, and organochlorine concentrations in lake trout, walleye, and whitefish from selected tribal fisheries in the Upper Great Lakes region. Environ Toxicol. 2002;17:513–519. doi: 10.1002/tox.10092. [DOI] [PubMed] [Google Scholar]
- Gerstenberger SL, Dellinger JA, Hansen LG. Concentrations and frequencies of polychlorinated biphenyl congeners in a Native American population that consumes Great Lakes fish. J Toxicol Clin Toxicol. 2000;38:729–746. doi: 10.1081/clt-100102386. [DOI] [PubMed] [Google Scholar]
- Gerstenberger SL, Tavris DR, Hansen LK, Pratt-Shelley J, Dellinger JA. Concentrations of blood and hair mercury and serum PCBs in an Ojibwa population that consumes Great Lakes region fish. J Toxicol Clin Toxicol. 1997;35:377–386. doi: 10.3109/15563659709043370. [DOI] [PubMed] [Google Scholar]
- Gilbert ME. Perinatal exposure to polychlorinated biphenyls alters excitatory synaptic transmission and short-term plasticity in the hippocampus of the adult rat. Neurotoxicol Teratol. 2003;24:851–860. doi: 10.1016/S0161-813X(03)00073-1. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Crofton KM. Developmental exposure to a commercial PCB mixture (Aroclor 1254) produces a persistent impairment in long-term potentiation in the rat dentate gyrus in vivo. Brain Res. 1999;850:87–95. doi: 10.1016/s0006-8993(99)02107-1. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- Hansen LG. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect. 1998;106(Suppl 1):171–189. doi: 10.1289/ehp.98106s1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hany J, Lilienthal H, Sarasin A, Roth-Harer A, Fastabend A, Dunemann L, Lichtensteiger W, Winneke G. Developmental exposure of rats to a reconstituted PCB mixture or aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol Appl Pharmacol. 1999;158:231–243. doi: 10.1006/taap.1999.8710. [DOI] [PubMed] [Google Scholar]
- Harrad S, Ren J, Hazrati S, Robson M. Chiral signatures of PCB#s 95 and 149 in indoor air, grass, duplicate diets and human faeces. Chemosphere. 2006;63:1368–1376. doi: 10.1016/j.chemosphere.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Grandjean P, Weihe P, Nielsen F, Budtz-Jorgensen E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006;3:e311. doi: 10.1371/journal.pmed.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbuckle KC, Clyde W, Pearson RF, Swackhamer DL, Eisenreich SJ. Assessing annual water-air fluxes of polychlorinated biphenyls in Lake Michigan. Environ Sci Technol. 1995;29:869–877. doi: 10.1021/es00004a006. [DOI] [PubMed] [Google Scholar]
- Humphrey HE, Gardiner JC, Pandya JR, Sweeney AM, Gasior DM, McCaffrey RJ, Schantz SL. PCB congener profile in the serum of humans consuming Great Lakes fish. Environ Health Perspect. 2000;108:167–172. doi: 10.1289/ehp.00108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HM, Green PG, Young TM. Tidal salt marsh sediment in California, USA. Part 1: occurrence and sources of organic contaminants. Chemosphere. 2006;64:1383–1392. doi: 10.1016/j.chemosphere.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Inglefield JR, Mundy WR, Shafer TJ. Inositol 1,4,5-triphosphate receptor-sensitive Ca2+ release, store-operated Ca2+ entry, and cAMP responsive element binding protein phosphorylation in developing cortical cells following exposure to polychlorinated biphenyls. J Pharmacol Exp Ther. 2001;297:762–773. [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Dose-response in perinatal exposure to polychlorinated biphenyls (PCBs): the Michigan and North Carolina cohort studies. Toxicol Ind Health. 1996;12:435–445. doi: 10.1177/074823379601200315. [DOI] [PubMed] [Google Scholar]
- Jaraczewska K, Lulek J, Covaci A, Voorspoels S, Kaluba-Skotarczak A, Drews K, Schepens P. Distribution of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers in human umbilical cord serum, maternal serum and milk from Wielkopolska region, Poland. Sci Total Environ. 2006;372:20–31. doi: 10.1016/j.scitotenv.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Jursa S, Chovancova J, Petrik J, Loksa J. Dioxin-like and non-dioxin-like PCBs in human serum of Slovak population. Chemosphere. 2006;64:686–691. doi: 10.1016/j.chemosphere.2005.10.048. [DOI] [PubMed] [Google Scholar]
- Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc Natl Acad Sci U S A. 2007;104:7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SA, Kim JH, Park M, Cho IH, Yoo HJ. Association of GABRB3 Polymorphisms with Autism Spectrum Disorders in Korean Trios. Neuropsychobiology. 2007;54:160–165. doi: 10.1159/000098651. [DOI] [PubMed] [Google Scholar]
- Knerr S, Schrenk D. Carcinogenicity of “non-dioxinlike” polychlorinated biphenyls. Crit Rev Toxicol. 2006;36:663–694. doi: 10.1080/10408440600845304. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Tilson HA. Neurochemical effects of environmental chemicals: in vitro and in vivo correlations on second messenger pathways. Ann N Y Acad Sci. 2000;919:97–105. doi: 10.1111/j.1749-6632.2000.tb06872.x. [DOI] [PubMed] [Google Scholar]
- Komuro H, Kumada T. Ca2+ transients control CNS neuronal migration. Cell Calcium. 2005;37:387–393. doi: 10.1016/j.ceca.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Koopman-Esseboom C, Weisglas-Kuperus N, de Ridder MA, Van der Paauw CG, Tuinstra LG, Sauer PJ. Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants’ mental and psychomotor development. Pediatrics. 1996;97:700–706. [PubMed] [Google Scholar]
- Kravchenko MO, Moskalyuk AO, Kolodin YO, Veselovsky NS, Fedulova SA. Activation of ryanodine receptors influences the paired-pulse depression in cultured rat hippocampal neurons. Fiziol Zh. 2004;50:50–56. [PubMed] [Google Scholar]
- Krnjevic K, Xu YZ. Dantrolene depolarizes hippocampal neurons in slices from rats. Can J Physiol Pharmacol. 1996;74:241–250. [PubMed] [Google Scholar]
- Liang Y, Yuan LL, Johnston D, Gray R. Calcium signaling at single mossy fiber presynaptic terminals in the rat hippocampus. J Neurophysiol. 2002;87:1132–1137. doi: 10.1152/jn.00661.2001. [DOI] [PubMed] [Google Scholar]
- Lin F, Xin Y, Wang J, Ma L, Liu J, Liu C, Long L, Wang F, Jin Y, Zhou J, Chen J. Puerarin facilitates Ca2+-induced Ca2+ release triggered by KCl-depolarization in primary cultured rat hippocampal neurons. Eur J Pharmacol. 2007;570:43–49. doi: 10.1016/j.ejphar.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Wong RO. Regulation of dendritic growth and plasticity by local and global calcium dynamics. Cell Calcium. 2005;37:403–409. doi: 10.1016/j.ceca.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, Ritchie MD, Delong GR, Abramson RK, Wright HH, Cuccaro ML, Hussman JP, Gilbert JR, Pericak-Vance MA. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet. 2005;77:377–388. doi: 10.1086/433195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier WE, Kodavanti PR, Harry GJ, Tilson HA. Sensitivity of adenosine triphosphatases in different brain regions to polychlorinated biphenyl congeners. J Appl Toxicol. 1994;14:225–229. doi: 10.1002/jat.2550140313. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology. 2001;159:11–21. doi: 10.1016/s0300-483x(00)00374-7. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. Neurochemical targets and behavioral effects of organohalogen compounds: an update. Crit Rev Toxicol. 2006;36:253–289. doi: 10.1080/10408440500534164. [DOI] [PubMed] [Google Scholar]
- Martin ED, Buno W. Caffeine-mediated presynaptic long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2003;89:3029–3038. doi: 10.1152/jn.00601.2002. [DOI] [PubMed] [Google Scholar]
- Meissner G. Regulation of mammalian ryanodine receptors. Front Biosci. 2002;7:d2072–2080. doi: 10.2741/A899. [DOI] [PubMed] [Google Scholar]
- Mellentine C, Jahnsen H, Abraham WC. Priming of long-term potentiation mediated by ryanodine receptor activation in rat hippocampal slices. Neuropharmacology. 2007;52:118–125. doi: 10.1016/j.neuropharm.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Miller SM, Green ML, Depinto JV, Hornbuckle KC. Results from the Lake Michigan Mass Balance study: concentrations and fluxes of atmospheric polychlorinated biphenyls and trans-nonachlor. Environ Sci Technol. 2001;35:278–285. doi: 10.1021/es991463b. [DOI] [PubMed] [Google Scholar]
- Mori F, Okada M, Tomiyama M, Kaneko S, Wakabayashi K. Effects of ryanodine receptor activation on neurotransmitter release and neuronal cell death following kainic acid-induced status epilepticus. Epilepsy Res. 2005;65:59–70. doi: 10.1016/j.eplepsyres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Narita K, Akita T, Hachisuka J, Huang S, Ochi K, Kuba K. Functional coupling of Ca2+ channels to ryanodine receptors at presynaptic terminals. Amplification of exocytosis and plasticity. J Gen Physiol. 2000;115:519–532. doi: 10.1085/jgp.115.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, Hong K, Mikoshiba K, Poo M-m, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- Ozcan M, Yilmaz B, King WM, Carpenter DO. Hippocampal long-term potentiation (LTP) is reduced by a coplanar PCB congener. Neurotoxicology. 2004;25:981–988. doi: 10.1016/j.neuro.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Pananceau M, Chen HX, Gustafsson B. Long-term potentiation induced by single volley activation: a mechanism for bicuculline-induced enhancement of synaptic field potentials in the CA1 hippocampal region. Neuroscience. 1997;79:95–101. doi: 10.1016/s0306-4522(96)00672-0. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW. Structure-Activity Relationship for Noncoplanar Polychlorinated Biphenyl Congeners toward the Ryanodine Receptor-Ca2+ Channel Complex Type 1 (RyR1) Chem Res Toxicol. 2006;19:92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Stambuk RA, Casida JE. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol Pharmacol. 1987;31:232–238. [PubMed] [Google Scholar]
- Pessah IN, Wong PW. Etiology of PCB Neurotoxicity: From nolecules to cellular dysfunction. In: Robertson L, Hansen L, editors. Progress In Polychlorinated Biphenyl Toxicology. New York, NY: Academic Press; 2001. pp. 179–184. [Google Scholar]
- Popescu BO, Oprica M, Sajin M, Stanciu CL, Bajenaru O, Predescu A, Vidulescu C, Popescu LM. Dantrolene protects neurons against kainic acid induced apoptosis in vitro and in vivo. J Cell Mol Med. 2002;6:555–569. doi: 10.1111/j.1582-4934.2002.tb00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte C, Albaiges J. Bioaccumulation patterns of hydrocarbons and polychlorinated biphenyls in bivalves, crustaceans, and fishes. Arch Environ Contam Toxicol. 1994;26:273–281. doi: 10.1007/BF00203552. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Redman SJ. Spatial segregation of neuronal calcium signals encodes different forms of LTP in rat hippocampus. J Physiol. 2006;570:97–111. doi: 10.1113/jphysiol.2005.098947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson M, Harrad S. Chiral PCB signatures in air and soil: implications for atmospheric source apportionment. Environ Sci Technol. 2004;38:1662–1666. doi: 10.1021/es0349002. [DOI] [PubMed] [Google Scholar]
- Roegge CS, Schantz SL. Motor function following developmental exposure to PCBS and/or MEHG. Neurotoxicol Teratol. 2006;28:260–277. doi: 10.1016/j.ntt.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella L, Carafoli E. Calcium signaling in the cell nucleus. FASEB J. 1997;11:1091–1109. [PubMed] [Google Scholar]
- Schantz SL, Seo BW, Wong PW, Pessah IN. Long-term effects of developmental exposure to 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) on locomotor activity, spatial learning and memory and brain ryanodine binding. Neurotoxicology. 1997;18:457–467. [PubMed] [Google Scholar]
- Seegal RF, Bush B, Brosch KO. Comparison of effects of Aroclors 1016 and 1260 on non-human primate catecholamine function. Toxicology. 1991a;66:145–163. doi: 10.1016/0300-483x(91)90215-m. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Bush B, Brosch KO. Sub-chronic exposure of the adult rat to Aroclor 1254 yields regionally-specific changes in central dopaminergic function. Neurotoxicology. 1991b;12:55–65. [PubMed] [Google Scholar]
- Seegal RF, Bush B, Shain W. Lightly chlorinated ortho-substituted PCB congeners decrease dopamine in nonhuman primate brain and in tissue culture. Toxicol Appl Pharmacol. 1990;106:136–144. doi: 10.1016/0041-008x(90)90113-9. [DOI] [PubMed] [Google Scholar]
- Segal M, Korkotian E, Murphy DD. Dendritic spine formation and pruning: common cellular mechanisms? Trends Neurosci. 2000;23:53–57. doi: 10.1016/s0166-2236(99)01499-x. [DOI] [PubMed] [Google Scholar]
- Shain W, Bush B, Seegal R. Neurotoxicity of polychlorinated biphenyls: structure-activity relationship of individual congeners. Toxicol Appl Pharmacol. 1991;111:33–42. doi: 10.1016/0041-008x(91)90131-w. [DOI] [PubMed] [Google Scholar]
- Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimuta M, Yoshikawa M, Fukaya M, Watanabe M, Takeshima H, Manabe T. Postsynaptic modulation of AMPA receptor-mediated synaptic responses and LTP by the type 3 ryanodine receptor. Mol Cell Neurosci. 2001;17:921–930. doi: 10.1006/mcne.2001.0981. [DOI] [PubMed] [Google Scholar]
- Sperk G, Furtinger S, Schwarzer C, Pirker S. GABA and its receptors in epilepsy. Adv Exp Med Biol. 2004;548:92–103. doi: 10.1007/978-1-4757-6376-8_7. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Spontaneous Ca2+ spikes and waves in embryonic neurons: signaling systems for differentiation. Trends Neurosci. 1994;17:115–118. doi: 10.1016/0166-2236(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Tilson HA, Kodavanti PR, Mundy WR, Bushnell PJ. Neurotoxicity of environmental chemicals and their mechanism of action. Toxicol Lett. 1998;102–103:631–635. doi: 10.1016/s0378-4274(98)00271-9. [DOI] [PubMed] [Google Scholar]
- Trifaro JM, Vitale ML. Cytoskeleton dynamics during neurotransmitter release. Trends Neurosci. 1993;16:466–472. doi: 10.1016/0166-2236(93)90079-2. [DOI] [PubMed] [Google Scholar]
- Tuchman R, Rapin I. Epilepsy in autism. The Lancet Neurol. 2002;1:352–358. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- Vigh J, Lasater EM. Intracellular calcium release resulting from mGluR1 receptor activation modulates GABAA currents in wide-field retinal amacrine cells: a study with caffeine. Eur J Neurosci. 2003;17:2237–2248. doi: 10.1046/j.1460-9568.2003.02652.x. [DOI] [PubMed] [Google Scholar]
- Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- Wethington DM, 3rd, Hornbuckle KC. Milwaukee, WI, as a source of atmospheric PCBs to Lake Michigan. Environ Sci Technol. 2005;39:57–63. doi: 10.1021/es048902d. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta Physiol Scand. 1985;125:159–172. doi: 10.1111/j.1748-1716.1985.tb07703.x. [DOI] [PubMed] [Google Scholar]
- Wong PW, Brackney WR, Pessah IN. Ortho-substituted polychlorinated biphenyls alter microsomal calcium transport by direct interaction with ryanodine receptors of mammalian brain. J Biol Chem. 1997a;272:15145–15153. doi: 10.1074/jbc.272.24.15145. [DOI] [PubMed] [Google Scholar]
- Wong PW, Garcia EF, Pessah IN. ortho-substituted PCB95 alters intracellular calcium signaling and causes cellular acidification in PC12 cells by an immunophilin-dependent mechanism. J Neurochem. 2001;76:450–463. doi: 10.1046/j.1471-4159.2001.00022.x. [DOI] [PubMed] [Google Scholar]
- Wong PW, Joy RM, Albertson TE, Schantz SL, Pessah IN. Ortho-substituted 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) alters rat hippocampal ryanodine receptors and neuroplasticity in vitro: evidence for altered hippocampal function. Neurotoxicology. 1997b;18:443–456. [PubMed] [Google Scholar]
- Wong PW, Pessah IN. Ortho-substituted polychlorinated biphenyls alter calcium regulation by a ryanodine receptor-mediated mechanism: structural specificity toward skeletal- and cardiac-type microsomal calcium release channels. Mol Pharmacol. 1996;49:740–751. [PubMed] [Google Scholar]
- Yang D, Kim KH, Phimister AV, Bachstetter A, Ward T, Stackman R, Mervis R, Wisniewski A, Klein S, Kodavanti P, Anderson, Wayman G, Pessah IN, Lein PJ. Developmental exposure to polychlorinated biphenyls (PCBs) interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling Rats. Env Health Perspect. 2009;117:426–435. doi: 10.1289/ehp.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- Zimanyi I, Pessah IN. Pharmacological characterization of the specific binding of [3H]ryanodine to rat brain microsomal membranes. Brain Res. 1991;561:181–191. doi: 10.1016/0006-8993(91)91594-q. [DOI] [PubMed] [Google Scholar]
- Zoeller RT. Environmental chemicals as thyroid hormone analogues: new studies indicate that thyroid hormone receptors are targets of industrial chemicals? Mol Cell Endocrinol. 2005;242:10–15. doi: 10.1016/j.mce.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Crofton KM. Thyroid hormone action in fetal brain development and potential for disruption by environmental chemicals. Neurotoxicology. 2000;21:935–945. [PubMed] [Google Scholar]
- Zucker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency. Final National Priorities List (NPL) sites as of Sep. 27, 2007. USEPA Office of Emergency and Remedial Response; Washington, DC: 2007. Available at http://www.epa.gov/superfund/sites/query/queryhtm/nplprop.htm. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
No significant difference in fEPSP slope was observed with perfusion of PCB95 at 10 nM (n=5) or 100nM (n=6) for 45~60 min.