Abstract
Background & Aims
Hepatic inflammation occurs immediately after cells are transplanted to the liver, but the mechanisms that underlie this process are not fully defined. We examined cyclooxygenase pathways that mediate hepatic inflammation through synthesis of prostaglandins (PG), prostacyclins, thromboxanes and other prostanoids following transplantation of hepatocytes.
Methods
We transplanted F344 rat hepatocytes into syngeneic dipeptidyl peptidase IV-deficient F344 rats. Changes in cyclooxygenase pathways were analyzed and specific pathways were blocked pharmacologically; the effects on cell engraftment and native liver cells were determined.
Results
Transplantation of hepatocytes induced hepatic expression of prostaglandin-endoperoxide synthases 1 and 2, which catalyze production of prostaglandin H2, as well as the downstream factor thromboxane synthase, which produces thromboxane A2 (a regulator of vascular and platelet responses in inflammation). Transplanted hepatocytes were in proximity with liver cells that express prostaglandin-endoperoxide synthases. The number of engrafted hepatocytes increased in rats given naproxen or celecoxib before transplantation, but not in rats given furegrelate (an inhibitor of thromboxane synthase) or clopodigrel (an anti-platelet drug). Naproxen and celecoxib did not prevent hepatic ischemia or activation of neutrophils, Kupffer cells or inflammatory cytokines, but they did induce hepatic stellate cells to express cytoprotective genes, vascular endothelial growth factor and hepatocyte growth factor, and matrix-type metalloproteinases and tissue inhibitor of metalloproteinase-1, which regulate hepatic remodeling.
Conclusions
Activation of cyclooxygenase pathways interferes with engraftment of transplanted hepatocytes in the liver. Pharmacological blockade of prostaglandin-endoperoxide synthases stimulated hepatic stellate cells and improved cell engraftment.
Keywords: Cell transplantation, Cyclooxygenase, Drugs, Hepatic stellate cell, Inflammation
INTRODUCTION
Insights into mechanisms regulating engraftment of transplanted hepatocytes will benefit strategies for liver repopulation and cell therapy. Multiple processes impede engraftment of hepatocytes arriving in liver sinusoids, including through activation of phagocytes and Kupffer cells, as well as interposition of the hepatic sinusoidal endothelial barrier.1,2 Consequently, the majority of transplanted cells, over 70-80%, do not engraft and are cleared from liver sinusoids within 24 h to 48 h after cell transplantation.3 The process of cell engraftment, where plasma membrane structures are restored and transplanted hepatocytes become integrated in the liver parenchyma, is completed over 3 to 7 days. Thereafter, transplanted hepatocytes survive in the liver parenchyma indefinitely and throughout the lifespan of rodents, except for allograft rejection.4,5 Strategies to improve engraftment of transplanted hepatocytes have included vasodilators for enhancing intrahepatic entry of cells, 6 damage to liver sinusoidal endothelial cells (LSEC),2 superior anchorage of cells to LSEC,7 depletion of Kupffer cells, 1 or activation of hepatic stellate cells (HSC).8 Although hepatic inflammation is a significant early event after cell transplantation in the liver, underlying mechanisms have not been well-defined.
Here, we examined cyclooxygenase pathways, which are ubiquitously active in cells for synthesizing prostaglandins (PG), such as PGH2, prostacyclins, such as PGI2, thromboxanes, such as, TXA2, as well as other prostanoids. 9 The prostaglandin-endoperoxide synthases (PTGS) 1 and 2 convert arachidonic acid to PGH2. In turn, constitutive and microsomal-types of prostaglandin E synthases (cPTGES, mPTGES) convert PGH2 to the significant mediator of tissue inflammation, PGE2, whereas thromboxane synthase (TXAS) catalyzes synthesis of TXA2, which transduces vascular and platelet-mediated inflammatory responses. As drugs are available to specifically block cyclooxygenase pathways, we reasoned that if hepatocyte transplantation activated these pathways, the contribution of given pathway members in cell engraftment could be elucidated. We performed studies in dipeptidyl peptidase IV deficient (DPPIV-) rats, since these animals offer superb models for demonstrating engraftment and proliferation of transplanted hepatocytes.1-8
MATERIALS AND METHODS
Animals
DPPIV-F334 rats of 6-8 weeks age and 120-150 g in weight were from the Special Animal Core of Marion Bessin Liver Research Center. F334 rats were from National Cancer Institute (Bethesda, MD). The Animal Care and Use Committee at Albert Einstein College of Medicine approved animal protocols according to institutional practices and the Guide for the Care and Use of Laboratory Animals (United States Public Health Service Publication, 1996).
Drugs and chemicals
We purchased naproxen (N-8280; Sigma Chemical Co., St. Louis, MO), furegrelate (70540; Cayman Chemical Co., Ann Arbor, MI), celecoxib (Cerebrex) (Pfizer Inc., New York, NY) and clopidogrel (Plavix) (Bristol-Myers Squibb/Sanofi Pharmaceuticals, New York, NY). Reagents and chemicals were from Sigma. Naproxen was dissolved in 20% ethanol to 2 mg/ml and diluted in normal saline. Furegrelate was dissolved to 2 mg/ml in normal saline. Celecoxib and clopidogrel were suspended in water to 10-30 mg/ml. Naproxen and furegrelate were injected intraperitoneally and celecoxib and clopidogrel were given by gavage. Drugs were typically given once 2 h before cell transplantation. In some studies, 7 additional doses of naproxen or celecoxib were given over 3 d after cell transplantation.
Cell isolation and transplantation
Hepatocytes were isolated form F344 rats by two-step collagenase perfusion of the liver. Cells were transplanted when >80% excluded 0.2% trypan blue dye. For transplantation, 1-2×107 freshly isolated hepatocytes were suspended in 0.5 ml serum-free RPMI 1640 medium and injected into the splenic pulp via left subcostal incision under ether anesthesia, according to approved animal protocols. Hemostasis was secured with a ligature around the lower pole of the spleen. Mortality ascribable to cell transplantation was not encountered.
Hepatic mRNA studies
Liver samples (~100 mg) were homogenized in Trizol Reagent (15596-018, Invitrogen, Carlsbad, CA) and total RNA was extracted according to manufacturer’s recommendations. RNAs (1 μg each) were reverse-transcribed (Omniscript RT PCR Kit, Qiagen, Valencia, CA) and amplified for 35 cycles in 50 μl of 1 × polymerase chain reaction (PCR) buffer, 10 U reverse transcriptase, 100 U RNase inhibitor, 1.25 μg oligo-dT primer, and dNTPs (Platinum PCR Kit, Invitrogen). PCR products were resolved in 1% agarose with ethidium bromide. PCR primers for PTGS1, PTGS2, cPTGES, mPTGES1 and 2, TXAS, 10 hepatocyte growth factor (HGF), 11 vascular endothelial growth factor (VEGF), 12 matrix-type metalloproteinase (MMP)-3,13 -9, and -13, tissue inhibitor of metalloproteinase (TIMP)-114 and β-actin, 15 were as described.
For quantitative real-time PCR (qRT-PCR), cDNAs were made from 1 μg total RNAs (C-03, RT2 First Strand Kit, SABiosciences Corp., Frederick, MD), and cDNAs amplified with RT2 Real-Time SYBR Green/Rox PCR master mix (PA-012-12, SABiosciences Corp.) Expression of 84 rat cytokines and chemokines was analyzed (PARN-022A, Rat Chemokines & Receptors PCR Array, SABiosciences Corp.), according to manufacturer’s instructions. Also, qRT-PCR was performed with SYBR Green with PCR primers and kits from SABiosciences Corp. for rat PTGS1, PTGS2, TXAS and β-actin mRNAs, as recommended by the manufacturer. These were chosen after initial studies with semi-quantitative RT-PCR and western blots. Differences in gene expression were analyzed by the delta-delta cycle threshold (Ct) method after normalizing normal control liver samples and test samples with β-actin followed by intergroup comparisons (n=3 each).
Western blots
Liver was homogenized in 0.25 M sucrose and 10 mM Tris-HCl buffer with Protease Inhibitor Cocktail Set III (539134, Calbiochem, Darmstadt, Germany). Protein content was measured with Bradford reagent. Equal amounts of proteins were resolved in 10% sodium dodecyl sulfate-polyacrylamide gels for polyvinylidine fluoride membrane transblots. Blots were probed with antibodies against PTGS1 (1:200; sc-1754, Santa Cruz Biotechnology Inc., Santa Cruz, CA), PTGS2 (1:200; sc-1747-R, Santa Cruz), TXAS (1:300; 160715, Cayman Chemical Co., Ann Arbor, MI), mPTGES1 (1:200; sc-20771, Santa Cruz), and β-tubulin (1:1000, Sigma). Antibody-binding was shown with 1:5,000 peroxidase-conjugated IgG (Sigma) followed by enzymatic chemiluminiscence (NEL104, Perkin Elmer LAS Inc., Boston, MA). Blots were regenerated with Restore Western Blot Stripping Buffer (21509, Pierce Biotechnology Inc., Rockford, IL) for serial reprobings. Density of bands was measured by ImageJ software (National Cancer Institute, Bethesda, MD). Data were normalized to β-tubulin bands. Normal liver served as denominator.
Tissue staining
Samples from multiple liver lobes were frozen in methylbutane at −80°C. Cryosections of 5 μm thickness were fixed in chloroform-acetone (1:1, vol/vol) at 4°C for 10 min, air-dried for 30 min and subjected to DPPIV histochemistry, as described.1-8 Transplanted cell numbers were determined by morphometry in 3-4 sections from each liver lobe per animal (n=3 each). Typically, 100 fields centered on consecutive portal areas were scored for transplanted cells under x100 magnification. Biochemical measurement of DPPIV activity was not sensitive enough to discriminate between changes in cell engraftment under our experimental conditions.
To stain PTGS1, PTGS2, TXAS and mPTGES1, cryosections were fixed in acetone for 10 min, blocked in 3% rabbit (PTGS1) or goat serum for 30 min at 37°C, and incubated with antibodies against PTGS1 (1:100; sc-1754, Santa Cruz), PTGS2 (1:100; sc-1747-R, Santa Cruz), TXAS (1:200; 160715, Cayman Chemical Co.); mPTGES1 (1:200; sc-20771, Santa Cruz) for 1 h at 37°C. After washing with PBS, sections were incubated with anti-goat or anti-rabbit IgG (1:500; Sigma) for 30 min at 37°C, and developed with DAB+ (K3467, DakoCytomation, Dako North America Inc., Carpinteria, CA). In some studies, DPPIV histochemistry was undertaken first followed by immunostaining for PTGS1 and PTGS2.
To demonstrate γ-glutamyl transpeptidase (GGT), cryosections were fixed in chloroform-acetone for histochemistry with N-γ-L-glutamyl-4-methoxy-β-naphthylamide substrate (02410, Polysciences, Inc., Washington, PA), as described previously. 16 Tissue myeloperoxidase activity was demonstrated in cryosections fixed in 4% paraformaldehyde and 85% ethanol with Peroxidase Indicator Reagent (3901, Sigma) as recommended (procedure 390A, Sigma). To identify HSC, tissues were immunostained for desmin, and HSC per liver lobule in multiple consecutive areas centered on portal radicles were counted under x400 magnification, as described previously. 8
Phagocytic activity of Kupffer cells
Carbon was injected into spleen 1 h before sacrificing animals, as described,1 followed by grading of carbon incorporation in 25 liver lobules per tissue: grade 1, minimal carbon; grade 2, moderate carbon; grade 3, maximal carbon.
Cell culture studies
The CFSC-8B clone of rat HSC was from Dr. M. Rojkind. These cells were from cirrhotic Wistar rat and expressed VEGF under suitable conditions. 17 We cultured 1×105 cells in 35 mm dishes with DMEM, 10% fetal bovine serum (FBS) and antibiotics. Near-confluent cells were switched to serum-free medium. Cells were cultured for 16-18 h under normoxia (21% O2, 5% CO2) or hypoxia (5% O2, 5% CO2, 11% N2) with 0 to 10 μM naproxen. Cellular RNA was extracted with Trizol Reagent and RT-PCR was performed for VEGF, HGF and β-actin as described above. Release of VEGF in 50 μl culture medium was measured (Rat VEGF Quantikine ELISA Kit, RRV00, R&D Systems, Minneapolis, MN), according to the manufacturer. VEGF concentration was obtained from standards in 0 to 2000 pg/ml range. Experimental conditions were in triplicate with two replicate experiments. To demonstrate the effect of conditioned medium on TNF-α cytotoxicity, 5×104 primary rat hepatocytes were attached to rat tail collagen-coated 24-well dishes in DMEM with 10% FBS. After 6 h, cells were switched to conditioned medium from naproxen-treated CFSC-8B cells and incubated with 200 ng/ml actinomycin D followed by 10 ng/ml TNF-α for thiazolyl blue viability assays after 16-18 h, as described previously. 18
Experimental design
Initially, to examine effects of cell transplantation on cyclooxygenase pathway, 2×107 hepatocytes were transplanted and rats were sacrificed 6 h, 1 d, 2 d, 3 d and 7 d after cell transplantation (n=3-4). In these animals, changes in liver gene expression and integrity of various cell compartments, including phagocytes, Kupffer cells and HSC were analyzed. Subsequently, to test the effect of drugs on cyclooxygenase pathways, dose-ranging studies were performed (n=3), with 2, 4 or 6 mg/kg naproxen and 20, 30 or 40 mg/kg celecoxib, given either once or to a total of 8 doses over 3 d. Also, animals were given 3, 6 and 9 mg/kg furegrelate and 5, 10 and 15 mg/kg clopidogrel 2 h before cell transplantation (n=3). To analyze changes in cell engraftment, transplanted cell numbers were determined 3 d after transplantation. All studies were repeated at least twice and were typically performed 3 to 4 times. Untreated control animals and sham-treated animals, where vehicle alone was injected intrasplenically, were included for comparisons. Studies were performed with CFSC-8B cells to demonstrate the effects of naproxen on VEGF expression and release, as well as the effects of the conditioned medium on TNF-α-induced hepatocyte cytotoxicity.
Statistical analysis
Data are shown as means±SD. Significances were analyzed by t-test or ANOVA with Tukey’s test for pairwise comparisons of mean responses as appropriate with SigmaStat 3.1 (Systat Software Inc, Point Richmond, CA). P<0.05 was considered significant.
RESULTS
Hepatocyte transplantation activated cyclooxygenase pathways
Transplanted hepatocytes were in portal vein radicles and sinusoids after 6 h (Fig. 1A), although owing to initial clearances, largely within 1 d, fewer transplanted cells were present in the liver at later times, although without changes in cell numbers during this subsequent period (Fig. 1B-1D). DPPIV staining in transplanted cells was diffuse at early times and DPPIV staining reverted to its normal linear pattern over 3 d to 7 d following integration of transplanted cells in the liver parenchyma (Fig. 1C-1D). Semi-quantitative RT-PCR showed increased expression of PTGS2 and mPTGES1 mRNAs after cell transplantation compared with untreated normal animals, although these mRNAs were also upregulated in sham-treated animals (Supplementary Fig. 1). However, expression of PTGS1, cPTGES, mPTGES2, and TXAS mRNAs seemed unchanged. To better establish these findings, we performed qRT-PCR for PTGS1, PTGS2 and TXAS mRNAs (Fig. 1E). This showed greatest changes in PTGS2 expression 1 d after cell transplantation, mean 46-fold increase, compared with untreated normal liver, although the increase was mean 3.5-fold compared with sham-treated controls, p<0.05, ANOVA. PTGS1 expression increased after cell transplantation, though more modestly, mean 5-fold increase compared with untreated controls, p<0.05, and only mean 1.5-fold compared with sham-treated controls, p=n.s. Expression of TXAS increased least after cell transplantation, mean 1.4-1.8-fold above untreated controls or sham-treated controls, with only marginal significances. Western blots indicated that total hepatic PTGS1 protein increased by up to 2.8-fold compared with untreated normal liver, although less compared with sham-treated animals (Fig. 1F). Hepatic PTGS2 protein level increased up to 2.1-fold above untreated control rats. Also, TXAS protein level rose, although mPTGES1 protein level was unchanged. These findings suggested differences in expression of cyclooxygenase genes at mRNA and protein levels, suggesting post-transcriptional or alterntive control mechanisms, perhaps similar to differences observed in other organ systems.19
Figure 1. Cell transplantation and perturbations in cyclooxygenase pathways.
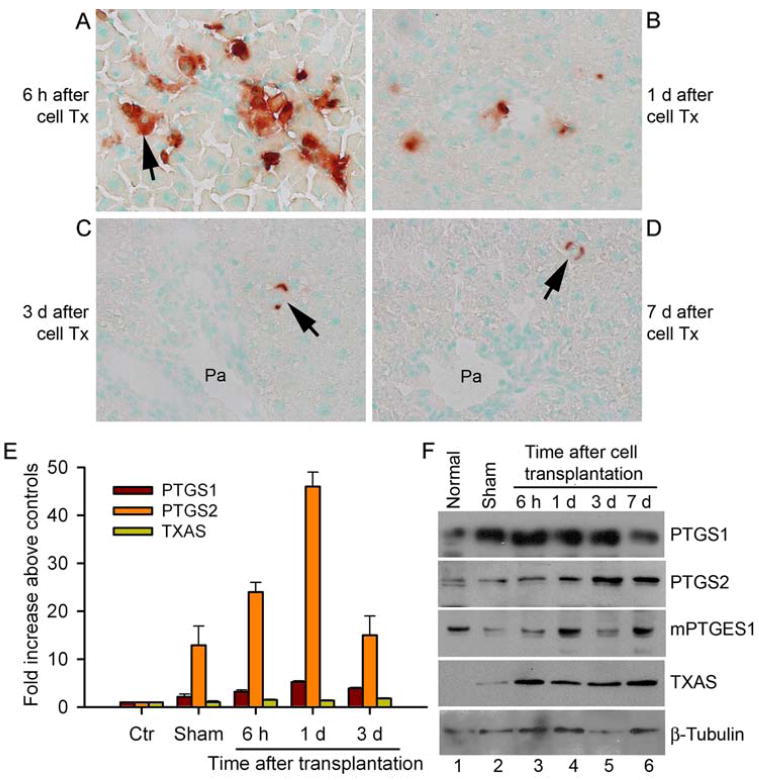
(A-D) Histochemical staining for DPPIV showing transplanted cells (arrows, red color) in the liver 6 h, 1 d, 3 d and 7 d after transplantation. Note transplanted cells in sinusoids and portal radicles after 6 h show diffuse membranous staining of DPPIV (A). By contrast, fewer transplanted cells were present at later times (B-D), and DPPIV staining was linear after 3 d and 7 d (arrows, C, D), indicating transplanted cells had integrated in liver parenchyma. Orig. mag. x400; methylgreen counterstain; Pa = portal area. (E) Real-time quantitative RT-PCR data showing changes in expression of PTGS1, PTGS2 and TXAS genes compared with untreated control animals and sham-treated animals as indicated. Expression of PTGS2 mRNA increased most. (F) Western blots showing protein analysis. Lane 1, unmanipulated normal liver; lane 2, sham-operated liver; lanes 3-6, livers after hepatocyte transplantation.
Tissue immunostaining demonstrated more PTGS1-expressing cells after hepatocyte transplantation (Fig. 2A-2F). PTGS1 was expressed in native hepatocytes, as well as sinusoidal cells, including HSC. By contrast, PTGS2-expressing cells were absent in untreated control rats, and appeared after cell transplantation, although PTGS2-expressing cells were less frequent than PTGS1-expressing cells, 0-2 cells versus 6-15 cells per lobule (Supplementary Fig. 2). Cells expressing PTGS1 and PTGS2 were often in proximity with transplanted cells (Fig. 2C, 2E and Supplementary Fig. 2C, 2E). We did not observe PTGS1 or PTGS2 expression in transplanted cells themselves. In sham-treated animals, PTGS1 or PTGS2-positive cells were occasionally observed in the liver (Supplementary Fig. 3), less than after cell transplantation, with 0-2 PTGS1-positive cells per lobule and only rare PTGS2-positive cells. Our efforts to demonstrate mPTGES and TXAS by immunostaining were unsuccessful.
Figure 2. Immunohistological studies of PTGS1 expression.
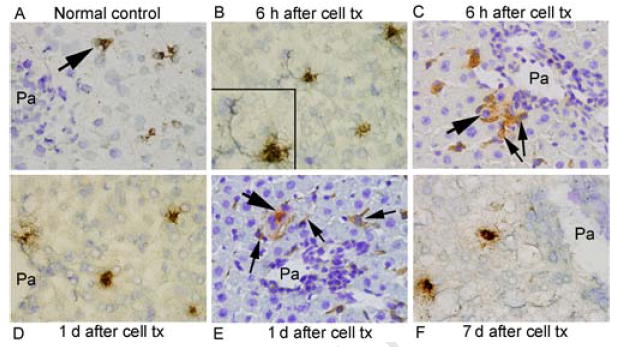
PTGS1 in normal rat liver (A) was expressed occasionally and was more frequently expressed after cell transplantation (arrows, B-F), including HSC (inset, B). Combined staining for DPPIV and PTGS1 showed that transplanted cells (thick arrows) and cells expressing PTGS1 (thin arrows) were often in proximity to one another (C,E). Orig. mag. x400; toluidine blue counterstain.
Inhibitors of PTGS1 and PTGS2 improved cell engraftment
Administration of naproxen or celecoxib once to rats improved cell engraftment (Supplementary Figure 4; Fig. 3A, 3B). In untreated control rats, we observed 60±27 transplanted cells per 100 liver lobules; after 2, 4 and 6 mg/kg naproxen, transplanted cells increased by 130±43%, 141±65% and 252±85%, respectively, p<0.05, ANOVA with Tukey’s test. In animals treated with 20, 30 and 40 mg/kg celecoxib, transplanted cell numbers increased by 114±48%, 120±38% and 151±79%, respectively, although this did not reach statistical significance. In animals treated over 3 d with 8 doses of naproxen (total 42 mg/kg) or celecoxib (total 320 mg/kg), cell engraftment again increased by 178±48% and 234±88%, respectively, compared with untreated controls, p<0.05, ANOVA with Tukey’s test, although this was not different from improved cell engraftment after single drug doses.
Figure 3. Changes in cell engraftment after treatment with PTS1 and PTS2 blockers.
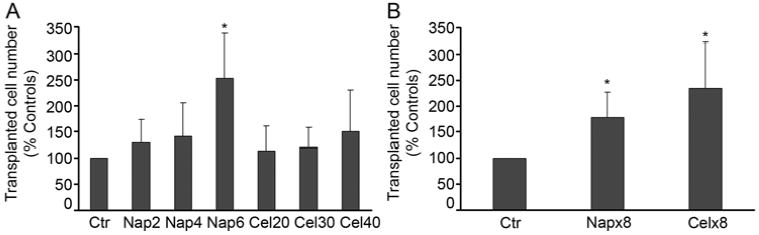
(A, B) Charts show morphometric quantitation of cell engraftment after single doses of 2, 4 and 6 mg/kg naproxen or 20, 30 and 40 mg/kg celecoxib (G) and multiple doses of these drugs (H). Asterisks = p<0.05 versus controls, ANOVA with Tukey test.
We found that superior cell engraftment in drug-treated rats was associated with lower or undetectable levels of PTGS2 and TXAS proteins after cell transplantation (Fig. 4). This indicated that naproxen and celecoxib altered cell transplantation-induced changes, particularly the TXA2 rather than the PGE2 limb of the cyclooxygenase pathway.
Figure 4. Changes in cyclooxygenase genes after drug treatments.
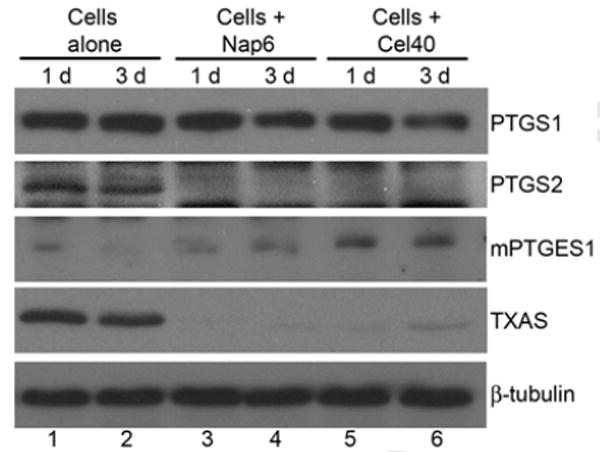
Shown are western blots with decreased expression of PTGS1, PTGS2 and TXAS proteins 1 d and 3 d after cell transplantation. Lanes 1, 2, transplantation of hepatocytes alone; lanes 3, 4, 6 mg/kg naproxen once before cell transplantation; lanes 5, 6, 40 mg/kg celecoxib once before cell transplantation. Note that PTGS1 expression declined slightly after drug treatments, whereas PTGS2 and TXAS expression decreased much more.
Inhibition of TXA2 mechanism and cell engraftment
To establish the contribution of TXA2 in cell engraftment, we treated rats with an inhibitor of TXAS, furegrelate, in doses of 3, 6 and 9 mg/kg, and the anti-platelet drug, clopidogrel, in doses of 5, 10 and 15 mg/kg, to block potential downstream effects of TXA2 via platelet aggregation. Drugs were given once 2 h before cell transplantation. Cell engraftment was unchanged 3 d after these drugs with morphometric analysis showing 97±22% and 95±17% of the transplanted cells in the liver of untreated control animals after highest doses of furegrelate and clopidogrel, respectively, p=n.s., ANOVA.
Regulation of cell transplantation-induced mechanisms by naproxen and celecoxib
To determine whether naproxen and celecoxib improved cell engraftment through vascular mechanisms, we studied hepatic activation of GGT, which is induced after cell transplantation and abolished by vasodilator drugs. 16 Neither naproxen nor celecoxib prevented cell transplantation-induced GGT expression (Supplementary Figure 5), despite improved cell engraftment, indicating these drugs did not alter hepatic ischemia-induced changes.
Next, we examined whether naproxen and celecoxib blocked hepatic inflammation following cell transplantation. Although neutrophils with myeloperoxidase activity accumulated in the liver within 6 h after cell transplantation (Fig. 5A, Supplementary Fig. 6), naproxen or celecoxib had no effect on neutrophil accumulation. Similarly, cell transplantation increased phagocytotic activity of Kupffer cells, whereas naproxen and celecoxib had no effect on Kupffer cell activation (Fig. 5B, Supplementary Fig. 7).
Figure 5. Cumulative morphometric analysis of myeloperoxidase-positive neutrophils and Kupffer cells in liver tissue 6 h after cell transplantation.
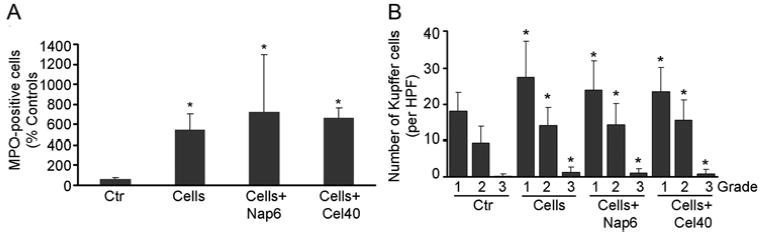
(A) Shows changes in the number of myeloperoxidase-positive neutrophils. (B) Shows numbers of carbon-containing Kupffer cells with grading as described in the text. Asterisks = p<0.05 versus untreated controls.
To analyze perturbations in inflammatory cytokines, we examined hepatic mRNAs of 84 chemokines, cytokines and relevant receptors by qRT-PCR. Cell transplantation resulted in 2-fold or greater expression of 29 genes (Supplementary Table 1). However, naproxen or celecoxib had no effect on these cytokine-chemokine responses.
Naproxen and celecoxib altered HSC
To demonstrate whether cell engraftment was improved through HSC, we initially determined phenotype changes with desmin immunostaining. Three days after cell transplantation, more HSC expressed desmin compared with untreated control rats (Fig. 6A-6B). Cell transplantation in animals treated with single doses of 6 mg/kg naproxen or 40 mg/kg celecoxib produced desmin expression in more HSC (Fig. 6C-6D). We observed 2±1 desmin-positive HSC per liver lobule in untreated control rats. The corresponding numbers of HSC in recipients of cells alone and in animals treated with naproxen and celecoxib were 12±5, 88±8 and 71±6 HSC per liver lobule, respectively, p<0.001, ANOVA. To determine whether desmin-positive phenotype of HSC was beneficial for cell engraftment, we analyzed expression of HGF, VEGF, MMPs and TIMP-1. These genes were expressed well in drug-treated rats (Fig. 6E, 6F).
Figure 6. Desmin staining to identify HSC.
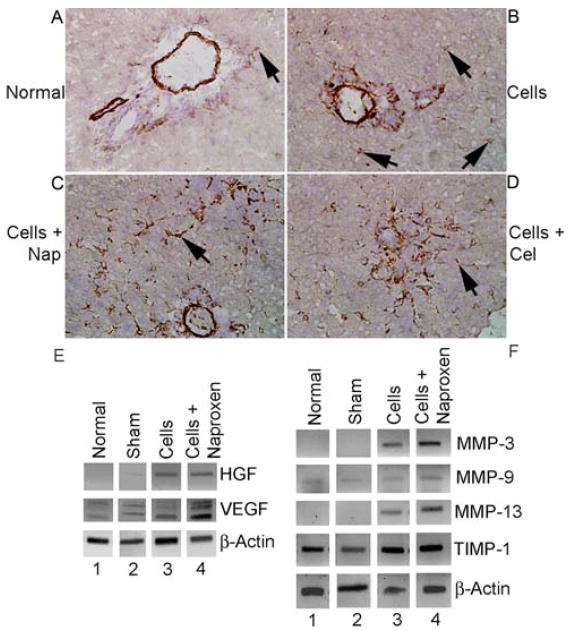
(A) Shows normal untreated rat without cell transplantation with desmin staining in major vessels in portal area as well as occasional desmin-positive HSC (arrow). (B) Shows rat 3 d after cell transplantation with interspersed HSC in periportal area (arrows). (C and D) Show rats 3 d after cell transplantation with 6 mg/kg naproxen or 40 mg/kg celecoxib with extensive increases in desmin-stained HSC. Orig. mag. x400; hematoxylin counterstain. (E) RT-PCR showing expression of HGF and VEGF mRNAs 3 d after cell transplantation with and without 6 mg/kg naproxen. Lane 1, untreated control liver; lane 2, cell transplantation alone; lane 3, cell transplantation after naproxen. (F) RT-PCR showing expression of MMP-3, -9 and -13, and TIMP-1 mRNAs. Lanes 1, 2, untreated controls; lanes 3, 4, cell transplantation alone; lanes 5, 6, cell transplantation after naproxen.
Studies of VEGF release in CFSC-8B rat HSC cells cultured with naproxen under normoxia and hypoxia condition indicated that both conditions, particularly the latter condition, induced expression of VEGF, as well as HGF, mRNAs (Fig. 7A). Increased release of VEGF was verified by protein measurements in culture medium following naproxen treatment (Fig. 7B). In CFSC-8B cells cultured with 1 μM naproxen under hypoxia conditions, VEGF release was 3-fold greater than drug-untreated controls, p<0.001, and exceeded 1 ng/ml. In cytotoxicity assays, when primary rat hepatocytes were cultured with actinomycin D and TNF-α, cell viability declined by 30±5% compared with untreated controls, p<0.05. By contrast, culture of hepatocytes with conditioned medium from CFSC-8B cells treated with naproxen dose-dependently abrogated TNF-α-induced cytotoxicity. Viability of hepatocytes treated with conditioned medium from CFSC-8B cells cultured with 1 μM naproxen was even higher than that of TNF-α untreated control hepatocytes.
Figure 7. Effects of naproxen on cytoprotective gene expression in CFSC-8B rat stellate cells cultured under normoxia and hypoxia conditions.
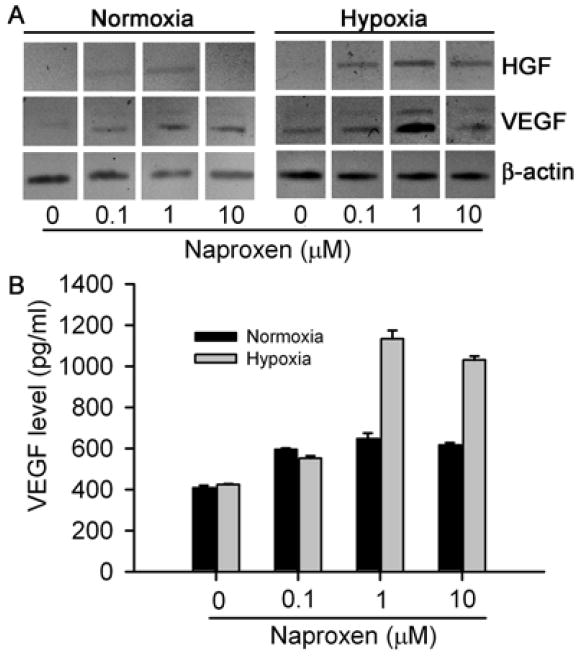
(A) Shows RT-PCR for cellular mRNAs indicating increases in expression of HGF and VEGF mRNAs with naproxen, up to 1 μM, especially under hypoxia conditions. (B) Shows VEGF protein levels in culture medium, which increased from 409±10 pg/ml and 424±5 pg/ml under basal normoxia and hypoxia conditions, respectively, to 648±26 pg/ml (1.8-fold) and 1133±42 pg/ml (2.8-fold) after culture with 1 μM naproxen under corresponding conditions, p<0.05, ANOVA.
DISCUSSION
These findings established that cell transplantation activated hepatic cyclooxygenase pathways during early clearance of transplanted cells from the liver. We found that mechanisms in cyclooxygenase-dependent cell clearance were complex, involving activation of PTGS1 and PTGS2 in native cells, whereas TXA2 or platelets were not involved. Sham-treatment with intrasplenic injection of vehicle alone also induced expression of cyclooxygenase genes, although it should be noteworthy that these components of intravascular perturbations cannot be completely separated from the procedure of cell transplantation. Blockade of PTGS1 and PTGS2 improved cell engraftment in the liver. This effect was mediated through HSC without reversal of ischemia-reperfusion events or phagocyte and macrophage responses after cell transplantation, which was unexpected, and opens new windows in the cellular roles of cyclooxygenase pathways.
After transplantation, engraftment of hepatocytes faces multiple hurdles, including microcirculatory alterations,6 with hepatic parenchymal disruption, loss of gap junctions in ischemic areas, and GGT expression as a reflection of oxidative stress and glutathione depletion.16 Since cyclooxygenase mechanisms may regulate hepatic vasculature through local production of PGI2, or impart cytoprotection against ischemia-induced changes, we examined alterations in these mechanisms. Naproxen or celecoxib did not prevent ischemic injury and GGT expression, indicating that hepatic changes induced by cell transplantation differed from other types of hepatic ischemia, where naproxen or celecoxib showed therapeutic efficacy. 20 The absence of an effect of furegrelate and clopidogrel in doses capable of inhibiting TXAS activity and platelet function, respectively, 21,22 on cell engraftment indicate the insignificance of TXA2 pathways in transplanted cell clearances.
We expected that naproxen and celecoxib will exert their beneficial effects, at least in part, by suppressing hepatic inflammation, especially since these drugs controlled inflammatory liver injury. 23,24 Activation of neutrophils and Kupffer cells, and expression of multiple cytokines-chemokines following cell transplantation was in agreement with hepatic inflammation. As PTGS1 and PTGS2 were often expressed in cells adjacent to transplanted hepatocytes, including HSC, this suggested opportunities for cell-cell interactions, along with potential for amplification of inflammatory perturbations. The underlying mechanisms regulating interactions between transplanted and native cells have not been established, although these would likely extend from changes in the hepatic environment to release of soluble signals from cells themselves. For instance, hypoxia after vascular occlusion, decreased ATP levels, release of mediators, such as endothelin-1, cytokines, MMP-9, or other molecules, from inflammatory cells, LSEC, parenchymal cells, and transplanted hepatocytes themselves, could have stimulated HSC. 25-28 It should be noteworthy that transplanted hepatocytes were previously demonstrated to express VEGF, 3 which plays major roles in activating HSC. 29 Inflammatory mediators, e.g., inducers of TNF-α or other signaling, 25,26,28 constitute plausible candidates for activating HSC. After cell transplantation in the liver, TNF-α expression was significantly upregulated, as also shown here; however, desmin expression in HSC was not prevented in cell transplantation studies with etanercept to block TNF-α. 8 Therefore, delineation of critical mechanisms in activation of HSC after cell transplantation requires more work.
Stimulation of HSC should offer an appropriate explanation for the efficacy of naproxen and celecoxib in improving hepatocyte engraftment in the liver since HSC were identified as regulators of cell engraftment. 8 Naproxen and celecoxib increased desmin expression in HSC after cell transplantation. This desmin-positive phenotype of HSC was similar to previous cell transplantation studies, where desmin was expressed without α-smooth muscle actin, presumably because this stimulus was transient and nonfibrogenic. Our studies with CFSC-8B cells verified that naproxen stimulated VEGF and HGF expression. In previous studies, activation of HSC produced expression of multiple cytokines, growth factors and MMPs associated with HSC, e.g., bFGF, HGF, TGF-β1, TNF-α, and VEGF, as well as MMP-3, -9 and -13, in particular, and TIMP-1, as well. 8 Cytoprotective molecules, e.g., HGF and VEGF should be of particular relevance in improving survival of transplanted hepatocytes, as shown by our findings of hepatocyte protection from cytokine toxicity by VEGF-containing CFSC-8B conditioned medium. Similarly, release of VEGF and MMPs would promote disruption of LSEC required for the entry of transplanted hepatocytes into the space of Disse and subsequently into the liver parenchyma followed by the involvement of TIMP-1 and other molecules in coordinating tissue remodeling during cell engraftment (Fig. 8).
Figure 8. Working model of cyclooxygenase pathways and hepatocyte engraftment in the liver.
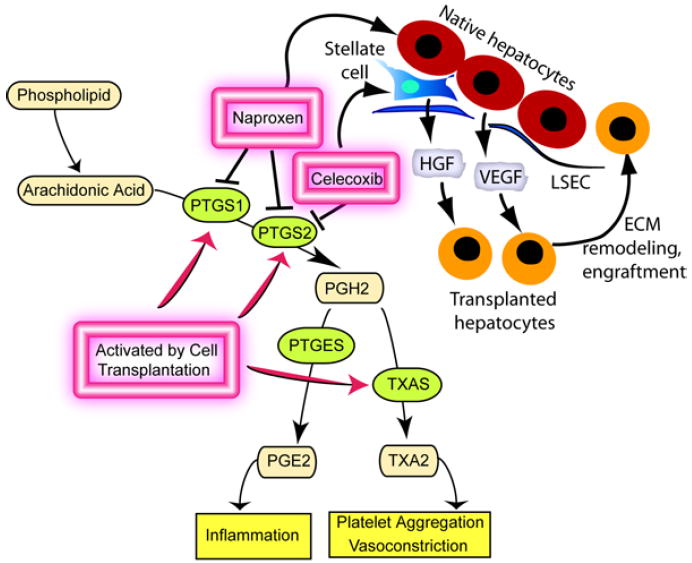
Cell transplantation induced hepatic expression of PTGS1, PTGS2, and other genes, although prostanoid limbs represented by TXA2 and PGE2 did not regulate cell engraftment. The studies showed that naproxen and celecoxib affected native liver cells, including HSC. Hepatic expression of pleiotropic factors, such as HGF and VEGF, which are both capable of cytoprotection, as well as of genes regulating extracellular matrix (ECM) remodeling provide relevant mechanisms to improve survival and engraftment of transplanted cells.
These findings of hepatic HGF, VEGF and MMP expression were different from other systems, where naproxen or celecoxib were found to inhibit these genes. For instance, celecoxib impaired HGF signaling in esophagus to delay ulcer healing,30 suppressed VEGF expression to impair growth of pancreatic adenocarcinoma,31 and of retinal pigment epithelial cells. 32 Similarly, celecoxib or naproxen decreased expression of MMPs-1, -2, -3, and -9 in chondrocytes, articular cartilage, meniscus and synovial cells.33,34 Therefore, cyclooxygenase pathways may subserve variable biological effects under tissue- and cell type-specific contexts.
This improvement in engraftment of transplanted hepatocytes by widely used PTGS1 and PTGS2 inhibitors, naproxen and celecoxib, will advance clinical strategies for cell therapy. Use of naproxen in a single dose before cell transplantation should be convenient. Relatively small drug doses were effective in our studies and no further advantage was found of administering drugs repeatedly over a longer period, suggesting that early and initial decrease in expression of PTGS1 and PTGS2 was sufficient. The lesser efficacy of celecoxib indicates that blocking PTGS2 alone will not be as effective for cell therapy.
Supplementary Material
Acknowledgments
Ms. Chaoying Zhang provided technical assistance.
Funding: Supported in part by NIH grants R01 DK46952, P30 DK41296 and P30 CA13330.
Footnotes
No conflicts of interest exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Joseph B, Malhi H, Bhargava KK, Palestro CJ, McCuskey RS, Gupta S. Kupffer cells participate in early clearance of syngeneic hepatocytes transplanted in the rat liver. Gastroenterology. 2002;123:1677–1685. doi: 10.1053/gast.2002.36592. [DOI] [PubMed] [Google Scholar]
- 2.Joseph B, Kumaran V, Berishvili E, Bhargava KK, Palestro CJ, Gupta S. Monocrotaline promotes transplanted cell engraftment and advances liver repopulation in rats via liver conditioning. Hepatology. 2006;44:1411–1420. doi: 10.1002/hep.21416. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Rajvanshi P, Sokhi RP, Slehria S, Yam A, Kerr A, Novikoff PM. Entry and integration of transplanted hepatocytes in liver plates occur by disruption of hepatic sinusoidal endothelium. Hepatology. 1999;29:509–519. doi: 10.1002/hep.510290213. [DOI] [PubMed] [Google Scholar]
- 4.Sokhi RP, Rajvanshi P, Gupta S. Transplanted reporter cells help in defining onset of hepatocyte proliferation during the life of F344 rats. Am J Physiol Gastroint Liver Physiol. 2000;279:G631–G640. doi: 10.1152/ajpgi.2000.279.3.G631. [DOI] [PubMed] [Google Scholar]
- 5.Wu YM, Joseph B, Gupta S. Immunosuppression using the mTOR inhibition mechanism affects replacement of the rat liver with transplanted cells. Hepatology. 2006;44:410–9. doi: 10.1002/hep.21277. [DOI] [PubMed] [Google Scholar]
- 6.Slehria S, Rajvanshi P, Ito Y, Sokhi R, Bhargava KK, Palestro CJ, McCuskey RS, Gupta S. Hepatic sinusoidal vasodilators improve transplanted cell engraftment and ameliorate microcirculatory perturbations in the liver. Hepatology. 2002;35:1320–1328. doi: 10.1053/jhep.2002.33201. [DOI] [PubMed] [Google Scholar]
- 7.Kumaran V, Joseph B, Benten D, Gupta S. Integrin and extracellular matrix interactions regulate engraftment of transplanted hepatocytes in the rat liver. Gastroenterology. 2005;129:1643–1653. doi: 10.1053/j.gastro.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Benten D, Kumaran V, Joseph B, Gupta S. Hepatocyte transplantation activates hepatic stellate cells with beneficial modulation of cell engraftment. Hepatology. 2005;42:1072–1081. doi: 10.1002/hep.20889. [DOI] [PubMed] [Google Scholar]
- 9.Khanapure SP, Garvey DS, Janero DR, Letts LG. Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Curr Top Med Chem. 2007;7:311–40. doi: 10.2174/156802607779941314. [DOI] [PubMed] [Google Scholar]
- 10.Bezugla Y, Kolada A, Kamionka S, Bernard B, Scheibe R, Dieter P. COX-1 and COX-2 contribute differentially to the LPS-induced release of PGE2 and TxA2 in liver macrophages. Prostagland Lipid Mediat. 2006;79:93–100. doi: 10.1016/j.prostaglandins.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, Yamanouchi K, Soeta C, Katakai Y, Harada R, Naito K, Tojo H. Skeletal muscle injury induces hepatocyte growth factor expression in spleen. Biochem Biophys Res Commun. 2002;292:709–714. doi: 10.1006/bbrc.2002.6706. [DOI] [PubMed] [Google Scholar]
- 12.Ishii H, Oota I, Arakawa T, Takuma T. Differential gene expression if vascular endothelial growth factor isoforms and their receptors in the development of the rat masseter muscle. Arch Oral Biol. 2002;47:505–510. doi: 10.1016/s0003-9969(02)00033-x. [DOI] [PubMed] [Google Scholar]
- 13.Haro H, Crawford HC, Fingleton B, MacDougall JR, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-3-dependent generation of a macrophage chemoattractant in a model of herniated disc resorption. J Clin Invest. 2000;105:133–141. doi: 10.1172/JCI7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-α and TGF-β1. J Hepatol. 1999;30:48–60. doi: 10.1016/s0168-8278(99)80007-5. [DOI] [PubMed] [Google Scholar]
- 15.Irani AN, Malhi H, Slehria S, Gorla GR, Volenberg I, Schilsky ML, Gupta S. Correction of liver disease following transplantation of normal hepatocytes in LEC rats modeling Wilson’s disease. Mol Ther. 2001;3:302–309. doi: 10.1006/mthe.2001.0271. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Rajvanshi P, Malhi H, Sokhi RP, Slehria S, Vasa SRG, Dabeva M, Shafritz DA, Kerr A. Cell transplantation causes loss of gap junctions and activates GGT expression permanently in host liver. Am J Physiol Gastroint Liver Physiol. 2000;279:G815–G826. doi: 10.1152/ajpgi.2000.279.4.G815. [DOI] [PubMed] [Google Scholar]
- 17.Ohayon O, Mawasi N, Pevzner A, Tryvitz A, Gildor T, Pines M, Rojkind M, Paizi M, Spira G. Halofuginone upregulates the expression of heparanase in thioacetamide-induced liver fibrosis in rats. Lab Invest. 2008;88:627–33. doi: 10.1038/labinvest.2008.30. [DOI] [PubMed] [Google Scholar]
- 18.Joseph B, Bhargava KK, Trunco G, Kumaran V, Palestro CJ, Gupta S. Regulation of hepatobiliary transport activity and noninvasive identification of cytokine-dependent liver inflammation. J Nucl Med. 2005;46:146–52. [PubMed] [Google Scholar]
- 19.Zhang Q, Collins V, Chakrabarty K, Rose JC, Wu WX. Regulation of the prostaglandin enzymatic system by estradiol and progesterone in nonpregnant sheep cervix. Reproduction. 2007;133:1027–34. doi: 10.1530/REP-06-0328. [DOI] [PubMed] [Google Scholar]
- 20.Ito Y, Katagiri H, Ishii K, Kakita A, Hayashi I, Majima M. Effects of selective cyclooxygenase inhibitors on ischemia/reperfusion-induced hepatic microcirculatory dysfunction in mice. Eur Surg Res. 2003;35:408–16. doi: 10.1159/000072174. [DOI] [PubMed] [Google Scholar]
- 21.Moussa O, Riker JM, Klein J, Fraig M, Halushka PV, Watson DK. Inhibition of thromboxane synthase activity modulates bladder cancer cell responses to chemotherapeutic agents. Oncogene. 2008;27:55–62. doi: 10.1038/sj.onc.1210629. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher WA, Bostwick JS, Ogletree ML, Stewart AB, Steinbacher TE, Hua J, Price LA, Wong PC, Rehfuss RP. Biomarker optimization to track the antithrombotic and hemostatic effects of clopidogrel in rats. J Pharmacol Exp Ther. 2007;322:369–77. doi: 10.1124/jpet.106.119156. [DOI] [PubMed] [Google Scholar]
- 23.Lebbe C, Reichen J, Wartna E, Sägesser H, Poelstra K, Meijer DK. Targeting naproxen to non-parenchymal liver cells protects against endotoxin induced liver damage. J Drug Target. 1997;4:303–10. doi: 10.3109/10611869708995846. [DOI] [PubMed] [Google Scholar]
- 24.Cazanave S, Vadrot N, Tinel M, Berson A, Lettéron P, Larosche I, Descatoire V, Feldmann G, Robin MA, Pessayre D. Ibuprofen administration attenuates serum TNF-alpha levels, hepatic glutathione depletion, hepatic apoptosis and mouse mortality after Fas stimulation. Toxicol Appl Pharmacol. 2008;15:231, 336–43. doi: 10.1016/j.taap.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Kojima N, Hori M, Murata T, Morizane Y, Ozaki H. Different profiles of Ca2+ responses to endothelin-1 and PDGF in liver myofibroblasts during the process of cell differentiation. Br J Pharmacol. 2007;151:816–27. doi: 10.1038/sj.bjp.0707269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruddell RG, Knight B, Tirnitz-Parker JE, Akhurst B, Summerville L, Subramaniam VN, Olynyk JK, Ramm GA. Lymphotoxin-beta receptor signaling regulates hepatic stellate cell function and wound healing in a murine model of chronic liver injury. Hepatology. 2008;49:227–239. doi: 10.1002/hep.22597. [DOI] [PubMed] [Google Scholar]
- 27.Yan C, Zhou L, Han YP. Contribution of hepatic stellate cells and matrix metalloproteinase 9 in acute liver failure. Liver Int. 2008;28:959–71. doi: 10.1111/j.1478-3231.2008.01775.x. [DOI] [PubMed] [Google Scholar]
- 28.Giannelli G, Bergamini C, Marinosci F, Fransvea E, Napoli N, Maurel P, Dentico P, Antonaci S. Antifibrogenic effect of IFN-alpha2b on hepatic stellate cell activation by human hepatocytes. J Interferon Cytokine Res. 2006;26:301–8. doi: 10.1089/jir.2006.26.301. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Lui EL, Friedman SL, Li L, Ye T, Chen Y, Poon RT, Wo J, Kok TW, Fan ST. PTK787/ZK22258 attenuates stellate cell activation and hepatic fibrosis in vivo by inhibiting VEGF signaling. Lab Invest. 2008 Dec 29; doi: 10.1038/labinvest.2008.127. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baatar D, Jones MK, Pai R, Kawanaka H, Szabo IL, Moon WS, Kitano S, Tarnawski AS. Selective cyclooxygenase-2 blocker delays healing of esophageal ulcers in rats and inhibits ulceration-triggered c-Met/hepatocyte growth factor receptor induction and extracellular signal-regulated kinase 2 activation. Am J Pathol. 2002;160:963–72. doi: 10.1016/S0002-9440(10)64918-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64:2030–8. doi: 10.1158/0008-5472.can-03-1945. [DOI] [PubMed] [Google Scholar]
- 32.Amrite AC, Ayalasomayajula SP, Cheruvu NP, Kompella UB. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci. 2006;47:1149–1160. doi: 10.1167/iovs.05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu SC, Yang SF, Lue KH, Hsieh YS, Li TJ, Lu KH. Naproxen, meloxicam and methylprednisolone inhibit urokinase plasminogen activator and inhibitor and gelatinases expression during the early stage of osteoarthritis. Clin Chim Acta. 2008;387:90–6. doi: 10.1016/j.cca.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Tsutsumi R, Ito H, Hiramitsu T, Nishitani K, Akiyoshi M, Kitaori T, Yasuda T, Nakamura T. Celecoxib inhibits production of MMP and NO via down-regulation of NF-kappaB and JNK in a PGE2 independent manner in human articular chondrocytes. Rheumatol Int. 2008;28:727–36. doi: 10.1007/s00296-007-0511-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


