Abstract
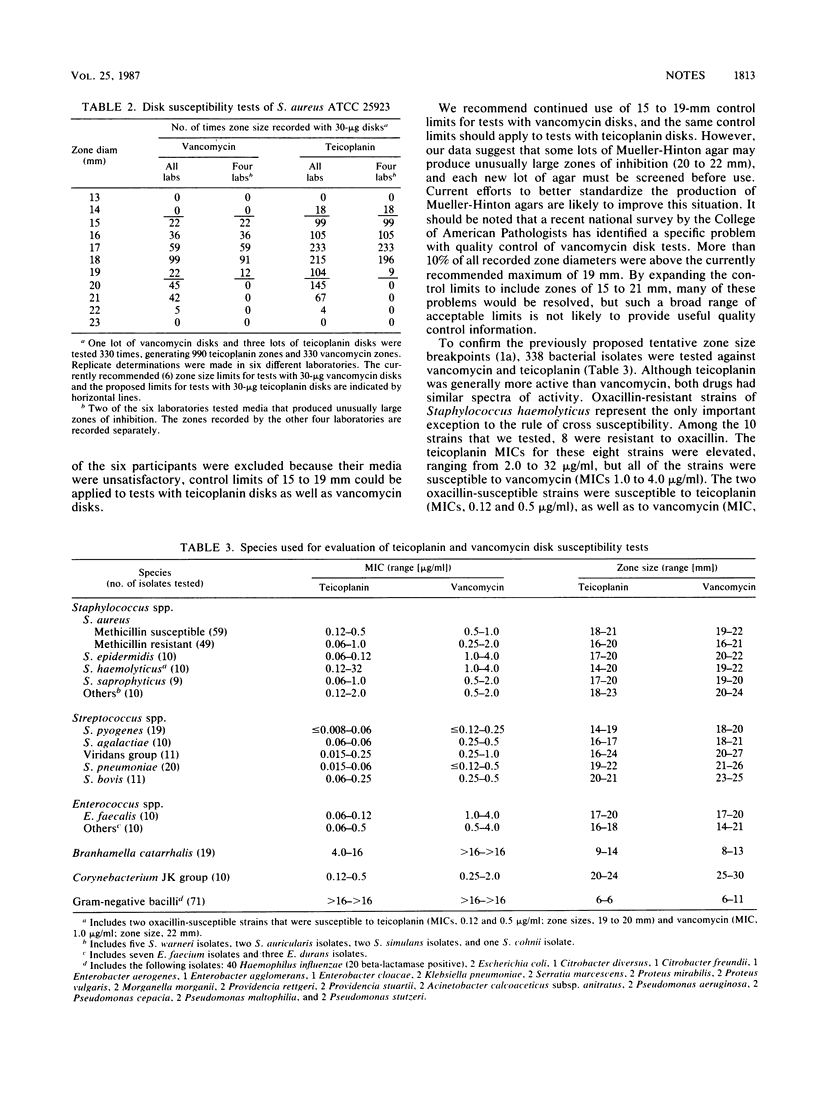
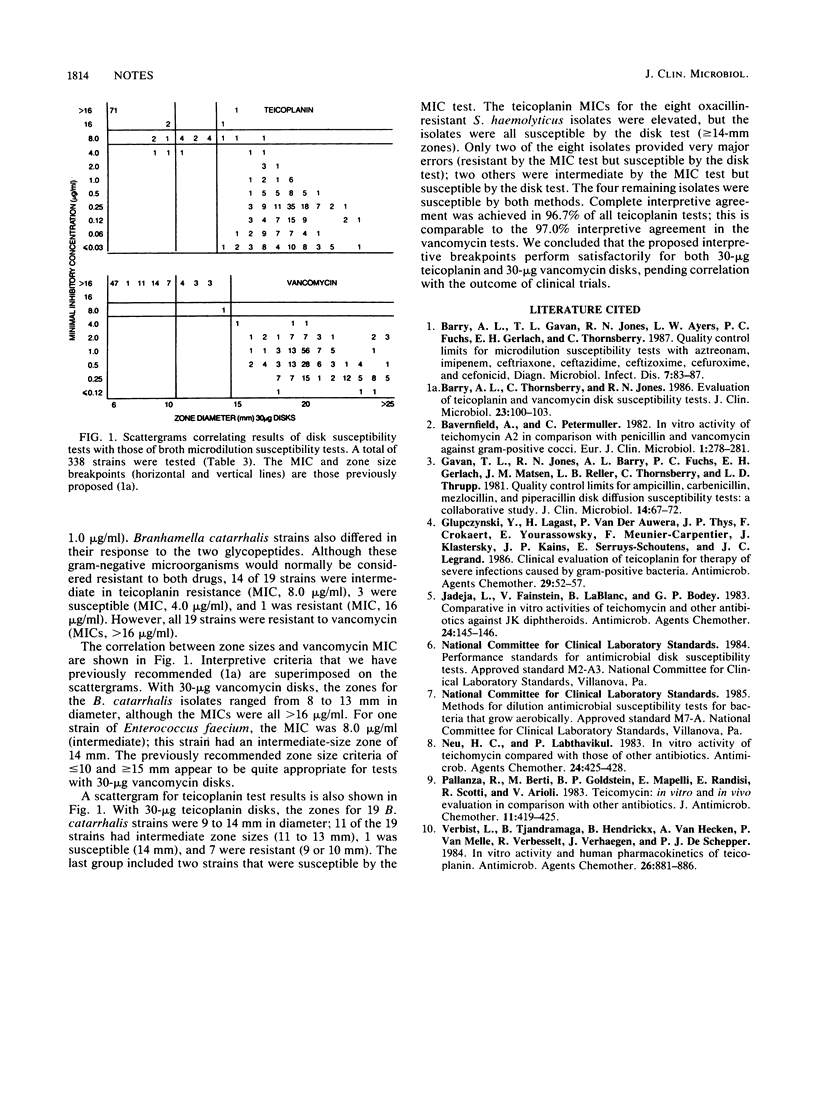
For monitoring the performance of teicoplanin susceptibility tests, the following quality control limits are recommended: Staphylococcus aureus ATCC 29213, MIC of 0.12 to 0.5 micrograms/ml; Enterococcus faecalis ATCC 29212, MIC of 0.06 to 0.25 micrograms/ml; and S. aureus ATCC 25923, zones 15 to 19 mm in diameter (30-micrograms disks). However, some lots of Mueller-Hinton agar provided unusually large zones of inhibition with both vancomycin and teicoplanin disks, and these lots should be excluded before routine use. Teicoplanin and vancomycin differed only in their activity against oxacillin-resistant strains of Staphylococcus haemolyticus, which had decreased susceptibility to teicoplanin but were fully susceptible to vancomycin. For tests with 30-micrograms teicoplanin disks, zones less than or equal to 10 and greater than or equal to 14 mm in diameter represent resistant and susceptible breakpoints, respectively.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Gavan T. L., Jones R. N., Ayers L. W., Fuchs P. C., Gerlach E. H., Thornsberry C. Quality control limits for microdilution susceptibility tests with aztreonam, imipenem, ceftriaxone, ceftazidime, ceftizoxime, cefuroxime, and cefonicid. Diagn Microbiol Infect Dis. 1987 May;7(1):83–87. doi: 10.1016/0732-8893(87)90076-9. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N. Evaluation of teicoplanin and vancomycin disk susceptibility tests. J Clin Microbiol. 1986 Jan;23(1):100–103. doi: 10.1128/jcm.23.1.100-103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind A., Petermüller C. In vitro activity of teichomycin A 2 in comparison with penicillin and vancomycin against gram-positive cocci. Eur J Clin Microbiol. 1982 Oct;1(5):278–281. doi: 10.1007/BF02019971. [DOI] [PubMed] [Google Scholar]
- Gavan T. L., Jones R. N., Barry A. L., Fuchs P. C., Gerlach E. H., Matsen J. M., Reller L. B., Thornsberry C., Thrupp L. D. Quality control limits for ampicillin, carbenicillin, mezlocillin, and piperacillin disk diffusion susceptibility tests: a collaborative study. J Clin Microbiol. 1981 Jul;14(1):67–72. doi: 10.1128/jcm.14.1.67-72.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glupczynski Y., Lagast H., Van der Auwera P., Thys J. P., Crokaert F., Yourassowsky E., Meunier-Carpentier F., Klastersky J., Kains J. P., Serruys-Schoutens E. Clinical evaluation of teicoplanin for therapy of severe infections caused by gram-positive bacteria. Antimicrob Agents Chemother. 1986 Jan;29(1):52–57. doi: 10.1128/aac.29.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadeja L., Fainstein V., LeBlanc B., Bodey G. P. Comparative in vitro activities of teichomycin and other antibiotics against JK diphtheroids. Antimicrob Agents Chemother. 1983 Aug;24(2):145–146. doi: 10.1128/aac.24.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. In vitro activity of teichomycin compared with those of other antibiotics. Antimicrob Agents Chemother. 1983 Sep;24(3):425–428. doi: 10.1128/aac.24.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanza R., Berti M., Goldstein B. P., Mapelli E., Randisi E., Scotti R., Arioli V. Teichomycin: in-vitro and in-vivo evaluation in comparison with other antibiotics. J Antimicrob Chemother. 1983 May;11(5):419–425. doi: 10.1093/jac/11.5.419. [DOI] [PubMed] [Google Scholar]
- Verbist L., Tjandramaga B., Hendrickx B., Van Hecken A., Van Melle P., Verbesselt R., Verhaegen J., De Schepper P. J. In vitro activity and human pharmacokinetics of teicoplanin. Antimicrob Agents Chemother. 1984 Dec;26(6):881–886. doi: 10.1128/aac.26.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]



