Abstract
Background
TACI expression on B cells is upregulated by TLR4.
Objective
To examine whether TACI synergizes with TLR4 in driving immunoglobulin (Ig) production by B cells and to examine the mechanism of this synergy.
Methods
Purified mouse naïve B cells were stimulated with the TACI ligand APRIL and with suboptimal concentrations of the TLR4 ligand LPS in the presence or absence of IL-4. Ig secretion was measured by ELISA. Surface IgG1+ (sIgG1+) B cells and CD138+ plasmacytoid cells were enumerated by FACS. Expression of γ1 and ε germ line transcripts (GLT), activation-induced cytidine deaminase (AICDA) and γ1 and ε mature transcripts was measured by RT-PCR.
Results
APRIL synergized with LPS in driving B cell proliferation and IgM, IgG1, IgG3, IgE and IgA production. This was mediated by TACI as it was preserved in BCMA-/-, but not TACI-/-, B cells. APRIL and LPS synergized to promote isotype switching as evidenced by increased expression of AICDA and γ1 and ε mature transcripts, and generation of sIgG1+ cells. More importantly, APRIL and LPS strongly synergized to drive the plasma cell differentiation program, as evidenced by increase in CD138+ cells and expression of Blimp-1, IRF-4 and the spliced form of XBP-1. TACI-/- mice had impaired IgM and IgG1 antibody responses to immunization with a suboptimal dose of the type I T independent antigen TNP-LPS.
Conclusions
These observations suggest that TACI cooperates with TLR4 to drive B cell differentiation and immunoglobulin production in vitro and in vivo.
Clinical Implications
Impaired synergy of TACI with TLRs may contribute to low serum immunoglobulin levels in CVID (Common Variable Immune Deficiency) patients with TACI mutations.
Capsule Summary
This work shows that TACI drives plasma cell differentiation in LPS stimulated cells and increases immunoglobulin production in vitro and in vivo.
Keywords: B cells, TLR, LPS, TACI, APRIL, immunoglobulin
INTRODUCTION
The TNF superfamily members BAFF (B cell activating factor belonging to TNF superfamily) and APRIL (a proliferation inducing ligand) play important roles in B cell development, homeostasis and activation. BAFF is expressed by neutrophils, monocytes and dendritic cells and promotes B lymphocyte survival and maturation 1. APRIL is expressed in monocytes/macrophages, DCs (Dendritic cells) and activated T cells 1. APRIL-/- mice have low serum IgA levels, impaired IgA antibody responses to oral immunization and decreased antibody response to the TD (T cell dependent) antigen tetanus toxoid 2, 3. BAFF and APRIL activate isotype switching in naïve B cells, albeit modestly compared to CD40 or TLR (Toll-like receptor) ligation 4, 5.
APRIL and BAFF share two receptors, TACI (transmembrane activator, calcium modulator, and cyclophilin ligand interactor) and BCMA (B cell maturation antigen) 6. TACI is expressed on a fraction of B cells 7, 8. BCMA is poorly expressed on resting B cells, but is upregulated on plasma cells (PCs) and germinal center B cells 9. A third receptor, BAFF-R is unique for BAFF and is expressed mainly on B cells 10. APRIL also binds to heparan-sulfated proteoglycans (HSPGs) 11, 12. TACI-/- mice have low serum IgM and IgA and have deficient antibody responses to the Type II T-independent (TI) antigens Pneumovax and TNP-Ficoll 13-15. TACI mediates isotype switching by APRIL 4. BCMA plays no detectable role in isotype switching 4.
TLRs recognize specific molecular patterns of microbial pathogens 16 and play an important role in the induction of adaptive immune responses, in part by promoting the maturation of antigen presenting DCs to fully activate T and B cells 17. The TLR4 ligand LPS, and the TLR9 ligand CpG, activate isotype switching in B cells in the presence of cytokines 18,19. LPS drives class switch recombination to IgG3 on its own 20.
B cells encounter antigens in secondary lymphoid organs, where they proliferate, undergo maturation and differentiation that includes class switch recombination (CSR), affinity maturation and differentiation into plasma cells or memory B cells. These processes are initiated and regulated by signals derived from ligand-receptor interactions that include CD40 ligand-CD40, BAFF/APRIL-TACI and TLR ligand-TLR interactions. The CD40L-CD40 interaction occurs in germinal centers and involve follicular helper T cells and their expression of ICOS and IL-2121. CD40 ligation upregulates TACI expression and synergizes with TACI ligation to enhance immmunoglobulin (Ig) production by naïve B cells 22. The TLR ligands TLR4 and TLR9 also upregulate TACI expression, and TLR9 synergizes with TACI to increase Ig production by B cells 18. In this study, we show that APRIL synergizes with suboptimal doses of LPS in driving naive mouse B cells to proliferate, generate plasmacytoid cells and secrete immunoglobulins in vitro. We also provide evidence for synergy between TACI and TLR4 in the in vivo antibody response to the prototypic TI type I antigen TNP-LPS, which focuses LPS on TNP specfic B cells, resulting in their activation and differentiation via TLR-4 mediated signaling in a T cell independent manner.
MATERIALS & METHODS
Mice
BALB/c mice were purchased from Charles River Laboratories. TACI-/-, BCMA-/- and genetically matched wild-type (WT) mice on Sv129xC57Bl6 background were previously described 14, 23. All mice were bred and housed in a specific pathogen-free animal facility. All experimental procedures performed on the animals were approved by Animal Care and Use Committee of the Children's Hospital, Boston.
Antibodies and Flow Cytometric Analysis
B cells were stained with anti-TACI-PE (Phycoerythrin) anti-BCMA-FITC (Fluorescein isothiocyanate) or anti-BAFF-R-FITC (R&D Systems), anti-B220-FITC anti-CD138-PE or anti-IgG1-PE (BD Pharmingen). For survival assays, B cells were stained with Annexin V-fluorescein isothiocyanate and propidium iodide (Bio Vision). Stained cells were analyzed by FACS (BD Facscalibur).
Proliferation and In vitro Immunoglobulin Production
Naïve B cells were negatively sorted from mouse splenocytes, cultured with APRIL (1 μg/ml) (R&D Systems), IL-4 (50 ng/ml) (R&D Systems), LPS (Escherichia coli 026:B6, Sigma) and assayed for proliferation and Ig production as previously described24.
RT-PCR Analysis
RNA extraction from 4-day cultures and PCR conditions used to detect Iε-Cε, Iμ-Cε, Iγ1-Cγ1, Iμ-Cγ1, AICDA and β2 microglobulin were previously describe 25.
Q-PCR Analysis
Real-time PCR reactions were run on cDNA using ABI Prism 7300 (Applied Biosystems) as detailed in the Online Repository Material.
In vivo antibody response to TNP-LPS
Genetically matched WT and TACI-/- mice were immunized i.p. with a single injection of 10 μg/mouse TNP(.4)-LPS (Biosearch Technologies). Sera were collected at day 14 post immunization and serial dilutions were analyzed for TNP specific IgM, IgA, IgG1 and IgG3 antibodies by ELISA.
Statistics
p values were calculated using the paired t test for in vitro data and two way ANOVA for in vivo data using PRISM software (Prism Software Corp).
RESULTS
APRIL enhances LPS driven Ig production in naïve B cells
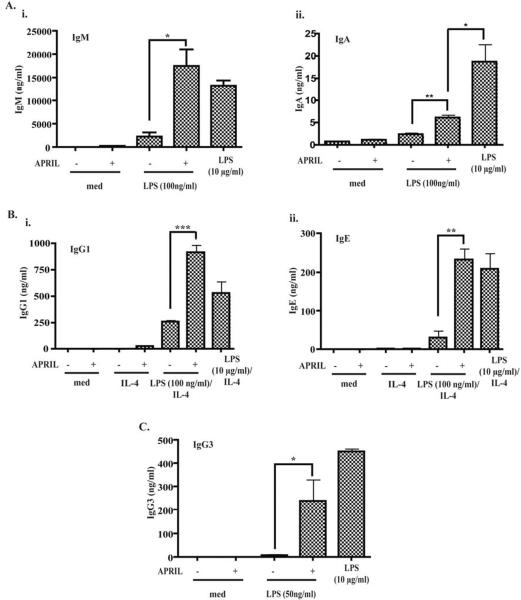
Initial experiments in which naïve B cells (95% B220+IgM+IgD+) were stimulated with a standard concentration of 10 μg/ml LPS did not reveal an enhancing effect of APRIL on Ig production (data not shown). We therefore examined the effect of APRIL on B cells stimulated with a suboptimal concentration of 100 ng/ml LPS. This concentration was selected based on pilot experiments, in which a range of LPS concentrations (50 ng/ml to 10 μg/ml) were tested for their ability to drive IgG1 and IgE synthesis in the presence of IL-4 (Online repository material, Figure 1A). Relative weak induction of proliferation and Ig production has been previously documented using 1 μg/ml APRIL compared to anti-CD40 and LPS 4. There was only a modest difference (<2.5 fold change) between the effects of different APRIL concentrations tested (range 50 ng/ml to 4 μg/ml) on B cell proliferation and production of IgG1, IgE and IgA (Online repository material, Table 1). Fig. 1A shows that APRIL (1 μg/ml) significantly enhanced IgM (~6 fold) and IgA (~2.7 fold) secretion in B cells stimulated with 100 ng/ml LPS to a level comparable to that induced by 10 μg/ml LPS. APRIL also significantly enhanced IgG1 and IgE synthesis driven by 100 ng/ml LPS+IL-4 (~2.5 fold and ~6 fold respectively) to levels comparable to those secreted by B cells stimulated with 10 μg/ml LPS+IL-4 (Figure 1B). Note that APRIL+IL-4 induced a weak, but detectable, IgG1 (24±10 ng/ml, n=3) and IgE (6±3 ng/ml, n=3) secretion, as previously described (6).
Figure 1. APRIL enhances LPS driven immunoglobulin production in naïve B cells.
A. Effect of APRIL (1 μg/ml) on IgM (A-i), IgA (A-ii), IgG1 (B-i), IgE (B-ii), and IgG3 (C) production by B cells stimulated with suboptimal concentrations of LPS. The right hand side bars depict the amounts of immunoglobulins secreted in cultures stimulated with 10 μg/ml LPS. Values represent mean±SD of at least 3 experiments. *p<0.05, **p<0.01, ***p<0.001.
Online Repository Material, Table 1.
APRIL dose response for proliferation, IgA, IgG1 and IgE production of purified splenic naïve B cells from Balb/c mice.
| Concentration of APRIL | 3H-thymidine incorporation (cpm) | 3H-thymidine incorporation with IL-4 (cpm) | IgA production (ng/ml) | IgG1 production (ng/ml) with IL-4 | IgE production (ng/ml) with IL-4 |
|---|---|---|---|---|---|
| 0 | 157 | 463 | 0 | 0 | 0 |
| 50 ng/ml | 232 | 1522 | 0 | 13.8 | 8.3 |
| 250 ng/ml | 364 | 1526 | 1.0 | 18.6 | 13.5 |
| 1 μg/ml | 451 | 1413 | 1.5 | 20.3 | 8.7 |
| 4 μg/ml | 386 | 1394 | 2.5 | 29.9 | 5.4 |
Values represent the mean of triplicate cultures.
LPS is a potent inducer of IgG3 synthesis 20. Based on pilot experiments (Online repository material, Figure 1B) we chose an LPS concentration of 50 ng/ml as the suboptimal LPS concentration to examine synergy with APRIL in inducing IgG3 synthesis. Fig. 1C shows that APRIL significantly enhanced IgG3 secretion by B cells stimulated with 50 ng/ml LPS (~25 fold), to a level comparable to that obtained with 10 μg/ml LPS. Taken together, these results indicate that APRIL synergizes with LPS to drive immunoglobulin synthesis.
APRIL enhancement of LPS driven immunoglobulin production is dependent on TACI
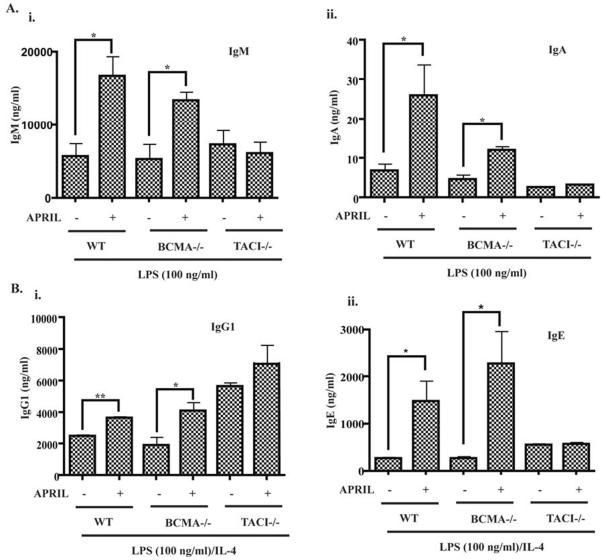
APRIL engages TACI, BCMA and HSPGs. To elucidate which of these receptors mediates the enhancing effect of APRIL on LPS driven immunoglobulin production, we examined the effect of APRIL on LPS induced Ig production in B cells from TACI-/- mice, BCMA-/- mice and WT controls on the same Sv129xC57Bl6 background. Fig. 2 shows that APRIL significantly enhanced LPS driven IgM, IgA, IgG1 and IgE secretion by B cells from BCMA-/- mice and WT controls, but not by B cells from TACI-/- mice. Variations in absolute amounts of immunoglobulins secreted by B cells from the 3 groups of mice studied, including higher IgG1 production by TACI-/- mice compared to WT controls, likely reflect their mixed Sv129xC57Bl6 background and/or, in the case of the increased IgG1 production in TACI-/- mice, the effect of increased stimulation by intestinal flora in an IgA deficient host. These results indicate that TACI mediates the synergy of APRIL with LPS.
Figure 2. APRIL enhancement of LPS driven immunoglobulin production is dependent on TACI.
Effect of APRIL (1 μg/ml) on IgM (A-i) and IgA (A-ii), IgG1 (B-i) and IgE (B-ii) production by naïve B cells from TACI-/- mice, BCMA-/- mice and genetically matched WT controls. Values represent mean±SD of at least 3 experiments. *p<0.05, **p<0.01.
APRIL and LPS synergize to increase B cell proliferation and survival
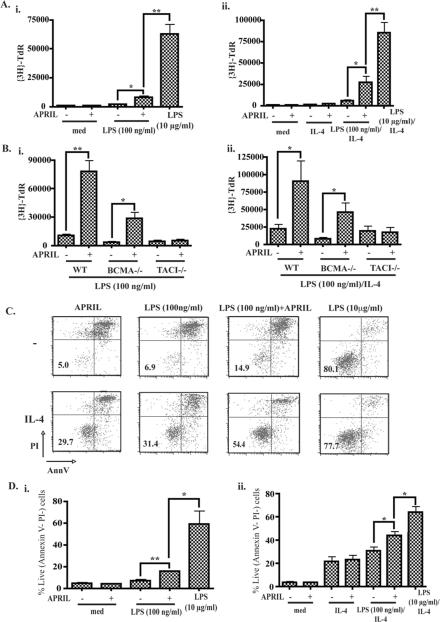
Class switch recombination (CSR) and subsequent immunoglobulin secretion is thought to require B cell proliferation 26. We tested the effect of APRIL on LPS driven proliferation of B cells from BALB/c WT mice by examining 3H-thymidine incorporation after 3 days in culture. Fig. 3A shows that APRIL enhanced the proliferation of naive B cells from WT BALB/c mice stimulated with 100 ng/ml LPS, both in the presence and absence of IL-4. APRIL caused a ~3.5 fold increase in 3H-thymidine incorporation in LPS stimulated cultures and a ~5 fold increase in LPS+IL-4 stimulated cultures. However, this proliferation remained significantly lower than that caused by 10 μg/ml LPS. APRIL caused a significant increase in the number of live cells in LPS stimulated cultures after three days of stimulation, which averaged 3.0±1.1 fold in the absence of IL-4 and 1.9±0.4 in the presence of IL-4 (data not shown). The effect of APRIL on B cell proliferation was mediated by TACI, because APRIL enhanced LPS and LPS/IL-4 driven proliferation of B cells from BCMA-/- mice, but not from TACI-/- mice (Fig. 3B). Variations in absolute number of 3H-thymidine incorporated into DNA of B cells from the 3 groups of mice studied likely reflect their mixed Sv129xC57Bl6 background.
Figure 3. APRIL and LPS synergize to increase proliferation and survival of naïve B cells.
A. Effect of APRIL on LPS driven proliferation of B cells in cultures without IL-4 (i) and with IL-4 (ii). B. Effect of APRIL on the proliferation of B cells from TACI-/-, BCMA-/- and WT mice in cultures without IL-4 (i) and with IL-4 (ii). C. FACS analysis of Annexin V and PI staining of B cells cultured for 3 days. D. Pooled results of 3 survival experiments. *p<0.05, **p<0.01.
We examined the effect of APRIL on the survival of B cells stimulated with LPS. We stained cells 3 days after stimulation with Annexin V and propidium iodide (PI), to identify live cells (Annexin V- PI-). Fig. 3C is a representative FACS analysis and shows that the percentage of live cells was substantially higher in cells stimulated with APRIL+LPS (100 ng/ml) compared to LPS (100 ng/ml), both in the absence and presence of IL-4. Pooled results from three experiments demonstrated that APRIL significantly increased the percentage of live cells in LPS±IL4 stimulated cultures (Fig. 3D). The percentage of live B cells in LPS (100 ng/ml) stimulated cultures was 7.0±1.8, and it rose to 15.8±0.7 with addition of APRIL. The percentage of live B cells in LPS (100ng/ml)+IL4 stimulated cultures was 30.9±4.8 and it rose to 44.1±4.9 with the addition of APRIL.
Increased B cell proliferation and survival may contribute to APRIL enhancement of LPS driven Ig production.
APRIL and LPS synergize in driving CSR
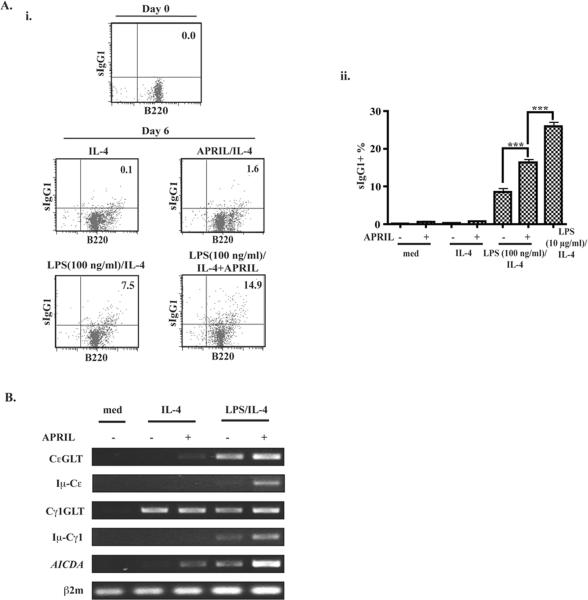
To determine whether APRIL synergizes with LPS in causing CSR, we first examined surface IgG1 expression in naïve B cells stimulated with LPS+IL-4 for 6 days in the presence or absence of APRIL. Fig. 4A shows that there was virtually no detectable sIgG1+ B cells in the starting population of sorted naïve B cells. APRIL+IL-4, induced a small but detectable increase in sIgG1 expression. Stimulation with 100 ng/ml LPS+IL-4 induced expression of sIgG1. Addition of APRIL to these cultures increased the percentage of sIgG1+ B cells. Pooled results from three experiments demonstrated that APRIL significantly increased the percentage of sIgG1+ cells (~ 2 fold) in cultures stimulated with 100 ng/ml LPS+IL-4. This percentage was however significantly lower than that achieved with 10 μg/ml LPS+IL-4 (Fig. 4A). The percentages of sIgG1+ cells are net percentages obtained after subtracting the percentage of cells that were stained by the isotype controls, which in all cases was <1.5%.
Figure 4. APRIL and LPS synergize to increase surface sIgG1 expression and to induce molecular events involved in CSR in naïve B cells.
A. Representative FACS analysis (i) and pooled results of 3 experiments (ii) of surface IgG1 expression in B cells stimulated for 6 days. *p<0.05, **p<0.01, ***p<0.001. B. Expression of CeGLT, Iμ-Cε, Cγ1GLT, Iμ-Cγ1 and AICDA mRNA by RT-PCR in B cells stimulated for 4 days. Similar results were obtained in 3 independent experiments.
Molecular events involved in CSR include expression of GLTs and AICDA, followed by deletional switch recombination and expression of Iμ-CH mature transcripts 27. We tested the effect of APRIL on molecular events involved in CSR to IgE and IgG1 driven by 100 ng/ml LPS+IL-4. Fig. 4B shows that as previously reported, IL-4 alone does not induce CεGLT in naïve mouse B cells 4. CεGLT was weakly induced by APRIL+IL-4. CεGLT induction by LPS +IL-4 was stronger and was slightly enhanced by APRIL. Cγ1GLT was induced by IL-4, but was not consistently upregulated by addition of APRIL, 100 ng/ml LPS or both. APRIL+IL-4 induced AICDA as previously described 4. APRIL clearly synergized with 100 ng/ml LPS+IL-4 in inducing AICDA, mature Iμ-Cε transcripts and mature Iμ-Cγ1 transcripts. Expression of Iμ-Cγ1 transcripts in APRIL+IL-4 stimulated B cells was only detected with increased number of cycles of RT-PCR amplification (data not shown). Taken together the above data suggest that APRIL synergizes with LPS in causing CSR.
APRIL strongly synergizes with LPS to induce plasma cell differentiation
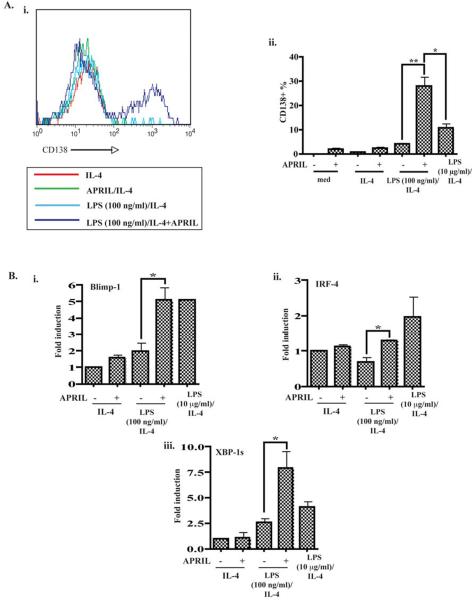
Plasma cells secrete immunoglobulins at a high rate. We investigated whether APRIL synergizes with LPS in the generation of cells that express syndecan-1/CD138, a marker for plasmacytoid cells. Fig. 5A shows that there was few CD138+ cells in cultures stimulated with APRIL, APRIL+IL-4 or 100 ng/ml LPS+IL-4. Addition of APRIL markedly increased (by ~4 fold) the percentage of CD138+ cells in cultures stimulated with 100 ng/ml LPS+IL-4 to a level significantly higher than that induced by 10 μg/ml LPS+IL-4. APRIL also significantly increased the percentage of CD138+ cells in cultures stimulated with 100 ng/ml LPS alone by ~ 2.5 fold (data not shown). The percentages shown are net percentages of positive cells.
Figure 5. APRIL synergizes with LPS+IL-4 to increase the surface CD138 expression and to induce mRNA expression of Blimp-1, IRF-4 and the spliced form of XBP-1.
A. Representative FACS analysis (i) and pooled results of 3 experiments (ii) of surface CD138 expression in B cells stimulated for 6 days. B. Q-PCR analysis of mRNA levels for Blimp-1 (i), IRF-4 (ii), and XBP-1(s) (iii) in B cells. *p<0.05, **p<0.01, ***p<0.001.
Differentiation of B cells into CD138+ plasma cells correlates with increased expression of the transcription factors Blimp-1 and IRF-4 28. We measured mRNA levels for Blimp-1 and IRF-4 in IL-4 stimulated cultures by Q-PCR. Fig. 5B shows that LPS (100 ng/ml) induced a 2.5 fold increase in Blimp-1 mRNA levels, while APRIL had no detectable effect. However, APRIL synergized with LPS (100 ng/ml) to cause a significant increase (~3 fold) in Blimp-1 mRNA expression and to a lesser extent in IRF-4 mRNA expression. Blimp-1 and IRF-4 synergize to induce XBP-1 splicing and plasma cell differentiation 28. Fig. 5B shows that APRIL synergized with LPS (100ng/ml)+IL-4 to induce a significant increase (2.5 fold) in the expression of XBP-1(s) (spliced form of XBP-1). Taken together these results indicate that APRIL strongly drives the plasma cell differentiation program in LPS stimulated B cells.
APRIL was recently shown to induce Bcl-XL expression in plasmacytoid cells and to be important for their survival in vivo3. Mcl-1 has also been reported to be induced by APRIL and to be important for plasma cell survival 12, 29. Addition of APRIL to LPS stimulated B cells had no detectable effect on Bcl-xL expression by CD138+ cells as determined by intracellular staining (Online repository material, Figure 2) or on Bcl-xL and Mcl-1 mRNA expression as determined by Q-PCR (data not shown).
TACI deficient mice have impaired antibody response to immunization with a suboptimal dose of TNP-LPS
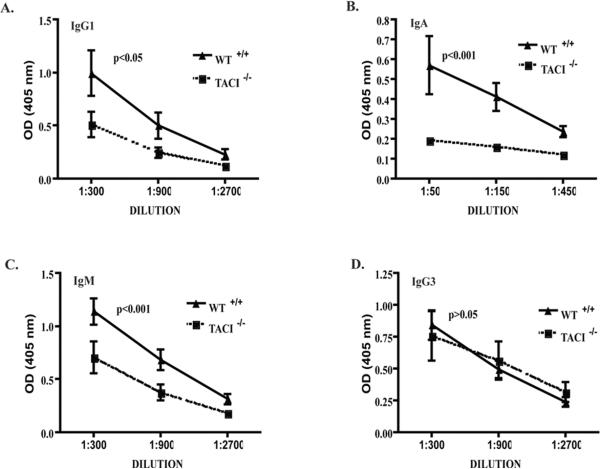
Antigen specific B cells responding to the TI antigen TNP-LPS in vivo receive signals via TLR4 and potentially via TACI from DC derived BAFF/APRIL. We reasoned that if TACI ligation synergizes with TLR4 in driving the response of B cells to type I TI antigens in vivo, TACI-/- mice could exhibit an impaired antibody response to a suboptimal dose of TNP-LPS. Consistent with published data 13, initial experiments revealed that TACI-/- mice and WT controls mounted comparable antibody responses to immunization with a dose of 50 μg TNP-LPS (data not shown). We then examined the response of these mice to a suboptimal dose of 10 μg TNP-LPS per mouse. Fig. 6 shows that TACI-/- mice had impaired IgM, IgA and IgG1 antibody responses to 10 μg of TNP-LPS compared to WT controls. TACI-/- mice immunized with 10 μg TNP-LPS + 40 μg LPS similarly showed a significant defect in TNP specific IgM, IgA and IgG1 antibody responses compared to WT mice (Online repository material, Figure 3). This suggests that TNP and LPS need to be physically linked for an optimal Ab response, possibly because linking TNP to LPS focuses the LPS signal on TNP specific B cells. There was no impairment of the IgG3 antibody response to this dose in TACI-/- mice. These results suggest that TACI synergizes with TLR4 in driving the antibody response to suboptimal amounts of type I TI antigen in vivo.
Figure 6. Impaired antibody response to immunization with low dose TNP-LPS (10 μg/mouse) in TACI-/- mice.
IgG1 (A), IgA (B), IgM (C) and IgG3 (D) anti-TNP antibody levels of TACI-/- and WT are shown. Results represent mean±SE of 9 mice. Dilution curves were compared by ANOVA.
DISCUSSION
We demonstrate that TACI synergizes with TLR4 in enhancing immunoglobulin production in vitro and promotes the antibody response to TI type I antigen in vivo.
APRIL synergized with suboptimal concentrations of LPS to enhance the production of all Ig isotypes examined, which included IgM, IgA, IgG3, IgG1 and IgE (Fig. 1). For all these isotypes, the amounts of immunoglobulin secreted in response to a suboptimal concentration of LPS+APRIL were comparable to those induced by the maximal concentration of 10 μg/ml LPS. There is both indirect and direct evidence that the effect of APRIL on LPS driven Ig production was mediated via TACI. BCMA is poorly expressed, if any, by splenic B cells and is not upregulated by LPS (18 and our unpublished observations). More importantly, APRIL enhanced LPS driven Ig production in BCMA deficient B cells, but not in TACI deficient B cells (Fig. 2). These observations argue against a role for BCMA or HSPGs, and for an exclusive role of TACI in the synergistic effect of APRIL on LPS driven immunoglobulin production by naïve B cells.
APRIL synergized with LPS in inducing B cell proliferation and survival, which may have contributed to increased immunoglobulin production. The ability of APRIL to enhance the survival of primary murine B cells activated by LPS is consistent with its ability to promote increased viability of human B cells stimulated with anti-IgM antibodies 12 and of chronic lymphocytic leukemia B cells 30, and is consistent with its anti-apoptotic effect on malignant human B cells 31, 32, 33. CSR induced by LPS is dependent on proliferation 34. Given this fact that, it is likely that B-cell proliferation is necessary for enhanced Ig secretion by LPS stimulated B cells upon addition of APRIL.
APRIL promoted isotype switching induced by LPS+IL-4 as evidenced by a significant increase in the percentage and numbers of sIgG1+ cells (Fig. 4A). APRIL had minimal effect on the expression of Cε and Cγ1 germ line transcripts induced by LPS+IL-4. However, it strongly enhanced the expression of AICDA and of mature post switch Iμ-Cε and Iμ-Cγ1 transcripts (Fig. 4B). These results suggest that APRIL enhances LPS driven CSR by upregulating AICDA expression. An additional effect on the viability of switched B cells is possible.
The most striking effect of addition of APRIL to B cell cultures stimulated with suboptimal concentration of LPS was an increase in the percentage of CD138+ cells which significantly exceeded that observed in cultures stimulated with optimal concentrations of LPS (Fig. 5A). The fact that B cells that differentiate more vigorously into plasmacytoid cells have less time to proliferate may explain the observation that generation of CD138+ cells was more potently enhanced than proliferation by addition of APRIL to LPS stimulated B cells. Since CD138+ cells produce large amounts of immunoglobulins compared to CD138- B cells, the increase in CD138+ cells probably accounts for most of the increased immunoglobulin production in B cell cultures stimulated with APRIL and LPS. Consistent with the increased percentages of CD138+ cells, APRIL synergized with LPS (100 ng/ml) in inducing Blimp-1, and to a lesser extent IRF-4, and in inducing increased expression of the spliced form of XBP-1 (Fig. 5B). Thus, APRIL synergized with LPS in activating the plasma cell differentiation program. The increase in plasmacytoid cells was not accompanied by a detectable increase in the expression of Bcl-xL (Online repository material, Figure 2) or Mcl-1 expression (data not shown), two factors that are important for plasma cell survival.
Synergy between TACI and TLR4 was demonstrated in vivo by the observation that TACI-/- mice on Sv129xC57Bl6 background, which were used for all in vitro experiments, have impaired IgM, IgA and IgG1 antibody responses to immunization with 10 μg TNP-LPS (Fig. 6), a dose five fold lower than the usual standard dose of 50 μg. These results were confirmed in TACI-/- on C57Bl6 background suggesting that TACI may be required for an optimal response to type I TI antigen. The equivalent dose of 10 μg TNP-LPS dose per mouse is 35 mg for a 70 kg man, a substantial dose of immunogen. The observation that the IgG3 response of TACI-/- mice to 10 μg TNP-LPS was unaffected, simply reflect the higher sensitivity of IgG3 isotype switching to LPS. Immunization of TACI-/- mice with doses of TNP-LPS lower than 10 μg may uncover synergy between TACI and TLR4 in the in vivo IgG3 response to type I TI antigen.
The present work shows that in addition to synergizing with CD40 and TLR9, TACI ligation synergizes with TLR4 in driving Ig production by B cells 18, 22. Upregulation of TACI has been invoked in the synergy of APRIL with CD40 and TLR9 in driving immunoglobulin production. Similarly TACI upregulation by TLR4 may contribute to the synergy of APRIL with LPS 18, 35. The mechanism by which TACI ligation enhances B cell activation by LPS remains to be determined. TACI has been reported to activate the canonical NF-κB pathway in B cell lines 30, 32. We were unable to detect NF-κB activation in primary B cells treated with APRIL, as assessed by IκB phosphorylation, IκB degradation and p65 translocation in a time course that spanned 5 min. to 12 hrs (data not shown). Furthermore, addition of APRIL to LPS (100 ng/ml) caused no significant, enhancement of p65 nuclear translocation. In addition, APRIL and LPS, alone or in combination, caused no detectable activation of the non-canonical NF-κB pathway, as measured by nuclear appearance of p52 (data not shown). TACI activates NF-AT via its interaction with CAML 36. It is possible that activation of the CAML - NF-AT pathway by TACI may contribute to its synergy with LPS.
Our data indicates that in addition to its ability to trigger isotype switching 4, 37, TACI plays an important role in plasma cell differentiation of B cells. Furthermore, TACI was shown to be important for the antibody response to suboptimal doses of type I antigen. It remains to be established whether TACI promotes the differentiation of TLR stimulated human B cells into plasma cells. Impairment of TACI driven plasmacytoid cell generation and Ig production by B cells stimulated with the TLR ligands, may contribute to the low serum immunoglobulin levels in CVID patients with TACI mutations.
Acknowledgements
We thank Haifa Jabara and Emmanuela Castigli for useful discussions and experimental guidance.
Acknowledgement of Funding This work was supported by March of Dimes grant #6-FY07-285 and NIH grant AI-031541.
Abbreviations
- BAFF
B cell activating factor belonging to TNF superfamily
- APRIL
a proliferation inducing ligand
- DC
Dendritic cell
- TD
T cell dependent
- TLR
Toll-like receptor
- TACI
transmembrane activator, calcium modulator, and cyclophilin ligand interactor
- BCMA
B cell maturation antigen
- BAFF-R
B cell activating factor belonging to TNF superfamily receptor
- HSPG
heparan-sulfated proteoglycans
- WT
Wild Type
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- CSR
Class Switch Recombination
- TI
T cell independent
- PE
Phycoerythrin
- FITC
Fluorescein isothiocyanate
- CVID
Common Variable Immune Deficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: R.J.B. is listed as a co-inventor on patents including the TACI gene.
REFERENCES
- 1.Mackay F, Ambrose C. The TNF family members BAFF and APRIL: the growing complexity. Cytokine Growth Factor Rev. 2003;14:311–24. doi: 10.1016/s1359-6101(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 2.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A. 2004;101:3903–8. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat A, Bossen C, et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early life bone marrow stromal cells. Blood. 2008 doi: 10.1182/blood-2007-09-110858. In press. [DOI] [PubMed] [Google Scholar]
- 4.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay F, Kalled SL. TNF ligands and receptors in autoimmunity: an update. Curr Opin Immunol. 2002;14:783–90. doi: 10.1016/s0952-7915(02)00407-7. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Marsters SA, Baker T, Chan B, Lee WP, Fu L, et al. TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice. Nat Immunol. 2001;2:632–7. doi: 10.1038/89782. [DOI] [PubMed] [Google Scholar]
- 8.Seyler TM, Park YW, Takemura S, Bram RJ, Kurtin PJ, Goronzy JJ, et al. BLyS and APRIL in rheumatoid arthritis. J Clin Invest. 2005;115:3083–92. doi: 10.1172/JCI25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Park CS, Yoon SO, Li L, Hsu YM, Ambrose C, et al. BAFF supports human B cell differentiation in the lymphoid follicles through distinct receptors. Int Immunol. 2005;17:779–88. doi: 10.1093/intimm/dxh259. [DOI] [PubMed] [Google Scholar]
- 10.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: A Tutorial on B Cell Survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 11.Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero TG, et al. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005;201:1375–83. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mongini PK, Inman JK, Han H, Fattah RJ, Abramson SB, Attur M. APRIL and BAFF promote increased viability of replicating human B2 cells via mechanism involving cyclooxygenase 2. J Immunol. 2006;176:6736–51. doi: 10.4049/jimmunol.176.11.6736. [DOI] [PubMed] [Google Scholar]
- 13.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–82. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 14.Yan M, Wang H, Chan B, Roose-Girma M, Erickson S, Baker T, et al. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol. 2001;2:638–43. doi: 10.1038/89790. [DOI] [PubMed] [Google Scholar]
- 15.Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18:279–88. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol. 2005;17:338–44. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Reis e Sousa C, Diebold SD, Edwards AD, Rogers N, Schulz O, Sporri R. Regulation of dendritic cell function by microbial stimuli. Pathol Biol (Paris) 2003;51:67–8. doi: 10.1016/s0369-8114(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 18.Katsenelson N, Kanswal S, Puig M, Mostowski H, Verthelyi D, Akkoyunlu M. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur J Immunol. 2007;37:1785–95. doi: 10.1002/eji.200636800. [DOI] [PubMed] [Google Scholar]
- 19.Phipps RP, Roper RL, Stein SH. Regulation of B-cell tolerance and triggering by macrophages and lymphoid dendritic cells. Immunol Rev. 1990;117:135–58. doi: 10.1111/j.1600-065x.1990.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 20.Rothman P, Lutzker S, Gorham B, Stewart V, Coffman R, Alt FW. Structure and expression of germline immunoglobulin gamma 3 heavy chain gene transcripts: implications for mitogen and lymphokine directed class-switching. Int Immunol. 1990;2:621–7. doi: 10.1093/intimm/2.7.621. [DOI] [PubMed] [Google Scholar]
- 21.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–66. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 22.Castigli E, Wilson SA, Elkhal A, Ozcan E, Garibyan L, Geha RS. Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. J Allergy Clin Immunol. 2007;120:885–91. doi: 10.1016/j.jaci.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu S, Lam KP. B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol Cell Biol. 2001;21:4067–74. doi: 10.1128/MCB.21.12.4067-4074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castigli E, Alt FW, Davidson L, Bottaro A, Mizoguchi E, Bhan AK, et al. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc Natl Acad Sci U S A. 1994;91:12135–9. doi: 10.1073/pnas.91.25.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabara H, Laouini D, Tsitsikov E, Mizoguchi E, Bhan A, Castigli E, et al. The binding site for TRAF2 and TRAF3 but not for TRAF6 is essential for CD40-mediated immunoglobulin class switching. Immunity. 2002;17:265–76. doi: 10.1016/s1074-7613(02)00394-1. [DOI] [PubMed] [Google Scholar]
- 26.Hasbold J, Lyons AB, Kehry MR, Hodgkin PD. Cell division number regulates IgG1 and IgE switching of B cells following stimulation by CD40 ligand and IL-4. Eur J Immunol. 1998;28:1040–51. doi: 10.1002/(SICI)1521-4141(199803)28:03<1040::AID-IMMU1040>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Manis JP, Tian M, Alt FW. Mechanism and control of class-switch recombination. Trends Immunol. 2002;23:31–9. doi: 10.1016/s1471-4906(01)02111-1. [DOI] [PubMed] [Google Scholar]
- 28.Igarashi K, Ochiai K, Muto A. Architecture and dynamics of the transcription factor network that regulates B-to-plasma cell differentiation. J Biochem. 2007;141:783–9. doi: 10.1093/jb/mvm106. [DOI] [PubMed] [Google Scholar]
- 29.Woodland RT, Fox CJ, Schmidt MR, Hammerman PS, Opferman JT, Korsmeyer SJ, et al. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood. 2008;111:750–60. doi: 10.1182/blood-2007-03-077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endo T, Nishio M, Enzler T, Cottam HB, Fukuda T, James DF, et al. BAFF and APRIL support chronic lymphocytic leukemia B-cell survival through activation of the canonical NF-kappaB pathway. Blood. 2007;109:703–10. doi: 10.1182/blood-2007-04-081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kern C, Cornuel JF, Billard C, Tang R, Rouillard D, Stenou V, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103:679–88. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 32.He B, Chadburn A, Jou E, Schattner EJ, Knowles DM, Cerutti A. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol. 2004;172:3268–79. doi: 10.4049/jimmunol.172.5.3268. [DOI] [PubMed] [Google Scholar]
- 33.Chiu A, Xu W, He B, Dillon SR, Gross JA, Sievers E, et al. Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. Blood. 2007;109:729–39. doi: 10.1182/blood-2006-04-015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deenick EK, Hasbold J, Hodgkin PD. Switching to IgG3, IgG2b, and IgA is division linked and independent, revealing a stochastic framework for describing differentiation. J Immunol. 1999;163:4707–14. [PubMed] [Google Scholar]
- 35.Acosta-Rodriguez EV, Craxton A, Hendricks DW, Merino MC, Montes CL, Clark EA, et al. BAFF and LPS cooperate to induce B cells to become susceptible to CD95/Fas-mediated cell death. Eur J Immunol. 2007;37:990–1000. doi: 10.1002/eji.200636698. [DOI] [PubMed] [Google Scholar]
- 36.von Bulow GU, Bram RJ. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science. 1997;278:138–41. doi: 10.1126/science.278.5335.138. [DOI] [PubMed] [Google Scholar]
- 37.Bossen C, Schneider P. BAFF, APRIL and their receptors: Structure, function and signaling. Semin Immunol. 2006;18:263–75. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]