Abstract
Purpose
This study will determine whether MTT assays accurately assess the effect of STI571 (Gleevec; Abl kinase inhibitor) on the viability of cancer cells containing highly active Abl kinases.
Methods
Growth kinetics, tritiated thymidine, fluorescent caspase, MTT, and Cell Titer Glo assays were used to determine the effect of STI571 on growth, proliferation, apoptosis, and viability of melanoma and breast cancer cells.
Results
STI571 inhibited growth and proliferation, and increased apoptosis. However, MTT assays indicated that STI571 increased cell viability. In contrast, STI571 induced a dose-dependent decrease in viability using Cell Titer Glo assays.
Conclusions
Doses of STI571 (1−10μM) required to inhibit endogenous Abl kinases interfere with the MTT assay, and therefore MTT cannot be used to determine the effect of STI571 on viability using these doses. Additionally, caution should be utilized when interpreting the results of MTT assays used to screen kinase inhibitors for anti-cancer activity, as drug effectiveness may be minimized.
Keywords: STI571, Gleevec, Abl, MTT, Cell Titer Glo, viability
INTRODUCTION
The Abl family of non-receptor tyrosine kinases (Abl kinases) includes two proteins, c-Abl and Arg, encoded by Abl1 and Abl2 genes, respectively [1]. c-Abl and Arg are highly homologous in their N-termini, where they contain SH3, SH2, and kinase domains, but are more divergent in their C-termini [1]. Abl kinases are known for their involvement in human leukemia, as c-Abl is translocated next to BCR, which results in a BCR-Abl fusion protein that drives the development of chronic myelogenous leukemia (CML) [2]. STI571 (Gleevec; imatinib) was developed to specifically inhibit BCR-Abl, and is FDA-approved to treat CML [3]. In addition to inhibiting BCR-Abl, STI571 also inhibits endogenous c-Abl and Arg [4]. We showed that endogenous Abl kinases are activated by growth factors (PDGF, platelet-derived growth factor; EGF, epidermal growth factor receptor), and promote proliferation, membrane ruffling, and migration in fibroblasts [5, 6]. Significantly, we recently demonstrated that Abl kinases also are activated downstream of deregulated growth factor receptors (PDGFR, EGFR, IGF-1R, ErbB2/Her-2) and Src family kinases in invasive breast cancer cells, and promote invasion, proliferation, and survival in response to nutrient deprivation [7, 8].
The MTT (3,-(4,5-dimethylthiazol-2-y))-2,5-diphenyl tetrazolium bromide) colorimetric assay is widely applied to assess cell viability, proliferation, and differentiation. The tetrazolium salt, MTT, is reduced to formazan, which can be analyzed colorimetrically. Reduction of MTT to formazan is due to cellular enzymatic activity not only in the mitochondria, but also in endosomes, and lysosomes [9, 10]. The MTT assay is commonly used to screen compounds for effects on viability; however, there are several reports of agents that increase MTT reduction to formazan without increasing cell viability, including drug efflux inhibitors, genistein, ursolic acid, resveratrol, and interferons [11-14]. Despite these reports, tetrazolium-based assays continue to be used for screening cell lines for the effectiveness of various drugs/compounds, many times without corroborating results using complementary assays.
Here, we report that STI571, an Abl kinase inhibitor, inhibits cell growth, proliferation, and induces apoptosis of two cell lines containing high Abl kinase activity: MDA-MB-435s melanoma cells and MDA-MB-468 breast cancer cells. However, MTT assays clearly show a dose-dependent increase in MTT reduction to formazan with STI571 treatment, which is inconsistent with proliferation and apoptosis assays. In contrast, we demonstrate that another viability assay, Cell Titer Glo, which measures cellular ATP and does not require reduction of a compound, is a better method for determining the effect on viability, as the results are consistent with tritiated thymidine and caspase assays.
MATERIALS AND METHODS
Reagents
STI571 (Gleevec; imatinib) was obtained from Novartis Pharmaceuticals (Basel, Switzerland), dissolved in water at a concentration of 10mM, and stored at −80°C. Doxorubicin was obtained from Sigma (St. Louis, MO) and dissolved in water.
Growth Kinetic Assay
Cells were plated in 6-well dishes in triplicate, so that cells were 30% confluent the next day when they were treated with STI571, and trypan blue-negative cells were counted on a hemacytometer on the indicated days. Cells were fed with fresh media and STI571 every third day.
Tritiated Thymidine Assay
Cells were plated in 12-well dishes in triplicate, and the next day the media was replaced with media containing STI571. Seventy-two hours later, cells were labeled with tritiated thymidine for 2 hours, harvested by washing with phosphate-buffered saline, 10% trichloroacetic acid (TCA), incubating in 10% TCA for 45 minutes, solubilizing radioactivity in 0.2N NaOH, and reading on a scintillation counter.
MTT Assay (Sigma; St. Louis, MO)
Cells were plated in triplicate in 96-well plates at a density of 2500 cells/well, and the next day the media was replaced with media containing STI571. After 72 hours, the media was replaced with 3-(4, 5-methylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT)-containing media (0.5mg/ml), incubated at 37°C for 4 hours, an equal volume of solubilization solution (10% SDS, 0.01M HCl) was added, and the plate was incubated at 37°C overnight to solubilize formazon crystals. Absorbance was measured at 570nm in a Biotek Synergy 2 plate reader (Biotek; Winooski, VT).
Cell Titer Glo Assay (Promega; Fitchburg, WI)
Cells were plated in triplicate in 96-well dishes (2500 cells/well), the day after plating the media was replaced with media containing STI571, and 72 hours later an equal volume of Cell Titer Glo reagent was added. Plates were rocked on a rotator, half of the total volume of each well was transferred to a 96-well opaque plate, rocked for 2 minutes, and total light emitted was measured ten minutes later on a Biotek Synergy 2 plate reader.
Fluorescent Caspase-3/7 Assay (Sigma; St. Louis, MO)
Cells were plated in 6-well dishes (60,000 cells/well), treated for 40hours with STI571, and lysed in 1X lysis buffer. Lysate (5μl) was added to an opaque 96-well plate, incubated with 200 μl of substrate (diluted 1:4), and fluorescence was assessed at 360nm (excitation)/460nm (emission) using a Biotek Synergy 2 plate reader. Relative Fluorescence Units (RFUs) were divided by the protein concentration of each sample, which was determined by Bradford assay (Biorad; Hercules, CA).
RESULTS
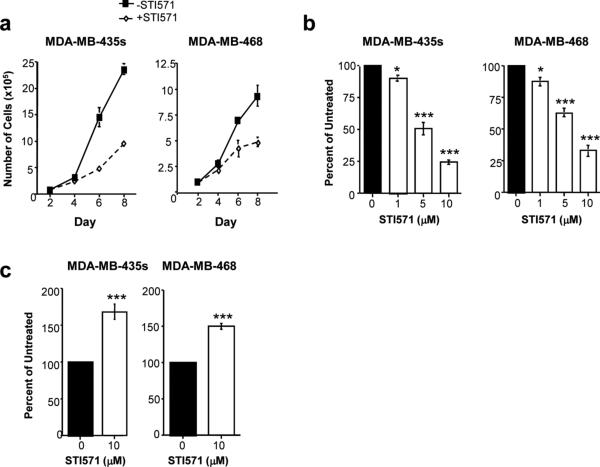
Previously, we showed that Abl kinases are activated in invasive breast cancer cells, and also in MDA-MB-435s cells [7]. MDA-MB-435 cells were originally thought to be a breast cancer cell line, but recently were shown to be M14 melanoma cells [15]. MDA-MB-435s is a highly invasive, spindle-shaped variant derived from MDA-MB-435. We demonstrated that treatment of cell lines containing active Abl kinases, grown in serum conditions, with the Abl kinase inhibitor, STI571 (Gleevec), dramatically inhibits cell growth [8]. The growth rate of MDA-MB-435s melanoma cells and MDA-MB-468 breast cancer cells in the absence or presence of STI571 is shown in Fig. 1a [8]. A dose of 10μM STI571 was used for this assay because we showed that 10μM was required to reduce phosphorylation/activity of active endogenous c-Abl by 65−75% [7]. To determine whether the decrease in cell growth was due to decreased proliferation, we performed tritiated thymidine proliferation assays. We found that proliferation was dramatically reduced in MDA-MB-435s and MDA-MB-468 cells treated with STI571, and these effects were dose-dependent (Fig. 1b) [8]. STI571 inhibits c-Kit and PDGF receptors in addition to Abl kinases [4]. PDGF receptors are not expressed in either cell line, while c-Kit is expressed in MDA-MB-468 but not MDA-MB-435s cells [7]. To determine whether STI571 induces apoptosis of cells grown in serum-conditions, we performed fluorescent caspase-3/7 assays. Significantly, treatment of MDA-MB-435s and MDA-MB-468 cells with STI571 increased caspase activity (Fig. 1c). Therefore, STI571 reduces the growth of cells in serum by inhibiting proliferation and inducing apoptosis.
Fig. 1. STI571 inhibits cell growth, proliferation, and induces apoptosis of cells containing highly active Abl kinases.
(a) MDA-MB-435s melanoma cells and MDAMB-468 breast cancer cells were plated in serum in triplicate 6-well dishes, STI571 was added the next day (Day 2), and cell growth was assessed by counting trypan blue-negative cells on a hemacytometer. Experiments shown are representative of three independent experiments. (b) Cell lines were plated in triplicate in 12-well dishes, the media was replaced the next day with media containing STI571, and tritiated thymidine incorporation was measured 72 hours later. Experiments shown are mean ± s.e.m. of three independent experiments. Values were normalized to values obtained in untreated wells and expressed as a percentage of untreated. *p<0.05, ***p<0.001 using Student t-tests. (c) Cells were plated in 6-well dishes, the media was replaced with media containing STI571 the next day, and caspase-3/7 activity was detected in detached and attached cells by fluorescent caspase assay, 40 hours after treatment. Mean ± s.e.m. of three independent experiments. ***p<0.001 using a Student t-test. Fig. 1a was adapted from Srinivasan and Plattner (2006) [7].
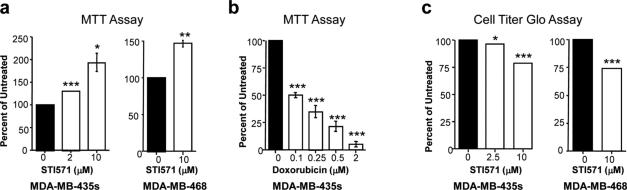
Since both proliferation and apoptosis are affected by STI571, viability assays should demonstrate a decrease in viability in the presence of STI571. To test this hypothesis, we utilized the MTT assay (Sigma; St. Louis, MO). MDA-MB-435s and MDA-MB-468 cells were grown in the absence or presence of STI571 in serum, and MTT was added 72 hours later (the same timepoint used for tritiated thymidine assays). Surprisingly, although there was a noticeable decrease in cell number in wells treated with STI571, there was increased rather than decreased MTT reduction to formazan in STI571-treated MDA-MB-435s and MDA-MB-468 cells, and the increase in formazan formation was dependent on the STI571 dose, which was observed with as little as 1μM STI571 (Fig. 2a, data not shown). These data clearly were inconsistent with growth kinetic, tritiated thymidine, and caspase assays, and also were inconsistent with what was observed by eye (Fig. 1, data not shown). To be certain that the MTT assay was working in our hands, we treated MDA-MB-435s cells with increasing doses of doxorubicin, a drug known to inhibit cell viability, and assessed the effect on reduction of MTT. As shown in Fig. 2b, increasing doses of doxorubicin induced decreased reduction of MTT, demonstrating that the MTT assay effectively assesses the ability of doxorubicin to inhibit viability.
Fig. 2. STI571 interferes with the ability of the MTT assay to measure cell viability.
MDA-MB-435s melanoma cells and MDA-MB-468 breast cancer cells were plated in 96-well dishes in triplicate, the media was replaced the next day with media containing STI571 (a,c) or doxorubicin (b), and 72 hours later the cells were used either in MTT (a,b) or Cell Titer Glo (c) viability assays. Experiments shown are mean ± s.e.m. for three independent experiments. *p<0.05, **p<0.005, ***p<0.001 using Student t-tests. Some error bars are too small to be visualized.
To determine whether the effect of STI571 on viability was specific to the tetrazolium-based assay, we assessed the effect of STI571 treatment on the viability of MDA-MB-435s and MDA-MB-468 cells using a different assay, Cell Titer Glo (CTG; Promega; Fitchburg, WI). Cell Titer Glo is a luminescent assay that quantitates the amount of cellular ATP present, which reflects the number of metabolically active, viable cells. Luciferase enzymatic activity requires ATP, and thus the amount of luminescent signal is proportional to the amount of ATP present in the lysate. Treatment of MDA-MB-435s and MDA-MB-468 cells with STI571 resulted in a decrease in Cell Titer Glo luminescence (Fig. 2c) consistent with growth kinetic, tritiated thymidine, and caspase assays (Fig. 1), although the decrease in luminescence was not as great as one would expect given the large effect of STI571 on proliferation and its additional effect on apoptosis. However, the Cell Titer Glo assay did detect a decrease in viability that was not observed with the MTT assay.
DISCUSSION
Tetrazolium salt assays (MTT, MTS, XTT) are often used to screen cell lines for sensitivity to drugs, due to the fact that they are relatively inexpensive, are quick, easy, and quantitative. However, our data clearly show that STI571 cannot reliably be used in MTT assays when utilizing doses of 1−10μM (Fig. 2; data not shown), as it interferes with the assay. MTT assays have frequently been used to study the effects of STI571. In leukemic cells, BCR-Abl is much more sensitive to STI571, and doses of 0.1−1μM inhibit BCR-Abl phosphorylation and/activity [4]. At these doses, STI571 may not affect the MTT assay or may not affect it to as great an extent as when higher doses are used. However, we and others have shown that higher doses of STI571 (5−10μM) are required to inhibit the activity of endogenous Abl kinases in non-transformed cells and in solid tumor cell lines [7, 16]. The MTT assay has been used frequently to determine the ability of STI571 to reduce viability in tumor cell lines. For example, one study used the MTT assay to determine the sensitivity of melanoma cells to STI571 [17]. This study concluded that M14 (MDA-MB-435) cells are resistant to the effects of STI571, while we show that MDA-MB-435s cells are sensitive to STI571, using both proliferation and apoptosis assays. We performed sTR analysis to be certain that the cells we are utilizing are indeed derived from M14 melanoma cells, and this was determined to be the case, as all loci matched those obtained for M14 cells (data not shown). It is possible that MDA-MB-435s cells are sensitive to STI571 and MDA-MB-435 (M14) cells are not; however it is more likely that the MTT assay was unable to detect sensitivity in the MDA-MB-435 cells (M14) since STI571 interferes with the assay. In another study, STI571 had no effect on reduction of MTT to formazan in c-Kit-expressing Ewing sarcoma cells at doses of ≤10μM STI571; however a second assay to corroborate MTT results were not utilized [18]. Therefore, MTT assays cannot reliably be utilized to determine the effect of STI571 on the viability of solid tumor cells if doses >1 μM are utilized, as STI571 interferes with the assay, which may lead to erroneous conclusions.
Other drugs/compounds, such as drug transporter inhibitors, interferons, resveratrol, and ursolic acid also interfere with MTT assays, and cause an underestimation of the cytotoxicity effects [11-14]. Genistein, a tyrosine kinase inhibitor, inhibits cell growth, but causes increased MTT reduction to formazan, most likely because it increases cell volume and mitochondrial number and activity [13]. It is possible that the tyrosine kinase inhibitor, STI571, may have a similar effect on cell volume or mitochondrial number and/or activity. Factors in the cell culture environment such as pH and glucose supply also influence MTT reduction to formazan [19]. Decreased concentrations of D-glucose, NADH, or NADPH in the culture medium can induce decreased reduction of MTT to formazan, and thus drugs that affect cell metabolism may cause alterations in mitochondrial activity and alter the validity of the assay [20].
Cell Titer Glo viability assays give results that are more consistent with proliferation and apoptosis assays; however, this assay also appears to underestimate the effects of STI571, which suggests that cell viability assays on their own are not as reliable as their use in combination with proliferation and apoptosis assays. In summary, our data emphasize that caution should be utilized when interpreting the results of tetrazolium assays for screening potential anti-cancer drugs, as the effectiveness of the drugs may be minimized by the assay.
ACKNOWLEDGEMENTS
This work was supported by National Institute of Health Grant P20 RR20171 from the National Center for Research Resources, and National Institute of Health/National Cancer Institute Grant 1R01CA116784 to R.P. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. We thank Elisabeth Buchdunger (Novartis Pharmaceuticals; Basel, Switzerland) for providing STI571, and Leann Fiore for critically reading the manuscript.
REFERENCES
- 1.Pendergast AM. The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res. 2002;85:51–100. doi: 10.1016/s0065-230x(02)85003-5. [DOI] [PubMed] [Google Scholar]
- 2.Pendergast AM. BCR-ABL protein domain, function, and signaling. In: Helmann R, editor. Chronic Myeloid Leukaemia: Biology and Treatment. Martin Dunitz Lt; London: 2001. pp. 19–39. [Google Scholar]
- 3.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 4.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 5.Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plattner R, Irvin BJ, Guo S, Blackburn K, Kazlauskas A, Abraham RT, York JD, Pendergast AM. A New Link Between the c-Abl Tyrosine Kinase and Phosphoinositide Signaling via PLC-γ1. Nat Cell Biol. 2003;5:309–319. doi: 10.1038/ncb949. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan D, Plattner R. Activation of abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 2006;66:5648–5655. doi: 10.1158/0008-5472.CAN-06-0734. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan D, Sims JT, Plattner R. Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene. 2008;27:1095–1105. doi: 10.1038/sj.onc.1210714. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem. 1997;69:581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- 10.Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993;303:474–482. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- 11.Bernhard D, Schwaiger W, Crazzolara R, Tinhofer I, Kofler R, Csordas A. Enhanced MTT-reducing activity under growth inhibition by resveratrol in CEMC7H2 lymphocytic leukemia cells. Cancer Lett. 2003;195:193–199. doi: 10.1016/s0304-3835(03)00157-5. [DOI] [PubMed] [Google Scholar]
- 12.Vellonen KS, Honkakoski P, Urtti A. Substrates and inhibitors of efflux proteins interfere with the MTT assay in cells and may lead to underestimation of drug toxicity. Eur J Pharm Sci. 2004;23:181–188. doi: 10.1016/j.ejps.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Pagliacci MC, Spinozzi F, Migliorati G, Fumi G, Smacchia M, Grignani F, Riccardi C, Nicoletti I. Genistein inhibits tumour cell growth in vitro but enhances mitochondrial reduction of tetrazolium salts: a further pitfall in the use of the MTT assay for evaluating cell growth and survival. Eur J Cancer. 1993;29A:1573–1577. doi: 10.1016/0959-8049(93)90297-s. [DOI] [PubMed] [Google Scholar]
- 14.Es-Saady D, Simon A, Jayat-Vignoles C, Chulia AJ, Delage C. MCF-7 cell cycle arrested at G1 through ursolic acid, and increased reduction of tetrazolium salts. Anticancer Res. 1996;16:481–486. [PubMed] [Google Scholar]
- 15.Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 melanoma cells--a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 16.Burton EA, Plattner R, Pendergast AM. Abl tyrosine kinases are required for infection by Shigella flexneri. EMBO J. 2003;22:5471–5479. doi: 10.1093/emboj/cdg512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayorga ME, Sanchis D, Perez de Santos AM, Velasco A, Dolcet X, Casanova JM, Baradad M, Egido R, Pallares J, Espurz N, Benitez D, Mila J, Malvehy J, Castel T, Comella JX, Matias-Guiu X, Vilella R, Marti RM. Antiproliferative effect of STI571 on cultured human cutaneous melanoma-derived cell lines. Melanoma Res. 2006;16:127–135. doi: 10.1097/01.cmr.0000215039.30812.9b. [DOI] [PubMed] [Google Scholar]
- 18.Hotfilder M, Lanvers C, Jurgens H, Boos J, Vormoor J. c-KIT-expressing Ewing tumour cells are insensitive to imatinib mesylate (STI571). Cancer Chemother Pharmacol. 2002;50:167–169. doi: 10.1007/s00280-002-0477-8. [DOI] [PubMed] [Google Scholar]
- 19.Marshall NJ, Goodwin CJ, Holt SJ. A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul. 1995;5:69–84. [PubMed] [Google Scholar]
- 20.Hayon T, Dvilansky A, Shpilberg O, Nathan I. Appraisal of the MTT-based assay as a useful tool for predicting drug chemosensitivity in leukemia. Leuk Lymphoma. 2003;44:1957–1962. doi: 10.1080/1042819031000116607. [DOI] [PubMed] [Google Scholar]