Abstract
Activins and inhibins are members of the transforming growth factor β superfamily that act on different cell types and regulate a broad range of cellular processes including proliferation, differentiation, and apoptosis. Here, we provide the first evidence that activins and inhibins regulate specific checkpoints during thymocyte development. We demonstrate that both activin A and inhibin A promote the DN3-DN4 transition in vitro, although they differentially control the transition to the DP stage. Whereas activin A induces the accumulation of a CD8+CD24hiTCRβlo intermediate subpopulation, inhibin A promotes the differentiation of DN4 to DP. In addition, both activin A and inhibin A appear to promote CD8+SP differentiation Moreover, Inhibin α null mice have delayed in vitro T cell development, showing both a decrease in the DN-DP transition and reduced thymocyte numbers, further supporting a role for inhibins in the control of developmental signals taking place during T cell differentiation in vivo.
Keywords: Thymocytes, Cell Differentiation, Activins, Inhibins, Knockout Mice
Introduction
T cell development occurs in the thymus and involves the close interaction between developing thymocytes and stromal cells. This interaction defines discrete anatomical microenvironments known as subcapsular, cortical, and medullary regions [1, 2]. Thymocytes mature from double negative (DN, CD4−CD8−), to double positive (DP, CD4+CD8+) and finally to single positive (SP, CD4+ or CD8+) cells. Immature DN thymocytes, have been subdivided into four subpopulations based on the expression of CD44 and CD25 surface markers: DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+) and DN4 (CD44−CD25−) [3–5]. At the DN3 stage, thymocytes that express an immature T cell receptor (pre-TCR) undergo the first developmental checkpoint (β selection) in thymocyte development, which leads to survival signals, proliferation, allelic exclusion, initiation of TCRα chain rearrangement, and expression of the CD4 and CD8 co-receptors [6, 7]. In addition, the DN4 subpopulation may pass through an intermediate stage where only CD8 is expressed (intermediate single positive, ISP) before the CD4+CD8+ double positive (DP) stage [8–10]. At the DP stage, αβ TCR recognition of self-peptides expressed on MHC-bearing stromal cells promotes positive or negative selection depending on the TCR avidity for the ligand [11, 12]. Those thymocytes surviving selection, give rise to CD4+ or CD8+ single positive thymocytes (SP) that migrate into the medulla and exit the thymus, homing to secondary lymphoid organs [13].
The molecular events regulating thymocyte differentiation are tightly controlled by the stromal cell compartment, not only through cell-cell contacts with thymocytes, but also through the production of soluble molecules such as chemokines, cytokines, and growth factors [14–16]. Transforming growth factor-β (TGF-β) superfamily members are recognized as important regulators of the immune system [17]; however, detailed studies characterizing their roles during thymocyte differentiation have only recently been suggested [18]. The TGF-β superfamily includes TGF-βs, BMPs (Bone morphogenetic proteins), activins and inhibins. These ligands share the same signaling mechanism, which involves binding of dimeric ligands to type I and type II receptors with serine/threonine kinase activity, leading to the phosphorylation of cytoplasmic proteins known as receptor SMADs (R-SMADs), which then heterodimerize with the co-SMAD (common SMAD, SMAD4) and translocate to the nucleus to regulate gene expression [19]. Among the subfamily members of TGF-β superfamily, TGF-βs and BMPs have been shown to regulate specific stages during T cell development More specifically, TGF-β1 appears to negatively regulate the transition DN1-DN2, but also promote the differentiation of mature CD8SP thymocytes, while BMPs inhibit thymocyte differentiation by regulating the DN1-DN2 and DN3-DN4 transitions, and therefore play roles in the control of the β selection checkpoint [20–23]. Nevertheless, recent evidence has demonstrated that activins and inhibins, as well as their receptors, are also expressed in the murine thymus [24]. In addition, activins are able to induce phosphorylation of Smad-2 (R-Smad) in thymocytes [25], suggesting that these molecules could also modulate thymocyte differentiation. Here, we present the first evidence demonstrating that activins and inhibins regulate and affect T cell differentiation. While both activins and inhibins promote DN3-DN4 transition, they differentially control the transition to the DP stage: activin A induces accumulation of CD8+ISP, whereas inhibin A promotes the differentiation of DN4 to the DP stage. Furthermore, activin A and inhibin A appear to promote CD8+ T cell differentiation at day 7 of culture, suggesting that they may regulate CD8 versus CD4 T lineage commitment. Finally, analysis of inhibin α null mice uncovered a delay in early thymocyte development and decreased thymocyte numbers in vitro, supporting the role of activins and inhibins during T cell development in vivo.
Materials and methods
Mice
C57BL/6 mice were bred and maintained in the mouse facility at the Instituto de Investigaciones Biomédicas, UNAM, in accordance with institutional guidelines. Eight to ten week old breeding pairs were used to obtain pregnant females, from which fetuses were harvested for fetal thymic organ cultures.
Inhibin α heterozygous mice (Inh α+/−) mutant mice were created as described previously [26]. Inh α+/− males and females were intercrossed to generate homozygous mutant mice (Inh α−/−) lacking all inhibins.
Fetal thymic organ cultures (FTOCs)
Fetal thymi were dissected from C57BL/6 mice on E14 and cultured on Millipore filters (8 μm pore size) in 10% SFB supplemented DMEM medium. Fetal thymi were cultured with or without human recombinant ligands, activin A (500 ng/ml Preprotech, Rocky Hill, NJ) or inhibin A (500 ng/ml DSL, Webster, TX) for 3–7 days, and medium was refreshed every third day. Fetal lobes were mechanically disaggregated and thymocytes stained for flow cytometric analysis. A 10 μl aliquot was taken from each sample before staining to calculate the total cell number at the end of each culture.
Flow cytometry
Cell suspensions of thymocytes were stained using a combination of the following directly conjugated antibodies (Pharmingen, BD Biosciences, San Jose, CA): anti-CD4APC, anti-CD4PE, anti-CD8FITC, anti-CD8Cy5, anti-CD25FITC, anti-CD44PE, anti-CD24FITC, and/or TCRβ PE and analyzed on a FACScalibur (BD Biosciences) with standard Flowjo software® (Tree Star, Inc., Ashland, OR).
Statistical Analysis
For FTOC using recombinant ligands, individual lobes from each fetus were compared (control lobe versus treated lobe), and Student’s T test for paired samples was used. P values ≤ 0.05 (**) were considered to be statistically significant. For the analysis of samples from inhibin α null mice (adult and fetal), unpaired Student’s T test was used. P values ≤0.1 (*) were considered to be statistically significant.
Results and Discussion
Activin A and inhibin A promote DN3 to DN4 thymocyte differentiation
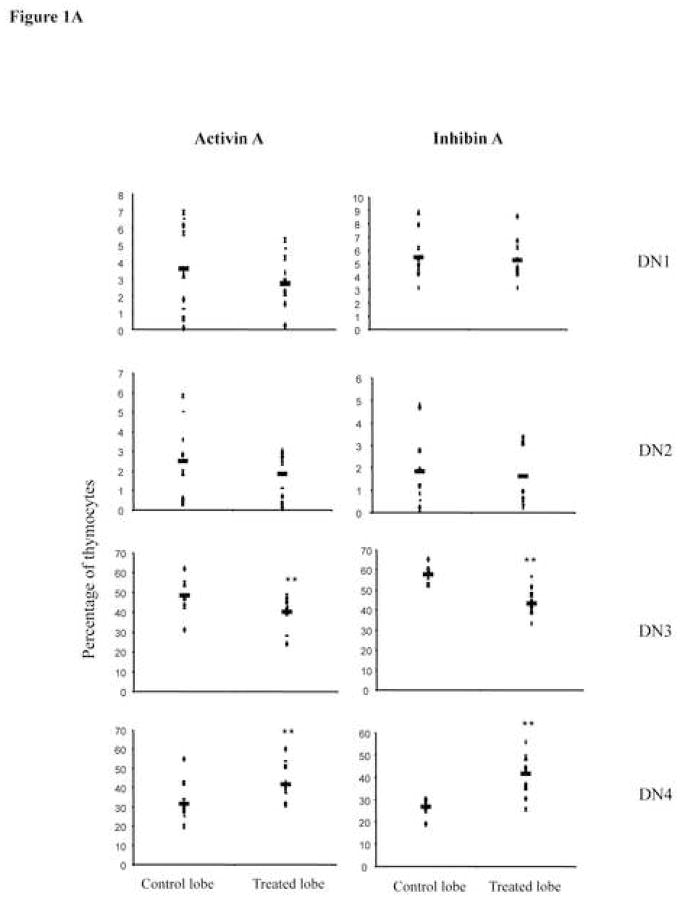
We have recently shown that activin and inhibin subunits are expressed in fetal and adult murine thymus, and that DN precursors are the subpopulation expressing higher levels of activin receptors [24]. In this study, we investigated the role of these ligands during early T cell development using fetal thymic organ cultures (FTOCs). We cultured 14-day fetal thymic lobes for 3 days in the presence of recombinant activin A or inhibin A, and performed surface staining using anti-CD44 and anti-CD25 to identify the DN thymocyte subpopulations generated in vitro. Although inhibins were initially described as antagonists of activin-mediated signals in the brain [27, 28], analysis of DN thymocyte subpopulations obtained after 3 days of treatment, showed that both activin A and inhibin A promoted DN3 to DN4 transition, significantly decreasing the DN3 subpopulation from 50 to 40 % and increasing DN4 subpopulation from 30 to 40% (Figure 1A). The percentage of each subpopulation correlated with cell numbers, as total thymocyte numbers did not show any statistically significant change in treated lobes compared with control lobes (not shown). This finding is relevant because it is the first evidence supporting a positive role for TGF-β superfamily members in mediating the earliest checkpoint during T cell differentiation (β-selection). In addition, our data supports previous reports where inhibins do not antagonize activin-mediated functions [29]. This observed promotion of early T cell development by activins and inhibins, is contrary to the reported roles of BMPs in the regulation of β-selection, similar to what has been previously described during dorso-ventral specification in Xenopus laevis [30]. According to previous reports, activin A and inhibin A have been shown to modulate proliferative responses as well as apoptosis in thymocytes and T cells [31–34]; therefore the possibility that treatment with the recombinant ligands is affecting these cellular processes, will need to be addressed in the future.
Figure 1.
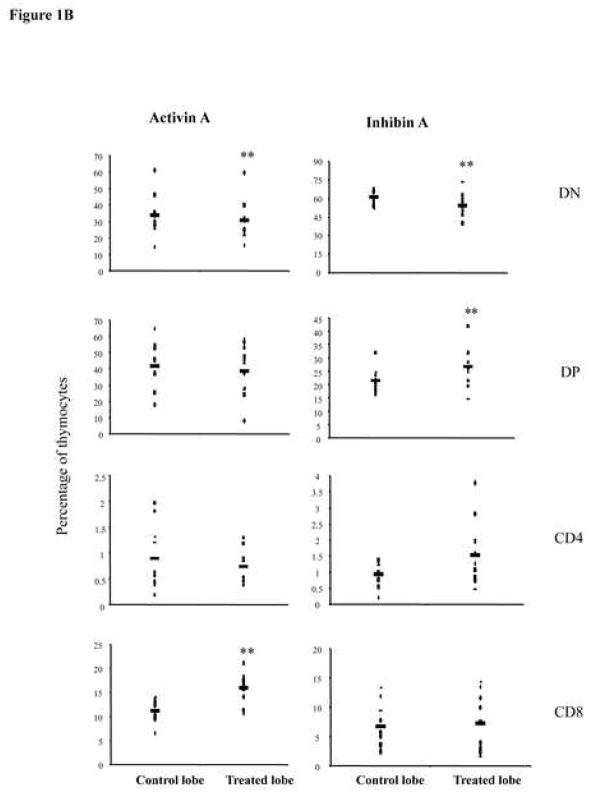
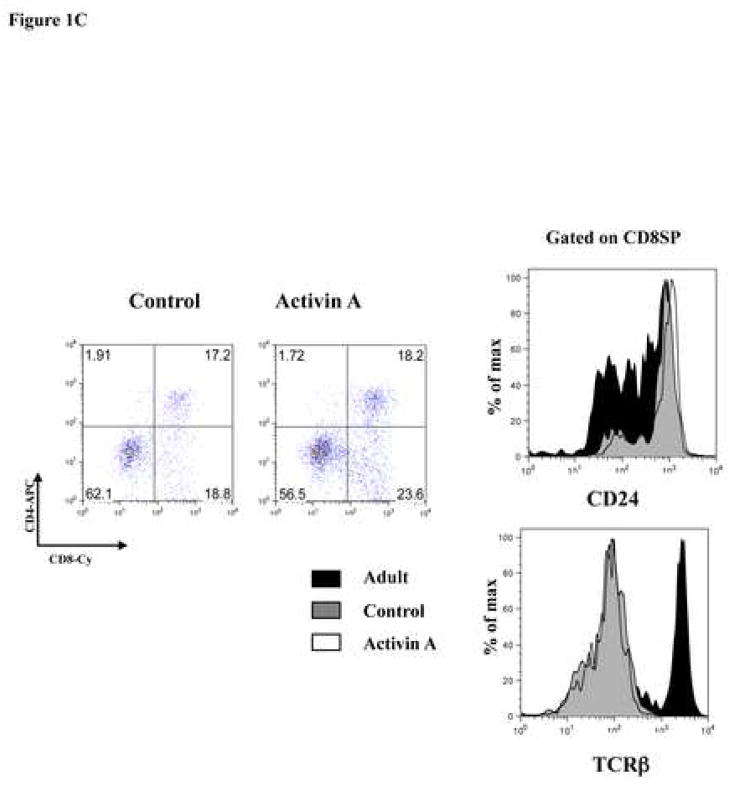
Activin A and inhibin A regulate early thymocyte development. E14 FTOC were performed for three days with 500 ng/ml of activin A or inhibin A and analyzed by flow cytometry. A, thymocytes were stained with anti CD25-FITC and anti CD44-PE coupled antibodies to differentiate between DN1, DN2, DN3, and DN4 subpopulations. Data represents percentage of DN thymocytes. B, Thymocytes stained with anti CD4-PE and anti CD8-Cy coupled antibodies. Data represents percentage of DN, DP, CD4 and CD8 thymocyte subpopulations. The average (−) of ten individual mice from at least four independent experiments is shown. ** Statistical differences (p ≤ 0.05) using paired T-test between control and treated lobes. C, Representative dot plot of thymic precursors from day 3 FTOCs with or without 500 ng/ml of activin A. Thymocytes were stained with anti CD4-APC, anti CD8-Cy, anti CD24-FITC and anti TCRβ-PE. Histograms represent CD24 and TCRβ expression of FTOC-derived and adult thymus CD8+ gated cells. Data obtained from a representative mouse (n= 10), from two independent experiments.
Activin A and inhibin A differentially regulate the transition from DN4 to DP
The ability of activin A and inhibin A to promote early T cell development was further analyzed using the CD4 and CD8 markers at day 3 of culture. Activin A treatment leads to a significant reduction in the percentage of DN precursors from 33 to 30%, while DP and CD4+ subpopulations were not significantly affected. At the same time, we observed an increase from 10% to 15% in CD8SP cells (Figure 1B, left panel). Characterization of this CD8+ subpopulation with anti-CD24 and anti-TCRβ specific antibodies identified these precursors as TCRβlow/CD24high expressing cells, corresponding to ISP precursors (Figure 1C). Inhibin A-treated lobes also resulted in a reduction from 60 to 55 in the percentage of total DNs. However, in contrast to activin A, inhibin A favoured the transition to the DP stage, increasing this subpopulation from 20% in the control to 25% in the inhibin A treated lobe (Figure 1B, right panel). Our data indicate that while inhibin A promoted DN4-DP transition, activin A delays this transition, promoting the accummulation of CD8+ ISPs. Thus, although both ligands seem to promote DN3 to DN4 transition, their mechanisms were different and only inhibin A induced increased maturation to the DP stage at day 3 of culture, providing a functional role for inhibin A, which presumably is the most likely physiological ligand present in the thymus as we previously reported [24].
Role of activin A and inhibin A in CD4 versus CD8 T cell commitment
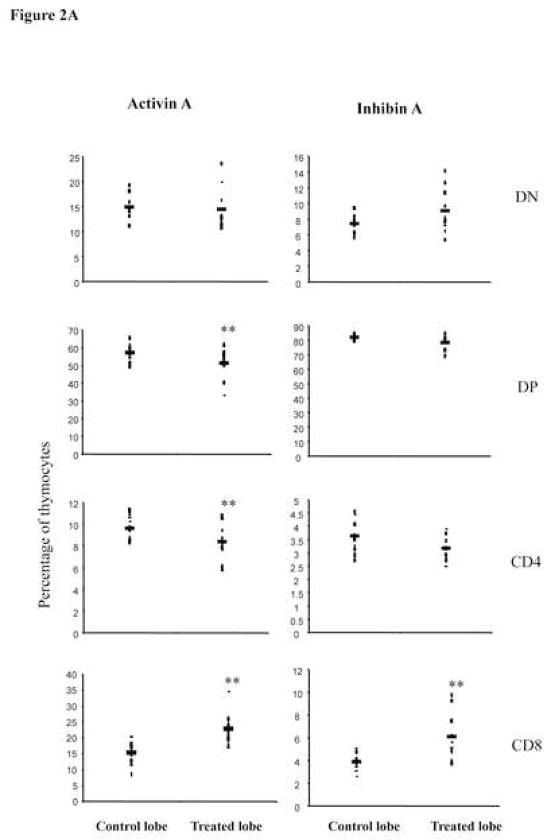
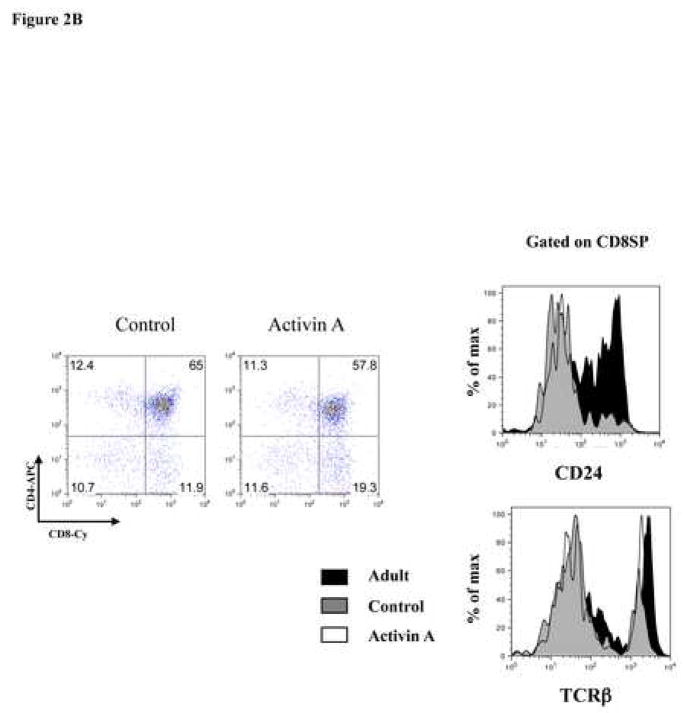
To determine the roles of activin A and inhibin A during positive selection, we analyzed the generation of SP thymocytes at day 7 of culture. Consistent with the data obtained at day 3 of culture, treatment of thymic lobes with activin A or inhibin A also promoted T cell development (Figure 2A). Interestingly, activin A treatment led to an increase in CD8+ T cells (15% in control cultures to 25% in treated cultures) which was accompanied by a decrease in DPs and CD4+ SPs. CD8+ cells generated in these cultures were mostly mature CD8SP thymocytes as determined by CD24 and TCRβ expression (CD24low TCRhigh) (Figure 2B), suggesting that activin A might be promoting CD8+ T lineage commitment. In addition, analysis of inhibin A-treated lobes also showed a significant increase (two-fold) in CD8SP cells, although it did not significantly reduce the percentages of DPs and CD4SP (Figure 2A). Finally, no statistically significant differences in total cell numbers were observed between treated and untreated lobes after 7 days of culture (data not shown). Altogether, our data demonstrate that activin A (and potentially inhibin A) promotes thymocyte differentiation towards the CD8 SP lineage similar to TGF-βs [20].
Figure 2.
Activin A and inhibin A promote CD8 SP. A, E14 FTOC were performed for seven days with 500 ng/ml of activin A or inhibin A and analyzed by flow cytometry. Thymocytes were stained with anti CD4-PE and anti CD8-Cy coupled antibodies. Data represents percentage of thymocytes. The average (−) of ten individual mice from at least four independent experiments is shown. ** Statistical differences (p ≤ 0.05) using paired T-test between control and treated lobes. B, Representative dot plot of thymic precursors from 7 day FTOCs, with or without 500 ng/ml of activin A. Thymocytes were stained with anti CD4-APC, anti CD8-Cy, anti CD24-FITC and anti TCRβ-PE. Histogram represents CD24 and TCRβ expression of FTOC derived and adult CD8+ gated cells, data from a representative mouse (n=10) from two independent experiments.
Inhibin α null mice show impaired early T cell development
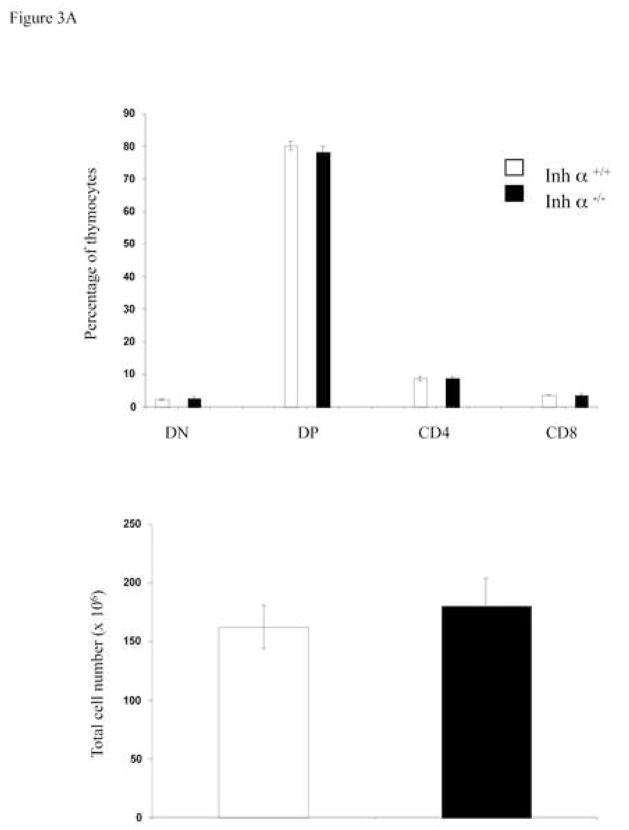
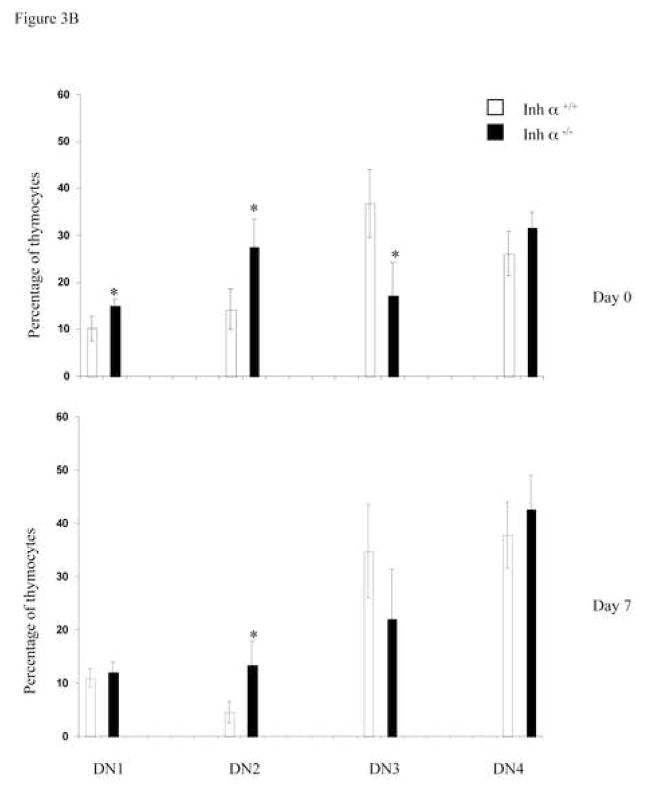
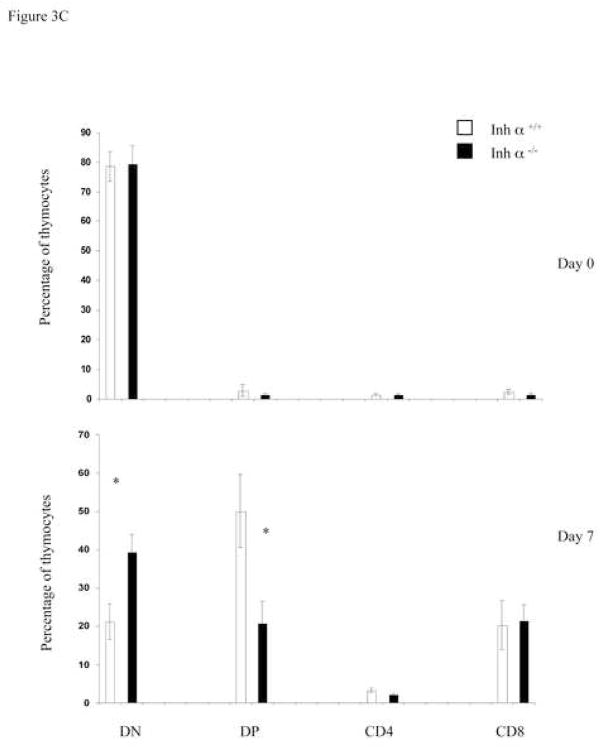
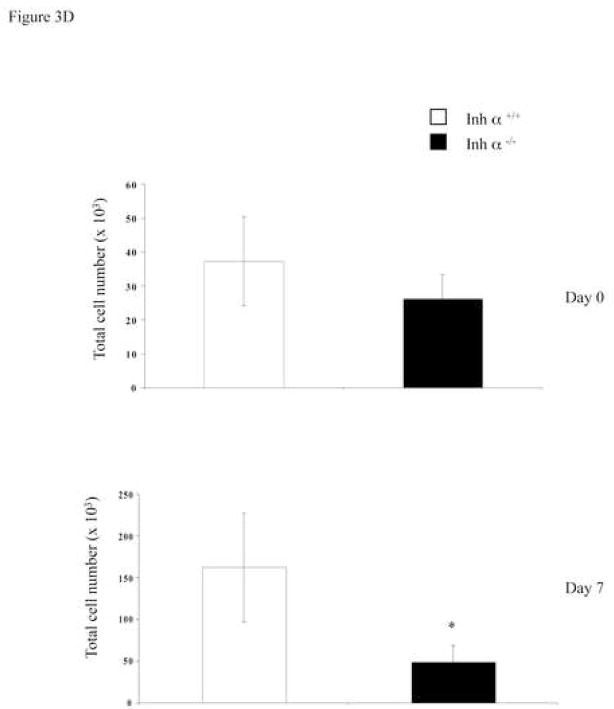
We have previously reported that inhibins appear to be the major ligands expressed during early thymocyte development [24]. To confirm the role of inhibins during T cell development in vivo, we analyzed the thymi of inhibin α null mice. All inhibins are dimeric proteins that share a common inhibin α subunit, and thus, inhibin α knockout mice are deficient in all inhibin ligands [26]. As shown in Figure 3A, the absence of inhibins in adult mice did not affect the percentage or the cell number of thymic precursors. However, since inhibin α−/− female mice are sterile [26], and expression of the ligand has been reported in the placenta of heterozygous pregnant females [35, 36], it is possible that the roles of inhibins during T cell differentiation was masked by the presence of the ligand from a maternal source. Therefore, we performed FTOCs using fetal thymus from inhibin α−/− and wild type mice. As shown in Figure 3B (upper panel), analysis of thymocyte subpopulations from E14 fetal thymus (Day 0 of culture) with CD44 and CD25 markers, showed an increase from 10 to 15% in early DN1 and from 14 to 27% in DN2, as well as a decrease from 36 to 17% in DN3 precursors in the thymus of Inhibin α−/− mice compared with wild type littermates. After 7 days of culture (in the absence of an exogenous source of inhibin), a significant increase (three-fold) in DN2 thymocytes was also observed (Figure 3B, lower panel). Analysis of E14 thymic lobes (day 0 of culture) with CD4 and CD8 markers, showed that the percentages of thymocyte subpopulations were equivalent in wild type thymi compared with Inhibin α−/− thymi (Figure 3C, upper panel). However, after 7 days of culture, Inhibin α−/− thymi demonstrated impaired in vitro development, showing an increase from 21% to 39% in DNs, accompanied by a decrease from 50% to 20% in DP precursors (Figure 3C, lower panel). Additionally, the absence of inhibins led to a significant reduction in the absolute thymocyte numbers generated after 7 days of culture compared to wild type thymi (Figure 3D). The impaired early thymocyte development observed in inhibin α−/− fetal thymi provides further evidence in support of the role of inhibins in the promotion of thymocyte differentiation in vivo, and corroborates the in vitro studies with these ligands (see Figure 1B).
Figure 3.
Inhibin α−/− mice show impaired early T cell development. A, Phenotypic analysis of thymocytes from 6–8 week old inhibin α−/− mice and wild type littermates, after staining with anti CD4-PE and anti CD8-Cy coupled antibodies. Data represents percentage and total cell number of thymocytes in inhibin α−/− mice and wild type littermates. The average and standard error of twelve individual mice from six independent experiments is shown. B, and C, E14 FTOC were performed for seven days with thymic lobes from inhibin α−/− mice and control littermates, thymocytes were stained with anti CD44-PE, anti CD25-FITC (B) anti CD4-APC and anti CD8-Cy (C). D, Total thymocyte numbers obtained from inhibin α−/− or wild type FTOC at day 0 and day 7 of culture. The average and standard error of seven individual mice, from four independent experiments is shown. * Statistical differences (p ≤ 0.1) using unpaired T-test.
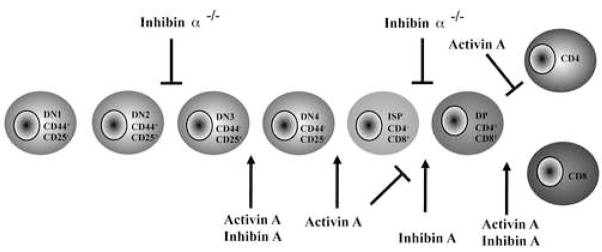
In summary, we provide the first evidence that activins and inhibins regulate T cell development (Figure 4). Moreover, inhibins do not appear to antagonize activin-mediated functions in thymocytes. On the contrary, during early thymocyte development, both activin A and inhibin A positively regulate the DN3 to DN4 transition, potentially antagonizing the effect of BMPs at this developmental checkpoint [21–23]. However, in later stages of thymocyte development, both activin A and inhibin A promote CD8 SP differentiation, similarly to what was previously reported for TGF-β1[20]. It is worth noting that activin/inhibin receptors are also expressed on thymic stromal cells [24], and, therefore, we cannot exclude the possibility that these ligands may also control stromal cell development and/or function. Our data provides a model in which a balance between BMPs, TGFβs, activins, and inhibins, as well as their controlled receptor expression, regulates different processes taking place during thymocyte development. Finally, the work presented here, together with previous evidence, supports non-redundant roles of TGF-β family members in the regulation of T cell differentiation, despite their highly conserved signaling mechanisms.
Figure 4.
Schematic representation of the proposed role of activins and inhibins during thymocyte development. Activin A and inhibin A promote the transition from DN3 to DN4, potentially antagonizing the previously reported effects of BMPs at this developmental checkpoint. Inhibin A promotes the transition to the DP stage while activin A induces the accumulation of an ISP subpopulation. At later stages of development, both activin A and inhibin A promote thymocyte maturation, favouring the differentiation of CD8SP mature thymocytes. During early fetal stages, the absence of inhibins delays the DN2-DN3 and DN-DP transitions and also reduces the total number of thymocytes.
In the future, it will be interesting to address the roles of activins and inhibins in the regulation of cellular responses in thymocytes, including proliferation, cell cycle progression, and apoptosis. A more detailed study of the interactions between TGF-β superfamily members with other morphogens (i.e, Sonic Hedgehog, Wnt) expressed in the thymus [37] is required to have an integrated understanding of the signals controlling thymocyte differentiation in vivo.
Acknowledgments
We want to thank Ramsés Chávez for technical assistance, MVZ Georgina Diaz and MVZ Jorge Omar Garcia Rebollar for animal care assistance, and Carlos Castellanos for technical support in the Flow Cytometry Unit. We also thank Dr. Adam Williams for critical reading of the manuscript.
This work was supported by Grants 42797 and 53484 from CONACyT, IN225208 and IN220808 from DGAPA, UNAM, and National Institutes of Health, USA (CA60651 to M.M.M.). PL was supported by a doctoral fellowship (203753) and from DGEP, UNAM. GA is recipient of doctoral fellowship 208213 from CONACyT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller JF. Immunological function of the thymus. Lancet. 1961;2:748–9. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 2.Scollay R, Godfrey DI. Thymic emigration: conveyor belts or lucky dips? Immunol Today. 1995;16:268–73. doi: 10.1016/0167-5699(95)80179-0. discussion 273–4. [DOI] [PubMed] [Google Scholar]
- 3.Lesley J, Trotter J, Hyman R. The Pgp-1 antigen is expressed on early fetal thymocytes. Immunogenetics. 1985;22:149–57. doi: 10.1007/BF00563512. [DOI] [PubMed] [Google Scholar]
- 4.Ceredig R, Lowenthal JW, Nabholz M, MacDonald HR. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985;314:98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- 5.Ceredig R, Rolink T. A positive look at double-negative thymocytes. Nat Rev Immunol. 2002;2:888–97. doi: 10.1038/nri937. [DOI] [PubMed] [Google Scholar]
- 6.Kruisbeek AM, Haks MC, Carleton M, Michie AM, Zuniga-Pflucker JC, Wiest DL. Branching out to gain control: how the pre-TCR is linked to multiple functions. Immunol Today. 2000;21:637–44. doi: 10.1016/s0167-5699(00)01744-8. [DOI] [PubMed] [Google Scholar]
- 7.Aifantis I, Mandal M, Sawai K, Ferrando A, Vilimas T. Regulation of T-cell progenitor survival and cell-cycle entry by the pre-T-cell receptor. Immunol Rev. 2006;209:159–69. doi: 10.1111/j.0105-2896.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 8.Paterson DJ, Williams AF. An intermediate cell in thymocyte differentiation that expresses CD8 but not CD4 antigen. J Exp Med. 1987;166:1603–8. doi: 10.1084/jem.166.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald HR, Budd RC, Howe RC. A CD3− subset of CD4−8+ thymocytes: a rapidly cycling intermediate in the generation of CD4+8+ cells. Eur J Immunol. 1988;18:519–23. doi: 10.1002/eji.1830180405. [DOI] [PubMed] [Google Scholar]
- 10.Shortman K, Wilson A, Egerton M, Pearse M, Scollay R. Immature CD4− CD8+ murine thymocytes. Cell Immunol. 1988;113:462–79. doi: 10.1016/0008-8749(88)90042-1. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin KK, Trenchak BP, Altman JD, Davis MM. Negative selection of T cells occurs throughout thymic development. J Immunol. 1999;163:689–98. [PubMed] [Google Scholar]
- 12.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 13.Gabor MJ, Godfrey DI, Scollay R. Recent thymic emigrants are distinct from most medullary thymocytes. Eur J Immunol. 1997;27:2010–5. doi: 10.1002/eji.1830270827. [DOI] [PubMed] [Google Scholar]
- 14.Savino W, Mendes-da-Cruz DA, Silva JS, Dardenne M, Cotta-de-Almeida V. Intrathymic T-cell migration: a combinatorial interplay of extracellular matrix and chemokines? Trends Immunol. 2002;23:305–13. doi: 10.1016/s1471-4906(02)02224-x. [DOI] [PubMed] [Google Scholar]
- 15.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–35. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 16.Anderson G, Jenkinson WE, Jones T, Parnell SM, Kinsella FA, White AJ, Pongrac’z JE, Rossi SW, Jenkinson EJ. Establishment and functioning of intrathymic microenvironments. Immunol Rev. 2006;209:10–27. doi: 10.1111/j.0105-2896.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 17.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 18.Licona-Limon P, Soldevila G. The role of TGF-beta superfamily during T cell development: new insights. Immunol Lett. 2007;109:1–12. doi: 10.1016/j.imlet.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 20.Plum J, De Smedt M, Leclercq G, Vandekerckhove B. Influence of TGF-beta on murine thymocyte development in fetal thymus organ culture. J Immunol. 1995;154:5789–98. [PubMed] [Google Scholar]
- 21.Hager-Theodorides AL, Outram SV, Shah DK, Sacedon R, Shrimpton RE, Vicente A, Varas A, Crompton T. Bone morphogenetic protein 2/4 signaling regulates early thymocyte differentiation. J Immunol. 2002;169:5496–504. doi: 10.4049/jimmunol.169.10.5496. [DOI] [PubMed] [Google Scholar]
- 22.Graf D, Nethisinghe S, Palmer DB, Fisher AG, Merkenschlager M. The developmentally regulated expression of Twisted gastrulation reveals a role for bone morphogenetic proteins in the control of T cell development. J Exp Med. 2002;196:163–71. doi: 10.1084/jem.20020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nosaka T, Morita S, Kitamura H, Nakajima H, Shibata F, Morikawa Y, Kataoka Y, Ebihara Y, Kawashima T, Itoh T, Ozaki K, Senba E, Tsuji K, Makishima F, Yoshida N, Kitamura T. Mammalian twisted gastrulation is essential for skeleto-lymphogenesis. Mol Cell Biol. 2003;23:2969–80. doi: 10.1128/MCB.23.8.2969-2980.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Licona P, Chimal-Monroy J, Soldevila G. Inhibins are the major activin ligands expressed during early thymocyte development. Dev Dyn. 2006;235:1124–32. doi: 10.1002/dvdy.20707. [DOI] [PubMed] [Google Scholar]
- 25.Rosendahl A, Speletas M, Leandersson K, Ivars F, Sideras P. Transforming growth factor-beta- and Activin-Smad signaling pathways are activated at distinct maturation stages of the thymopoeisis. Int Immunol. 2003;15:1401–14. doi: 10.1093/intimm/dxg139. [DOI] [PubMed] [Google Scholar]
- 26.Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–9. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- 27.McLachlan RI, Robertson DM, de Kretser D, Burger HG. Inhibin--a non-steroidal regulator of pituitary follicle stimulating hormone. Baillieres Clin Endocrinol Metab. 1987;1:89–112. doi: 10.1016/s0950-351x(87)80054-x. [DOI] [PubMed] [Google Scholar]
- 28.Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature. 1986;321:779–82. doi: 10.1038/321779a0. [DOI] [PubMed] [Google Scholar]
- 29.Woodruff TK, Mather JP. Inhibin, activin and the female reproductive axis. Annu Rev Physiol. 1995;57:219–44. doi: 10.1146/annurev.ph.57.030195.001251. [DOI] [PubMed] [Google Scholar]
- 30.Whitman M. Smads and early developmental signaling by the TGFbeta superfamily. Genes Dev. 1998;12:2445–62. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- 31.Hedger MP, Drummond AE, Robertson DM, Risbridger GP, de Kretser DM. Inhibin and activin regulate [3H]thymidine uptake by rat thymocytes and 3T3 cells in vitro. Mol Cell Endocrinol. 1989;61:133–8. doi: 10.1016/0303-7207(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 32.Petraglia F, Sacerdote P, Cossarizza A, Angioni S, Genazzani AD, Franceschi C, Muscettola M, Grasso G. Inhibin and activin modulate human monocyte chemotaxis and human lymphocyte interferon-gamma production. J Clin Endocrinol Metab. 1991;72:496–502. doi: 10.1210/jcem-72-2-496. [DOI] [PubMed] [Google Scholar]
- 33.Hedger MP, Phillips DJ, de Kretser DM. Divergent cell-specific effects of activin-A on thymocyte proliferation stimulated by phytohemagglutinin, and interleukin 1 beta or interleukin 6 in vitro. Cytokine. 2000;12:595–602. doi: 10.1006/cyto.1999.0597. [DOI] [PubMed] [Google Scholar]
- 34.Valderrama-Carvajal H, Cocolakis E, Lacerte A, Lee EH, Krystal G, Ali S, Lebrun JJ. Activin/TGF-beta induce apoptosis through Smad-dependent expression of the lipid phosphatase SHIP. Nat Cell Biol. 2002;4:963–9. doi: 10.1038/ncb885. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi M, Endo H, Tasaka K, Miyake A. Mouse growth hormone-releasing factor secretion is activated by inhibin and inhibited by activin in placenta. Biol Reprod. 1995;53:368–72. doi: 10.1095/biolreprod53.2.368. [DOI] [PubMed] [Google Scholar]
- 36.Petraglia F, Sawchenko P, Lim AT, Rivier J, Vale W. Localization, secretion, and action of inhibin in human placenta. Science. 1987;237:187–9. doi: 10.1126/science.3299703. [DOI] [PubMed] [Google Scholar]
- 37.Ciofani M, Zuniga-Pflucker JC. The Thymus as an Inductive Site for T Lymphopoiesis. Annu Rev Cell Dev Biol. 2007;23:463–493. doi: 10.1146/annurev.cellbio.23.090506.123547. [DOI] [PubMed] [Google Scholar]