Abstract
BACKGROUND
Volatile anesthetics act primarily in the spinal cord to produce immobility but their exact site of action is unclear. Between 0.8 and 1.2 minimum alveolar anesthetic concentration (MAC), isoflurane does not depress neurons in the dorsal horn, suggesting that it acts at a more ventral site within the spinal cord such as in premotor interneurons and motoneurons. We hypothesized that isoflurane, halothane, and propofol would exert a greater depressant effect on nociceptive responses of ventral horn neurons when compared with dorsal horn neurons.
METHODS
Rats were anesthetized with isoflurane or halothane and responses of dorsal (<1200 μm deep) and ventral (>1200 μm) lumbar neurons to noxious mechanical stimulation of the hindpaw were determined at 0.8 and 1.2 MAC. In a third group anesthetized with isoflurane at 0.8 MAC, we administered 5 mg/kg propofol while recording responses from dorsal horn or ventral horn neurons.
RESULTS
Dorsal horn neuronal responses were not significantly affected when either isoflurane or halothane was increased from 0.8 to 1.2 MAC; propofol also had no significant effect. On the other hand, with increased isoflurane or halothane concentration, responses of ventral horn neurons were depressed by 60% and 45%, respectively. Propofol profoundly depressed (>90%) ventral horn neurons.
CONCLUSIONS
These data suggest that, in the peri-MAC range, isoflurane, halothane, and propofol have little or no effect on neuronal responses to noxious mechanical stimulation in the spinal dorsal horn but depress such responses in the ventral horn. Immobility produced in the 0.8–1.2 MAC range by these anesthetics appears to result from a depressant action in the ventral horn.
Immobility is one of the defining hallmarks of general anesthesia (1). Several studies have documented the importance of volatile anesthetic action in the spinal cord to produce immobility (2–5); however, we do not completely understand precisely how and where anesthetic action in the spinal cord produces this essential behavioral end point. Older studies indicate that anesthetic action in the dorsal horn might be important (6,7). However, we have more recently shown, in several studies, that isoflurane has little to no depressant effect on dorsal horn neurons in the 0.8–1.2 minimum alveolar anesthetic concentration (MAC) range (8,9). In fact, although both halothane and isoflurane depressed neuronal responses in the 0–0.8 MAC range, only halothane caused further depression in the 0.8–1.2 MAC range (9). These data suggest that, in the peri-MAC range, anesthetics, in particular isoflurane, might produce immobility largely via action at ventral spinal neurons, such as premotor interneurons or motoneurons. Indeed, in vitro studies show that anesthetics do depress motoneurons (10,11), and we have shown evidence suggesting isoflurane’s action on lamprey locomotor interneurons (12).
The purpose of the present study was to probe anesthetic sensitivity of neurons located in the dorsal and ventral horns of the rat spinal cord. We hypothesized that neurons located in the ventral horn would be more depressed by isoflurane, halothane, and propofol when compared with neurons located in the dorsal horn.
METHODS
The local animal care and use committee at the University of California, Davis, approved this study. In brief, the experimental paradigm was to determine responses to noxious stimulation in spinal neurons located in the dorsal horn and compare these responses with those from neurons situated ventral to the dorsal horn. We determined these responses at 0.8 and 1.2 MAC isoflurane and halothane, as well as after bolus administration of propofol in isoflurane-anesthetized rats.
Adult male rats were anesthetized in an acrylic chamber using either isoflurane (4%) or halothane (3%). Anesthesia was maintained via mask while a tracheostomy was placed. A 14-gauge plastic catheter was inserted into the trachea and mechanical ventilation initiated. End-tidal gases were sampled using a calibrated agent analyzer (Rascal II, Ohmeda, Salt Lake City, UT). End-tidal carbon dioxide was kept at approximately 30–40 mm Hg. Temperature was monitored using a rectal probe and was maintained at approximately 37°C–38°C. A carotid artery and jugular vein were cannulated for measuring arterial blood pressure and administration of drugs and fluids, respectively. Ligation of a carotid artery does not alter cerebral blood flow in the rat (13). Mean arterial blood pressure was maintained in the approximately 80–120 mm Hg range. A laminectomy was performed at T12–L3 to expose the lumbar spinal segments. After this, the MAC for each animal was determined as previously described (8). The anesthetic concentration was stabilized for at least 15 min and a hemostat was applied to the tail and oscillated (±45°) at 1 Hz for 1 min or until the animal displayed gross and purposeful movement, such as pawing of the extremities or movement of the head towards the stimulus. The anesthetic concentration was increased or decreased 0.2% (depending on whether purposeful movement occurred or not), and after waiting 15–20 min for equilibration, the clamp was applied again. This process was continued until two anesthetic concentrations were found that just permitted and just prevented movement. The MAC was the average of these concentrations.
The rat was placed into a stereotaxic frame (Kopf, Tujunga, CA) and the spinal column secured using vertebral clamps rostral and caudal to the laminectomy. The head was also secured using ear bars and incisor clamp. The dura was reflected and the spinal cord exposed. Warm agar was poured onto the spinal cord and a small window was made to expose the cord. Single unit activity was obtained using a tungsten microelectrode (approximately 10 MΩ, FHC, Bowdoinham, ME) lowered into the spinal cord with a micromanipulator (Kopf, Tujunga, CA). The signal was amplified (Tucker-Davis, Alachua, FL) band-pass filtered from 300 to 5000 Hz and fed to a computer for recording (Chart5, ADInstruments, CO Springs, CO) onto a hard-drive. The signal-to-noise ratio was at least four and was usually much more. Pancuronium (0.2–0.3 mg/kg every 1–2 h) was administered to minimize movement-related disruption of the recording. We sought neurons that responded to stimulation of the hindpaw. The search stimulus was a combination of touching the paw as well as mild pinching. Neurons were classified as wide dynamic range if they responded to innocuous and noxious mechanical stimuli at a progressively increasing firing rate and nociceptive-specific if they only responded to the latter.
We recorded neuronal responses to a noxious pinch applied to the receptive field on the hindpaw. We used a small forceps instrumented with a force transducer (Sensotec, Columbus, OH) near the proximal end of the forceps. The pinch was applied for 5 s and maintained at a constant force as determined by a transducer monitor readout (Sensotec, Columbus, OH), which was displayed continually on the Chart recording along with unit activity. The intensity of the pinch force (range, 35–55 N) was established for each individual unit based on its receptive field and response level. Although the intensity of the stimulus varied from unit to unit, for each unit the force of each pinch stimulus applied to the hindpaw was constant. When applied to the investigator’s skin, pinches in the force range used presently (35–55 N) all elicited moderate to strong pain.
We studied three groups. One group was anesthetized with isoflurane (n = 18), the second group was anesthetized with halothane (n = 10). In the third group, rats were anesthetized with isoflurane and then administered propofol (n = 7). This facilitated data collection, as administering propofol as the sole anesthetic is challenging in terms of measuring and maintaining propofol plasma concentrations. In the isoflurane and halothane groups, we recorded neuronal responses to the noxious pinch at 0.8 and 1.2 MAC as referenced to each animal’s individually determined MAC value. We waited at least 15 min to equilibrate at the new anesthetic concentration before recording neuronal responses. At each anesthetic concentration, a series of 3–5 pinch stimuli was delivered at a minimum interstimulus interval of 4 min. After recording responses at 1.2 MAC, in most animals we returned to 0.8 MAC and obtained responses again for the same neuron. In the propofol group we maintained isoflurane at 0.8 MAC and, after recording control neuronal responses, administered propofol 5 mg/kg IV. We chose this dose based on pilot data as well as our prior work (14). We recorded responses 1, 4, 7, 10, and 13 min after propofol administration.
In most animals, two units were recorded: one dorsal (depth <1200 μm) and one ventral (>1200 μm), with this depth (1200 μm) representing the approximate demarcation between the dorsal horn and the intermediate zone and ventral horn. The order of recording dorsal and ventral units was counterbalanced across experiments. In most rats, an electrolytic lesion was made at the last recording site (direct current, 8 V for 30–45 s). Animals were killed with an anesthetic overdose and IV potassium chloride, after which the lumbar spinal cord was removed and postfixed in 10% formalin for >2 wk. The lumbar spinal cords were cut in 50-μm frozen sections, mounted on microscope slides, and examined under the light microscope to identify lesions. A photomicrograph was taken of the spinal cord slice (with lesion), and the photomicrograph was overlaid on to a representative section of the fourth lumbar segment from the atlas of Paxinos and Watson (15). The size of the photomicrograph was adjusted to match the drawing of the lumbar segment. The person identifying the lesion and transferring the lesion site was blinded to experimental group and recording depth.
Neuronal activity was quantified using the histogram program of Chart5 (ADInstruments, CO Springs, CO). We counted the number of action potentials during the 30-s period before pinching (spontaneous activity) and for the 60-s period beginning with application of the pinch (total activity). We also separately analyzed the response during the period of pinching. This permitted analysis of the response during the period when movement would be most likely to occur. Evoked activity was total activity minus spontaneous activity. Total and evoked activities at 0.8 MAC were compared with those at 1.2 MAC. In the propofol group, we compared the control activity (recorded during 0.8 MAC isoflurane anesthesia in the absence of propofol) to the average of the evoked responses recorded after administration of propofol. Because some of the data were not normally distributed (based on the Kolmogorov–Smirnov test), we used the Wilcoxon’s signed rank test for statistical comparisons. A P < 0.05 was considered significant.
RESULTS
Responses were recorded from 70 neurons in 39 rats; 30 neurons (14 dorsal horn, 16 ventral horn) were recorded in the isoflurane group, 25 in the halothane group (11 dorsal horn, 14 ventral horn), and 15 neurons in the propofol group (8 dorsal horn, 7 ventral horn). The MAC in the isoflurane group was 1.3% ± 0.2%, whereas the halothane MAC was 1.0% ± 0.2%. The isoflurane MAC in the propofol group was 1.3% ± 0.2%. The mean recording depths of the dorsal and ventral neurons were 746 ± 184 μm and 1646 < 283 μm, respectively, for the isoflurane group; 580 ± 252 μm and 1766 ± 242 μm in the halothane group; and 689 ± 180 μm and 1869 ± 270 μm in the propofol group. In 25 animals, lesion sites were verified histologically and are shown in Figure 1 for the dorsal (open circles) and ventral (filled circles) sites. In all cases shown in Figure 1, the dorsal or ventral location of the unit based on microdrive micrometer readings was consistent with the location of the lesion. One neuron in the propofol group was located in the ventral horn based on the microdrive reading, but a lesion was found in the dorsal horn. Because of this discrepancy, we excluded this neuron from the propofol data analysis, leaving the 15 neurons noted above.
Figure 1.
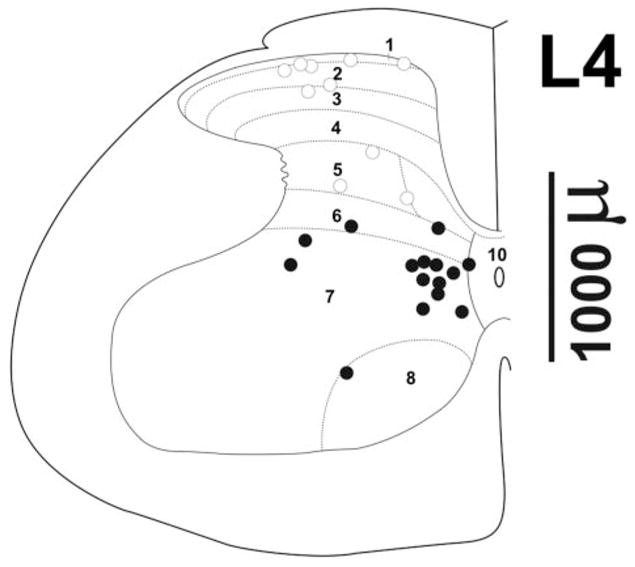
Recording sites. (A) Recording sites determined from histologically verified lesions are shown overlaid on a representative drawing of the L4 lumbar spinal cord [taken from the atlas of Paxinos and Watson (15)]. Open circles: sites <1200 μm based on micrometer reading; filled circles: depths >1200 μm. The approximate recording depth can be estimated from the depth marker at right.
In the isoflurane group, the neurons in the dorsal horn had low spontaneous activity (at 0.8 MAC, median = 0.18 Hz, range 0–13 Hz; at 1.2 MAC, median = 0.1 Hz, range 0–10.5 Hz). The spontaneous activity of ventral neurons tended to exhibit two distinct patterns, with either no or little spontaneous activity or high spontaneous activity that occasionally waxed and waned: at 0.8 MAC, median = 0 Hz, range 0–30 Hz; at 1.2 MAC, median = 0 Hz, range 0–53 Hz. In the halothane group, at 0.8 MAC, spontaneous activity in the dorsal horn neurons was low (median = 0.3 Hz, range 0–11 Hz); at 1.2 MAC, median = 0 Hz, range 0–0.9 Hz). In the ventral horn neurons, at 0.8 MAC, median spontaneous activity = 0 Hz, range 0–19 Hz; at 1.2 MAC, median = 0 Hz, range 0–5 Hz. In the propofol group with background isoflurane anesthesia, spontaneous activity, when present, was markedly depressed by propofol (data not reported).
Increasing the isoflurane concentration from 0.8 to 1.2 MAC did not have a significant effect on evoked activity of neurons in the dorsal horn; however, evoked activity of deeper neurons in the ventral horn was significantly (P < 0.05) suppressed by 60% (Fig. 2B). Analysis of responses for the 5-s period during stimulation revealed similar depression. Individual responses in Figure 3 show that a neuron in the ventral horn was more sensitive to the increased isoflurane concentration compared with a neuron in the dorsal horn.
Figure 2.
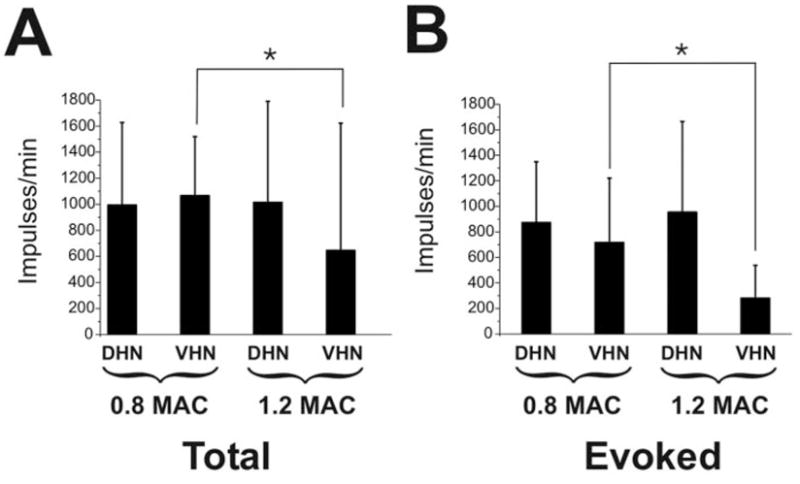
Summary data for isoflurane animals. (A) Shown is the total number of action potentials (mean, standard deviation) for the 60-s period after application of the noxious mechanical stimulus at 0.8 and 1.2 minimum alveolar anesthetic concentration (MAC) for dorsal horn neurons (DHNs) and ventral horn neurons (VHNs). (B) Same as (A) but for the evoked response (total number action potentials minus spontaneous activity). *P < 0.05. Note that the VHNs, as compared to the DHNs, are more depressed by the change to 1.2 MAC.
Figure 3.
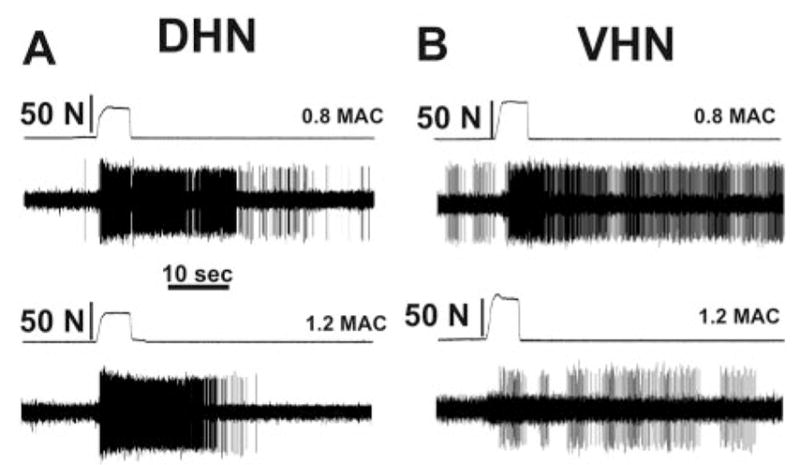
Individual examples of neuronal responses to noxious mechanical stimulation. Shown are action potentials recorded from lumbar neurons in animals anesthetized with isoflurane. Changing the isoflurane concentration from 0.8 to 1.2 minimum alveolar anesthetic concentration (MAC) did not appreciably affect the responses of the dorsal horn neuron (DHN, shown in A); the response at 0.8 MAC was 1472 impulses and was 1339 impulses at 1.2 MAC. Increasing isoflurane from 0.8 to 1.2 MAC decreased the response of a ventral horn neuron (VHN, shown in B) from 829 impulses to 204 impulses (different animal than in A).
Increasing the halothane concentration had no effect on dorsal horn neuronal responses, but depressed responses in the ventral horn neurons by 45% (P < 0.05) (Fig. 4). For halothane and isoflurane, after return to 0.8 MAC, the neuronal responses were not significantly different from the responses at the first 0.8 MAC condition.
Figure 4.
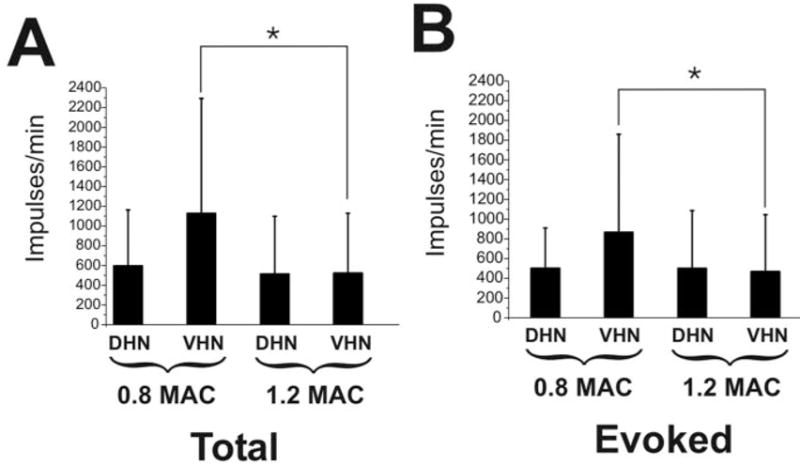
Summary data for halothane animals. (A) Shown is the total number of action potentials (mean, standard deviation) for the 60-s period after application of the noxious mechanical stimulus at 0.8 and 1.2 minimum alveolar anesthetic concentration (MAC) for dorsal horn neurons (DHNs) and ventral horn neurons (VHNs). (B) Same as (A) but for the evoked response (total number action potentials minus spontaneous activity). *P < 0.05. Note that the VHNs, as compared to the DHNs, are more depressed by the change to 1.2 MAC.
In the propofol group, the dorsal horn neurons were transiently depressed, although this did not reach statistical significance (P = 0.13 for the 1 min response), whereas deeper ventral neurons were profoundly depressed (90% depression at 1 min postpropofol, P < 0.05; Fig. 5). Furthermore, the ventral neurons remained markedly depressed. The average of the responses of the ventral neurons after propofol was only 8% of the prepropofol control responses (P < 0.05).
Figure 5.
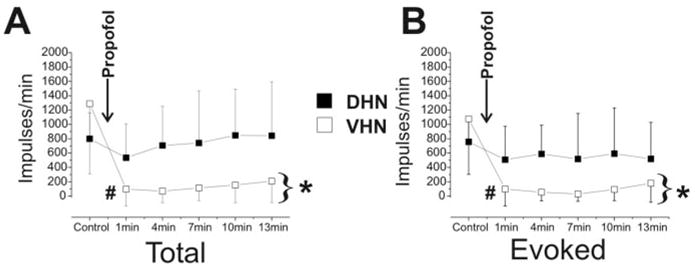
Summary data for propofol animals. (A) Shown is the total number of action potentials (mean, standard deviation) for the 60-s period after application of the noxious mechanical stimulus for dorsal horn neurons (DHNs, ■) and ventral horn neurons (VHNs, □). Data are from the control period before propofol and the responses 1, 4, 7, 10, and 13 min after propofol (5 mg/kg). (B) Same as (A) but for the evoked response (total number action potentials minus spontaneous activity). *P < 0.05, average of all postpropofol responses compared to control responses. #P < 0.05, 1 min postpropofol response compared with control responses. Note that the VHNs, when compared with the DHNs, are more depressed by the administration of propofol.
DISCUSSION
The purpose of the present study was to determine the extent of depression of nociceptive spinal neuronal responses by three common anesthetics, relative to neuronal location in the spinal cord. The results confirm the hypothesis that neurons in the ventral horn are more depressed by isoflurane, halothane, and propofol when compared with neurons in the dorsal horn. We discuss these findings in relation to the ability of these anesthetics to produce immobility.
Several lines of evidence suggest that anesthetics act primarily in the spinal cord to produce immobility. Anesthetic requirements increase 100%–300% when isoflurane, halothane, thiopental, and experimental anesthetics (such as hexafluorobenzene) are selectively delivered to the brain (2,3,16). After decerebration, or if the spinal cord is transected (using hypothermia to prevent spinal shock), isoflurane MAC is unchanged (4,5). Thus, although these data indicate that the spinal cord is an important site of anesthetic action, it is unclear where in the spinal cord this action might occur. We hasten to point out, however, that there is some evidence that anesthetics such as thiopental, propofol, and isoflurane can modulate nociception and immobility at supraspinal sites (16–19).
Numerous studies over several decades, including in vivo studies (6,8) as well as in vitro work (20,21), have focused on the dorsal horn as the site where anesthetics might act. These in vitro studies compared dorsal horn neuronal activity in control conditions (i.e., without isoflurane) with activity with 1 MAC isoflurane (20,21). These data are consistent with our finding that isoflurane depressed dorsal horn neuronal responses to noxious stimulation in the 0–0.8 MAC range (9), however, no further depression occurred in the 0.8–1.2 MAC range (8,9). This lack of effect of isoflurane between 0.8 and 1.2 MAC is important, as vigorous movement occurs at 0.8 MAC, whereas none occurs at 1.2 MAC. Halothane, on the other hand, depressed dorsal horn neuronal responses across the 0–1.2 MAC concentration range in a concentration-dependent manner, including the step from 0.8 to 1.2 MAC (8,9), although in the present study we did not see depression in this latter range. A possible explanation for this discrepancy might be the different stimulation techniques used. In prior studies we used noxious thermal (8) or electrical stimuli (9), whereas in the present study we used a mechanical stimulus. In our prior study, in which a range of thermal stimulus intensities was given (8), the depressant effects of halothane on dorsal horn neurons were most evident in the moderate stimulus intensity range and less evident at the highest stimulus intensity. A submaximal stimulus might reveal subtle differences among anesthetics that are not apparent with a supramaximal stimulus. Thus, the thermal and electrical stimuli used previously were most likely not supramaximal, whereas the mechanical stimulus used presently was of relatively greater intensity, approaching the supramaximal level of stimulation normally used in MAC studies. Because the focus of the present study was to investigate the relationship between neuronal location and anesthetic depression, we did not further examine the role of stimulus type.
Anesthetics also act on more ventral neurons, such as locomotor/reflex interneurons and motoneurons. For example, motoneurons become hyperpolarized by anesthetics (11), which would render the motoneurons less excitable. In the lamprey, intersegmental coordination of fictive swimming (mediated by locomotor interneurons) is disrupted by minimum immobilizing concentrations of isoflurane (12). Thus, immobility might result from a combination of depression of neurons in the dorsal horn (possibly at least for halothane) as well as ventrally located neurons. The question arises whether neuronal depression depends on location of specific receptors at different laminar depths or if there is progressive non-specific neuronal depression in a dorsal-to-ventral polysynaptic neural circuit.
Why might anesthetics differ in their effects at different laminar depths? Data from labeling studies suggest some laminar differences with respect to various neurotransmitter receptors, such as the N-methyl-D-aspartate (NMDA), γ-aminobutyric acid, and glycine receptors (22). Hence, anesthetics that have divergent receptor effects might exhibit varied responses at different laminae.
Lamina-specific differences in anesthetic effects on dorsal horn neurons have been observed previously (23). For example, Kitahata et al. (7) reported that halothane and thiopental depressed neuronal single unit activity in Lamina I, V, and VI, but not IV. Similarly, N2O has laminar-specific effects on neuronal activity (24). These investigators also reported that ketamine depressed neuronal responses (25) in a laminar-specific manner. We recently observed that N2O depresses neurons in the ventral horn more than those in the dorsal horn (26). Interestingly, administration of MK-801, which blocks the NMDA receptor, did not depress spinal neuronal responses to noxious stimulation (26), although it does depress N2O MAC (27). These data suggest that MK-801 decreases MAC by depressing neurons in the ventral spinal cord, such as motoneurons. Headley et al. (28) reported that ketamine, which also blocks the NMDA receptor, did not depress dorsal horn neurons, but did depress motoneurons. In addition, differences in network connectivity among ventral locomotor interneurons compared with dorsal horn neurons might be important.
Isoflurane, halothane, propofol, N2O, methohexitone, and ketamine have divergent receptor actions (29), yet they all cause a similar pattern of neuronal depression, i.e., more depression of ventral horn when compared with dorsal horn neurons (28). A parsimonious explanation of these findings is that although there might be laminar differences with respect to different receptor types, regardless of the anesthetic, there is progressive depression in a polysynaptic neural circuit involved in the production of movement after noxious stimulation.
Changes in responses of dorsal horn neurons do not necessarily directly translate into an effect at ventrally located neurons. For example, halothane depresses dorsal horn neuronal responses to noxious stimulation, whereas naloxone reverses the depression, but not the immobility (30). Likewise, additional barbiturates in a barbiturate-anesthetized animal, or microinjection of morphine into the periaqueductal gray, cause depression of motor responses (e.g., tail-flick or hindpaw withdrawal reflex), but do not inhibit responses of most dorsal horn neurons (31,32). A cold-block of the spinal cord enhances dorsal horn neuronal responses, but motor responses are depressed (33). Collectively, these data indicate that neuronal modulation in the dorsal horn does not always influence motor output in the expected direction.
It is noteworthy that the neurons most strongly depressed by the anesthetics tended to be located ventromedially in laminae VII–VIII (Fig. 1), a location shown recently to contain a substantial population of neurons with long ascending projections to the cervical ventral horn gray matter (34). Conceivably, some of these neurons may be involved in intersegmental pathways that coordinate hindlimb and forelimb movements during locomotion. The greater susceptibility of such ventrally located neurons to anesthetic depression may partly account for the abolition of gross and purposeful movement to a supramaximal stimulus that defines MAC.
We have found that, in the peri-MAC range, isoflurane, halothane, and propofol depress neurons in the ventral spinal cord more than those located in the dorsal spinal cord. Indeed, the minimal (or absent) depression in the dorsally located neurons in the 0.8–1.2 MAC range suggests that immobility occurs as a result of action at ventral horn neurons. Nonetheless, depression of dorsal horn neurons in the 0–0.8 MAC range (9) might be critical to permit anesthetic action at ventrally located neurons to produce immobility.
References
- 1.Antognini JF, Carstens E. In vivo characterization of clinical anaesthesia and its components. Br J Anaesth. 2002;89:156–66. doi: 10.1093/bja/aef156. [DOI] [PubMed] [Google Scholar]
- 2.Antognini JF, Carstens E, Atherley R. Does the immobilizing effect of thiopental in brain exceed that of halothane? Anesthesiology. 2002;96:980–6. doi: 10.1097/00000542-200204000-00028. [DOI] [PubMed] [Google Scholar]
- 3.Antognini JF, Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993;79:1244 –9. doi: 10.1097/00000542-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Rampil IJ, Mason P, Singh H. Anesthetic potency (MAC) is independent of forebrain structures in the rat. Anesthesiology. 1993;78:707–12. doi: 10.1097/00000542-199304000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Rampil IJ. Anesthetic potency is not altered after hypothermic spinal cord transection in rats. Anesthesiology. 1994;80:606–10. doi: 10.1097/00000542-199403000-00017. [DOI] [PubMed] [Google Scholar]
- 6.De Jong RH, Wagman IH. Block of afferent impulses in the dorsal horn of monkey. A possible mechanism of anesthesia. Exp Neurol. 1968;20:352–8. doi: 10.1016/0014-4886(68)90078-2. [DOI] [PubMed] [Google Scholar]
- 7.Kitahata LM, Ghazi-Saidi K, Yamashita M, Kosaka Y, Bonikos C, Taub A. The depressant effect of halothane and sodium thiopental on the spontaneous and evoked activity of dorsal horn cells: lamina specificity, time course and dose dependence. J Pharmacol Exp Ther. 1975;195:515–21. [PubMed] [Google Scholar]
- 8.Jinks SL, Martin JT, Carstens E, Jung SW, Antognini JF. Peri-MAC depression of a nociceptive withdrawal reflex is accompanied by reduced dorsal horn activity with halothane but not isoflurane. Anesthesiology. 2003;98:1128–38. doi: 10.1097/00000542-200305000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuyo T, Dutton RC, Antognini JF, Carstens E. The differential effects of halothane and isoflurane on windup of dorsal horn neurons selected in unanesthetized decerebrated rats. Anesth Analg. 2006;103:753–60. doi: 10.1213/01.ane.0000230605.22930.52. [DOI] [PubMed] [Google Scholar]
- 10.Cheng G, Kendig JJ. Enflurane directly depresses glutamate AMPA and NMDA currents in mouse spinal cord motor neurons independent of actions on GABAA or glycine receptors. Anesthesiology. 2000;93:1075–84. doi: 10.1097/00000542-200010000-00032. [DOI] [PubMed] [Google Scholar]
- 11.Nicoll RA, Madison DV. General anesthetics hyperpolarize neurons in the vertebrate central nervous system. Science. 1982;217:1055–7. doi: 10.1126/science.7112112. [DOI] [PubMed] [Google Scholar]
- 12.Jinks SL, Atherley RJ, Dominguez CL, Sigvardt KA, Antognini JF. Isoflurane disrupts central pattern generator activity and coordination in the lamprey isolated spinal cord. Anesthesiology. 2005;103:567–75. doi: 10.1097/00000542-200509000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Salford LG, Siesjo BK. The influence of arterial hypoxia and unilateral carotid artery occlusion upon regional blood flow and metabolism in the rat brain. Acta Physiol Scand. 1974;92:130–41. doi: 10.1111/j.1748-1716.1974.tb05729.x. [DOI] [PubMed] [Google Scholar]
- 14.Mitsuyo T, Antognini JF, Carstens EE. Etomidate depresses lumbar dorsal horn neuronal responses to noxious thermal stimulation in rats. Anesth Analg. 2006;102:1169–73. doi: 10.1213/01.ane.0000204764.13637.d4. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic Press; 1998. pp. 9–78. [Google Scholar]
- 16.Antognini JF, Raines DE, Solt K, Barter LS, Atherley RJ, Bravo E, Laster MJ, Jankowska K, Eger EI., II Hexafluorobenzene acts in the spinal cord, whereas o-difluorobenzene acts in both brain and spinal cord, to produce immobility. Anesth Analg. 2007;104:822–8. doi: 10.1213/01.ane.0000255226.63909.32. [DOI] [PubMed] [Google Scholar]
- 17.Devor M, Zalkind V. Reversible analgesia, atonia, and loss of consciousness on bilateral intracerebral microinjection of pentobarbital. Pain. 2001;94:101–12. doi: 10.1016/S0304-3959(01)00345-1. [DOI] [PubMed] [Google Scholar]
- 18.Stabernack C, Zhang Y, Sonner JM, Laster M, Eger EI., II Thiopental produces immobility primarily by supraspinal actions in rats. Anesth Analg. 2005;100:128–36. doi: 10.1213/01.ANE.0000139353.97950.FA. [DOI] [PubMed] [Google Scholar]
- 19.Kingery WS, Agashe GS, Guo TZ, Sawamura S, Davies MF, Clark JD, Kobilka BK, Maze M. Isoflurane and nociception: spinal alpha2A adrenoceptors mediate antinociception while supraspinal alpha1 adrenoceptors mediate pronociception. Anesthesiology. 2002;96:367–74. doi: 10.1097/00000542-200202000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Haseneder R, Kurz J, Dodt HU, Kochs E, Zieglgansberger W, Scheller M, Rammes G, Hapfelmeier G. Isoflurane reduces glutamatergic transmission in neurons in the spinal cord superficial dorsal horn: evidence for a presynaptic site of an analgesic action. Anesth Analg. 2004;98:1718–23. doi: 10.1213/01.ANE.0000112309.80017.3F. [DOI] [PubMed] [Google Scholar]
- 21.Georgiev SK, Wakai A, Kohno T, Yamakura T, Baba H. Actions of norepinephrine and isoflurane on inhibitory synaptic transmission in adult rat spinal cord substantia gelatinosa neurons. Anesth Analg. 2006;102:124–8. doi: 10.1213/01.ane.0000184829.25310.38. [DOI] [PubMed] [Google Scholar]
- 22.Coggeshall RE, Carlton SM. Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Res Brain Res Rev. 1997;24:28–66. doi: 10.1016/s0165-0173(97)00010-6. [DOI] [PubMed] [Google Scholar]
- 23.Kitahata LM. Modes and sites of “analgesic” action of anesthetics on the spinal cord. Int Anesthesiol Clin. 1975;13:149–70. doi: 10.1097/00004311-197513010-00007. [DOI] [PubMed] [Google Scholar]
- 24.Taub A, Hoffert M, Kitahata LM. Lamina-specific suppression and acceleration of dorsal-horn unit activity by nitrous oxide: a statistical analysis. Anesthesiology. 1974;40:24–31. doi: 10.1097/00000542-197401000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Kitahata LM, Taub A, Kosada Y. Lamina-specific suppression of dorsal-horn unit activity by detamine hydrochloride. Anesthesiology. 1973;38:4–11. doi: 10.1097/00000542-197301000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Antognini JF, Atherley RJ, Dutton RC, Laster MJ, Eger EI, II, Carstens E. The excitatory and inhibitory effects of nitrous oxide on spinal neuronal responses to noxious stimulation. Anesth Analg. 2007;104:829–35. doi: 10.1213/01.ane.0000255696.11833.24. [DOI] [PubMed] [Google Scholar]
- 27.Eger EI, II, Liao M, Laster MJ, Won A, Popovich J, Raines DE, Solt K, Dutton RC, Cobos FV, Sonner JM. Contrasting roles of the N-methyl-D-aspartate receptor in the production of immobilization by conventional and aromatic anesthetics. Anesth Analg. 2006;102:1397–406. doi: 10.1213/01.ANE.0000219019.91281.51. [DOI] [PubMed] [Google Scholar]
- 28.Headley PM, Parsons CG, West DC. The role of N-methylaspartate receptors in mediating responses of rat and cat spinal neurones to defined sensory stimuli. J Physiol. 1987;385:169–88. doi: 10.1113/jphysiol.1987.sp016490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci. 1999;55:1278–303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You HJ, Colpaert FC, Arendt-Nielsen L. Nociceptive spinal withdrawal reflexes but not spinal dorsal horn wide-dynamic range neuron activities are specifically inhibited by halothane anaesthesia in spinalized rats. Eur J Neurosci. 2005;22:354–60. doi: 10.1111/j.1460-9568.2005.04234.x. [DOI] [PubMed] [Google Scholar]
- 31.Carstens E, Stelzer B, Zimmermann M. Microinjections of glutamate or morphine at coincident midbrain sites have different effects on nociceptive dorsal horn neurons in the rat. Neurosci Lett. 1988;95:185–91. doi: 10.1016/0304-3940(88)90654-4. [DOI] [PubMed] [Google Scholar]
- 32.Carstens E, Campell IG. Responses of motor units during the hind limb flexion withdrawal reflex evoked by noxious skin heating: phasic and prolonged suppression by midbrain stimulation and comparison with simultaneously recorded dorsal horn units. Pain. 1992;48:215–26. doi: 10.1016/0304-3959(92)90061-F. [DOI] [PubMed] [Google Scholar]
- 33.Jinks SL, Antognini JF, Carstens E. Isoflurane depresses diffuse noxious inhibitory controls in rats between 0.8 and 1.2 minimum alveolar anesthetic concentration. Anesth Analg. 2003;97:111–6. doi: 10.1213/01.ane.0000066259.39584.f7. [DOI] [PubMed] [Google Scholar]
- 34.Dutton RC, Carstens MI, Antognini JF, Carstens E. Long ascending propriospinal projections from lumbosacral to upper cervical spinal cord in the rat. Brain Res. 2006;1119:76–85. doi: 10.1016/j.brainres.2006.08.063. [DOI] [PubMed] [Google Scholar]


