Abstract
We sought to quantify patient morbidity throughout Pseudomonas aeruginosa bloodstream infection (PABSI) as a function of patient covariates. Individuals with PABSI were included in a retrospective, observational, cohort study. Morbidity was quantified by serial Sequential Organ Failure Assessment (SOFA) scores. Impact of active antimicrobial treatment was assessed as a function of changes in SOFA scores as the dependent variable. A total of 95 patients with PABSI were analyzed. Relative to baseline SOFA scores (day −2), scores following PABSI were increased by 37% on day 0 and 22% on day +2 but returned to baseline on day +7. Overall mortality was 37%, and mean length of hospital stay (post-culture) was 16 days. Most patients were appropriately treated, with n=83 (87%) receiving an active agent and n=61 (64%) receiving >1 agent. As a result, an effect of therapy on morbidity was not observed. Advanced age and elevated baseline SOFA scores predicted increased in-hospital mortality (p=0.01 and p<0.001, respectively) and morbidity at day +2 (p<0.05 and p<0.05, respectively) and day +7 (p<0.05 and p<0.001, respectively). Neutropenia was also associated with increased morbidity at day +2 (p<0.05). In treated PABSI, morbidity is highest the day of the diagnostic blood cultures and slowly returns to baseline over the subsequent seven days. Age and baseline severity of illness are the strongest predictors of morbidity and mortality. Since neither of these factors is modifiable, efforts to minimize the negative impact of PABSI should focus on appropriate prevention and infection control efforts.
Keywords: Pseudomonas aeruginosa, bloodstream infection, bacteremia, epidemiology
Introduction
Nosocomial bloodstream infections have become a common occurrence in modern medicine. Over 250,000 of these infections occur annually in the United States at a rate of 1.5 to 6.8 events per 1000 central line days (Pittet et al., 1997; Edwards et al. 2007). In this setting, Pseudomonas aeruginosa is a frequently identified pathogen, accounting for approximately 20% of nosocomial bloodstream infections caused by gram-negative bacteria (Wisplinghoff et al., 2004). In addition to being common, P. aeruginosa bloodstream infections (PABSIs) portend dire outcomes for patients. In a recent large multicenter study, PABSI was associated with crude mortality rates of 39% in all patients and 48% in intensive care unit patients (Wisplinghoff et al., 2004). Thus it is not surprising that investigators have devoted significant effort to identifying factors and treatment options associated with improved survival.
Poor outcomes in P. aeruginosa infections have been associated with both microbial and host factors. Bacterial attributes predisposing to mortality include a high degree of intrinsic virulence (Roy-Burman et al., 2001; Hauser et al., 2002), widespread antibiotic resistance (NNIS, 2004), and the fact that the organisms incur minimal fitness costs with multiple resistance mutations (Hocquet et al., 2007). Host factors that have been associated with poor outcomes include neutropenia, prolonged duration of bacteremia, pneumonic source, shock, renal failure, and metastatic foci of infection (Bisbe et al., 1988; Hilf et al., 1989; Mallolas et al., 1990; Chatzinikolaou et al., 2000; Micek et al., 2005; Lodise et al., 2007). A potentially modifiable variable, time to active antimicrobial treatment, is of contemporary importance as evaluations have demonstrated poorer outcomes with inadequate antimicrobial treatment (Chamot et al., 2003; Kang et al., 2003; Micek et al., 2005; Lodise et al., 2007).
Despite an abundance of information on risk factors for mortality, relatively little is known about the temporal course of PABSI. In particular, the morbidity of these infections over time has not been carefully characterized, nor have the organ systems most affected by systemic P. aeruginosa infection. Sequential Organ Failure Assessment (SOFA) scores have emerged as a useful tool to quantify morbidity and predict mortality in critically ill patients (Vincent et al., 1998). To better understand the dynamic nature of severity of illness during PABSI, we used SOFA scores to quantify morbidity in a serial fashion throughout the infection. Morbidity, mortality, and length of hospital stay were compared for patient and treatment variables to identify risk factors for poor outcomes.
Materials and Methods
Study Setting and Design
In this retrospective cohort study, we examined hospitalized patients identified by blood cultures submitted to the Northwestern Memorial Hospital (NMH) Microbiology Laboratory that were positive for P. aeruginosa between August 1999 and January 2003. NMH is an 897-bed academic medical center with a full range of clinical services. Patients were considered to have PABSI if ≥1 blood culture grew this organism. Exclusion criteria were a previous positive blood culture for P. aeruginosa as part of the study, incomplete documentation of medical care, or patient age less than 16 years. Patients with polymicrobial bacteremia were included in the study, but this variable was assessed as a covariate. This project was reviewed and approved by the Northwestern University Institutional Review Board (IRB Project #0247-001).
Inpatient electronic medical records, pharmacy and microbiology databases, and paper charts were reviewed. Recorded patient demographics included age, race, gender, neutropenia, and number of days from admission to the positive blood culture. Recorded characteristics of bacteremia included the suspected source of infection and whether bacteremia was polymicrobial. The following aspects of antimicrobial therapy were determined: active therapy ever, active therapy within 2 days, time to active therapy, therapy with ≥2 active antibiotics ever, time to therapy with ≥2 active antibiotics, class of active antibiotic, and number of active agents. Outcomes included morbidity, length of hospital stay post-infection for survivors, and in-hospital mortality. Morbidity was classified as component and composite SOFA scores on day 0, day +2, and day +7.
Microbiology
Blood cultures from hospitalized patients were processed by the NMH Microbiology Laboratory using the BACTEC® 9240 blood culture system (Becton Dickinson, Sparks, MD), with each set consisting of aerobic and anaerobic cultures. Genus and species identification of bacteria from positive cultures was performed by the Vitek II System (bioMérieux, Balmes-les-Grottes, France) or manual biochemical assays when necessary. Susceptibility testing was performed on all isolates by either the Vitek II or Etest (AB Biodisk, Solna, Sweden) to determine minimum inhibitory concentrations (MICs). Antimicrobial agents tested included piperacillin/tazobactam, ceftazidime, imipenem, meropenem, aztreonam, levofloxacin, ciprofloxacin, amikacin, tobramycin, and gentamicin. MICs were categorically interpreted according to Clinical and Laboratories Standards Institute (CLSI) guidelines (CLSI, 2007).
Definitions
Race was classified categorically as Caucasian, African-American, Asian, Hispanic, or other/unknown. Neutropenia was defined as an absolute neutrophil count <500 per mm 3 (Hughes et al., 2002). Polymicrobial bacteremia was defined as isolation of P. aeruginosa and an additional bacterium from the blood at the time of the diagnostic blood culture. A separate variable was created for polymicrobial bacteremia that excluded coagulase-negative staphylococci. Nosocomial bacteremia was defined as bacteremia acquired after 72 hours of hospitalization or in patients hospitalized at some point within the two weeks preceding the diagnostic blood culture. For each patient, the bacteremia was classified as complex, catheter-related, or other. Complex bacteremia was defined as bacteremia resulting from major organ infections, soft tissue infections, abscesses, or any necrotic infection, as previously described (Elting et al., 1997). Catheter-related infections were defined as PABSI with an intravascular catheter in place and in the absence of another attributable source.
Only antibiotics given during the hospitalization were reviewed. Active therapy was defined as therapy with at least one antibiotic to which the causative P. aeruginosa strain was susceptible as classified by the CLSI guidelines (CLSI, 2007). Specific doses were not reviewed, but all antibiotics were dosed by clinical pharmacists prospectively in an attempt to optimize efficacy while minimizing safety concerns. Active treatment within two days was defined as any active therapy administered within two calendar days of the collection of the blood specimen that ultimately grew P. aeruginosa. Time to therapy with ≥2 active agents was defined as the number of calendar days between collection of the blood specimen and the administration of more than one active agent. Active antibiotics were classified into categories of β-lactams, aminoglycosides, and fluoroquinolones.
Measures of Morbidity
Morbidity was quantified using SOFA scores (Vincent et al., 1998). Baseline morbidity was defined by the SOFA score two days prior to the diagnostic blood culture (day −2). Morbidity following PABSI was quantified by calculating SOFA scores at the following times: the day of the diagnostic culture (day 0), two days after the diagnostic blood culture (day +2), and seven days after the diagnostic blood culture (day +7). To calculate SOFA scores, the necessary laboratory and clinical data were collected from patients’ medical records for the appropriate day. If multiple values were obtained on a given day, the most abnormal value was used. If a value was missing from the day of interest, the measurement from the nearest day was used in its place. If no values were available, the measurement was assumed to be normal. Points were assigned for increasing degrees of failure of six different organ systems: respiratory, coagulation, hepatic, cardiovascular, central nervous system, and renal, as previously described (Vincent et al. 1998). Values were transformed into component dysfunction scores from 0 (normal) to 4 (the most abnormal) for each organ system (Vincent et al., 1998), thus providing a composite SOFA score between 0 to 24 points. Unless explicitly stated, the main analyses assumed that all patients who died had the maximal SOFA score (24 points) from the time of death forward. Other analyses only looked at SOFA scores for surviving patients. Length of stay post culture was used as a marker of morbidity; it was calculated only for surviving patients. Mortality was defined as death during the hospital admission.
Statistical Analysis
Data analysis was performed using Intercooled Stata, version 9.2 (Statacorp, College Station, TX). Univariate analyses were performed on all data. Continuous variables were evaluated with Students t-test, and categorical variables were evaluated with the Chi-square or Fisher’s exact test where appropriate. The primary endpoint for estimation of morbidity after infection was the change in SOFA score from day 0 to day +2 and day 0 to day +7. All tests were two-tailed, and p-values less than 0.05 were considered statistically significant.
Multivariate analysis was performed as a hierarchical regression by constructing a model consisting of variables commonly known or variables with a significant and plausible relationship with morbidity/mortality. The order in which the blocks were entered was based on theoretical or substantive considerations. The analysis included four separate hierarchical regression models, one for each dependent variable. Morbidity was measured as the change in SOFA score from day 0 to day +2 and day +7, and length of hospital stay post-bacteremia for surviving patients. Mortality was assessed as a dependent variable. The models predicting day 0 to day +2 change in SOFA score, day 0 to day +7 change in SOFA score, and length of stay post infection used ordinary least squares regression. The model predicting hospital death was performed with a logistic regression. Variables were iteratively removed according to block and significance level (p<0.05) until only variables with significant effects remained.
Actual model building was performed as follows: First, day −2 SOFA scores were forced into the model as a baseline measure of morbidity, prior to infection. In the second step, demographic factors were entered to control for exogenous patient characteristics. Insignificant demographic variables were then iteratively removed until only the significant variables remained. Because polymicrobial bacteremia, host neutropenia, and known nosocomial infection can confound the effect of active therapy on morbidity/mortality, measures of those parameters were entered third. Insignificant variables from the third step were then removed until only significant variables remained. The use of specific active antibiotics (β-lactam, aminoglycoside, and fluoroquinolone) was entered fourth. Finally, two variables of interest (active therapy by day +2 and dual active therapy) were added last to determine whether they had an impact beyond all other factors in the models.
Results
Patient Characteristics
A total of 108 occurrences of PABSI were assessed for patient enrollment into the study. Thirteen patients were subsequently excluded: 5 had incomplete medical records, 5 were less than 16 years of age, and 3 had been previously enrolled in the study. The remaining 95 patients with PABSI were analyzed. Patients had a mean age of 57.0 years, represented a number of different races, and were evenly divided by gender (54% male) (Table 1). PABSI occurred after a mean of 9.4 days of hospitalization. Few patients had catheter-related infections (12%); the majority of bacteremias (51%) were complex, resulting from extensive tissue infections. Twenty-seven percent of patients had polymicrobial bacteremia with secondary pathogens other than coagulase-negative staphylococci.
Table 1.
Demographic and Clinical Characteristics of the Study Population
| Variable | All Patients (n=95) |
|---|---|
| Age in Years, mean (SD) | 57.0 (19.0) |
| Ethnicity (n, %) | |
| Caucasian | 50 (52.6) |
| African-American | 15 (15.8) |
| Asian | 2 (2.1) |
| Hispanic | 6 (6.3) |
| Other or Unknown | 22 (23.2) |
| Gender, n (%) | |
| Male | 51 (53.7) |
| Baseline Morbidity | |
| Composite SOFA Score at Day −2, mean (SD) | 4.3 (0.4) |
| Composite SOFA Score at Day 0, mean (SD) | 6.1 (4.4) |
| Length of Hospital Stay Prior to Bacteremia, mean (SD) | 9.4 (15.4) |
| Neutropenia, n (%) | 17 (18.1) |
| Polymicrobial Bacteremia, n (%) | 34 (35.8) |
| Polymicrobial Bacteremia excluding CNS*, n (%) | 26 (27.4) |
| Catheter-Related Bacteremia, n (%) | 11 (11.6) |
| Complex Bacteremia, n (%) | 48 (50.5) |
coagulase-negative staphylococci
Morbidity and Mortality of PABSI
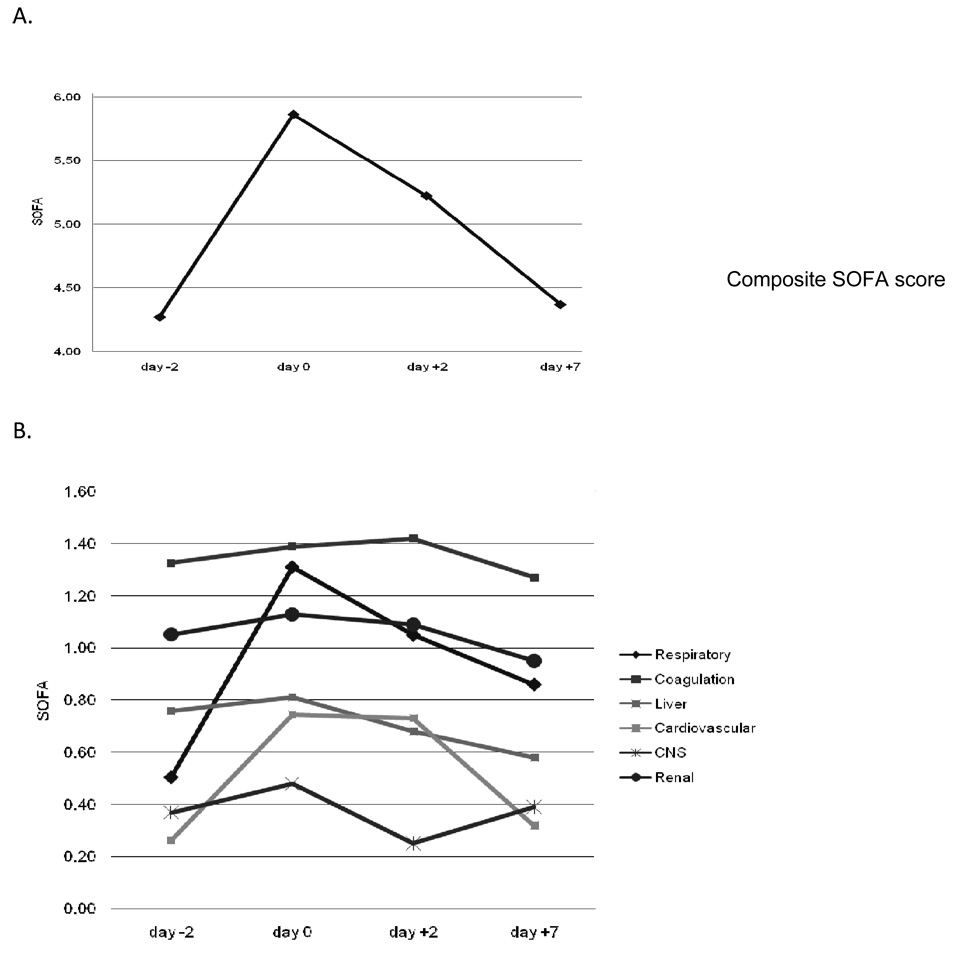
The morbidity experienced by patients with PABSI was examined as a function of time. Since patient drop-out due to mortality can bias the calculation of morbidity, only surviving patients were included in this analysis at each time point. The mean baseline composite SOFA score for all patients was 4.3 at day −2 (Table 1 and Fig. 1A). The composite SOFA score rose (i.e. morbidity increased) by 37% at day 0, the day of the diagnostic blood culture, and remained 22% above baseline at day +2. By day +7, the composite SOFA score had returned to only 2% above baseline. The component SOFA scores indicated that the majority of overall morbidity was due to cardiovascular and respiratory compromise (Fig. 1B).
Figure 1.
Change in SOFA scores over time for surviving patients with PABSI. A. Composite SOFA scores. B. Component SOFA scores. Only surviving patients are included in the analysis to avoid drop-out bias from patients who died. Day −2, n=95; day 0, n=94; day +2,n=85; day +7, n=79
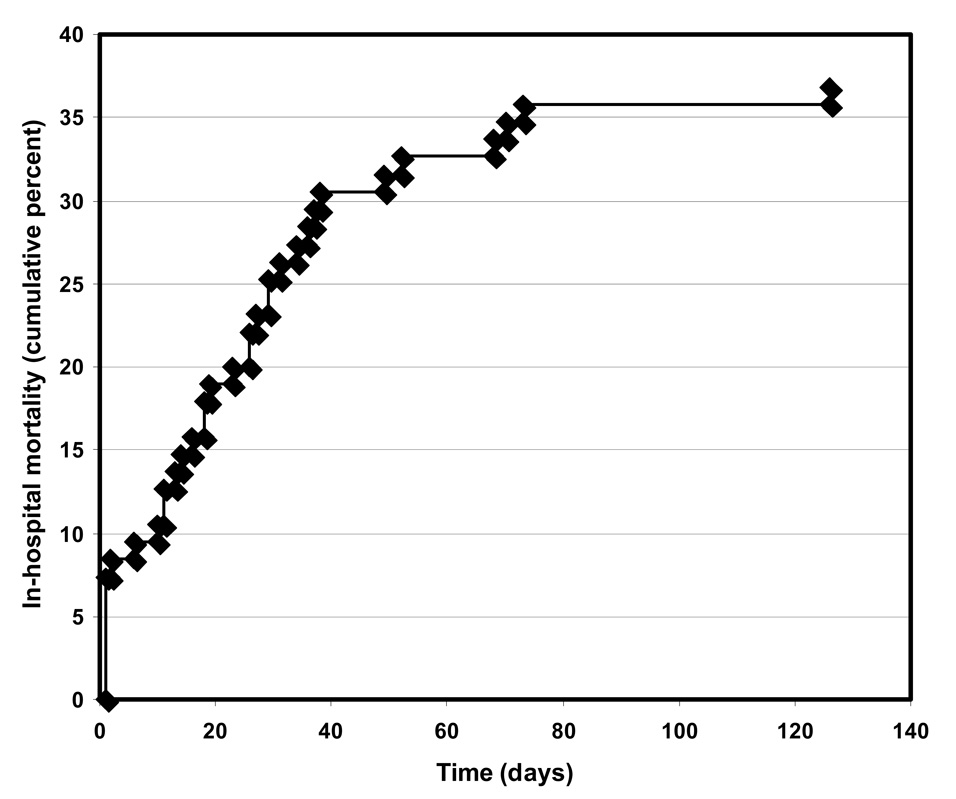
PABSI was associated with in-hospital mortality of 37% and, among surviving patients, a mean length of hospital stay after onset of bacteremia 16 days. The rate of in-hospital deaths was highest during the first day following the diagnostic blood culture, with subsequent deaths occurring at a relatively constant rate over the next 40 days (Fig. 2).
Figure 2.
In-hospital mortality over time of patients with PABSI. Time is measured from the day of the diagnostic blood culture.
Effect of Active Antimicrobial Therapy on Disease Severity
The administration of antimicrobial agents active against the causative agent of bacteremia has been shown to impact mortality in bacteremia (Vidal et al., 1996), so associations between this variable and outcomes were examined. All together, 83 (87%) of the 95 patients received active therapy (defined as at least one agent to which their P. aeruginosa isolate was susceptible); 22 (23%) received one active agent, 49 (52%) received two active agents, and 12 (13%) received 3 active agents. Of the 12 patients who did not receive active therapy, 3 (25%) were infected with P. aeruginosa strains that were resistant to all tested antimicrobial agents, and 2 (17%) were infected with strains resistant to all antimicrobial agents except aminoglycosides. Patients who received active therapy and those who did not receive active therapy were similar in age and ethnicity (data not shown).
As expected, the relatively small number of patients receiving inappropriate antimicrobial therapy limited our ability to show an effect of therapy on outcomes. In bivariate analysis, mortality did not differ between those who received active therapy and those who did not receive active therapy (36.1% vs. 41.7%, respectively; p=0.76) nor was mortality affected by receipt of dual active therapy (38.2% vs. 36.0%, respectively; p=0.83) (data not shown). Additionally, receipt of active therapy by day 2 was not predictive of mortality (p=0.29) nor was number of active antimicrobial agents analyzed as a continuous variable (p=0.72).
Since mortality is a rather crude measure of disease severity, we also examined the effect of antimicrobial therapy on morbidity. No statistically significant associations were noted between active or timely antibiotic therapy and SOFA scores (data not shown). However a trend toward improved composite SOFA scores was noted in those patients who received active therapy between day 0 and day +2 compared to those who did not receive active therapy during this time frame (1.0 vs. 2.4, respectively; p=0.28). Likewise a trend towards shorter mean length of hospital stay following bacteremia was noted for those who received active therapy compared to those who did not receive active therapy (15.3 days vs. 22.7 days, respectively; p=0.30).
Multiple reports have linked aminoglycoside monotherapy with poor outcomes in PABSI (Hilf et al., 1989; Kuikka et al., 1998; Chatzinikolaou et al., 2000). For this reason, we repeated our analysis defining aminoglycosides alone or in conjunction with a second inactive agent as inadequate therapy. Results were not significantly changed (data not shown).
Effect of Timing of Active Therapy on Disease Severity
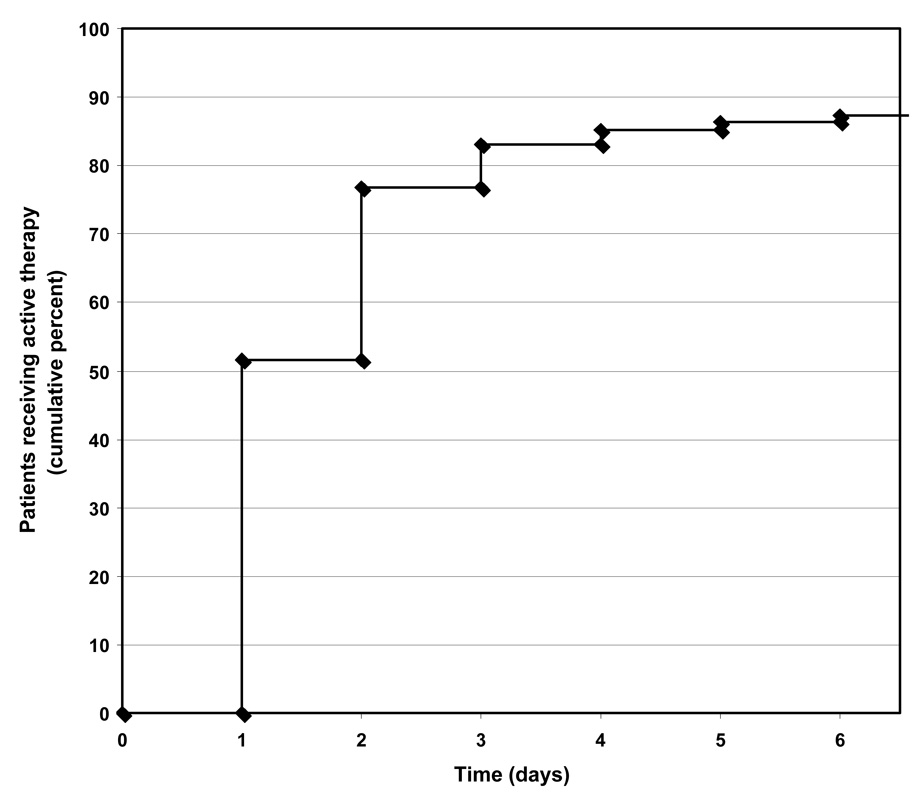
Delay in administration of appropriate antibiotic agents has also been shown to effect outcomes of bloodstream infections (Kang et al., 2003; Lodise et al., 2003). For this reason, the timing of antimicrobial therapy in our patient cohort was examined more directly. Patients generally were given active antimicrobial therapy rapidly, with 77% receiving active therapy by day +2 (Fig. 3). Mean times to active therapy and to therapy with ≥2 active agents were 1.6 days (SD 0.96 days) and 2.3 days (SD 1.52 days), respectively. Time to active therapy or time to therapy with ≥2 active agents was not associated with changes in mortality, length of hospital stay after bacteremia, or in SOFA scores between day 0 and day +2 (p= 0.41 and 0.43, respectively) or between day 0 and day +7 (p=0.71 and 0.39, respectively). Results did not appreciably change after controlling for age and severity of illness at baseline (day −2). Additionally, active therapy variables (any active therapy ever, dual active therapy ever, and number of active agents) and time to active therapy variables (time to active therapy and time to ≥2 active agents) did not appreciably impact the change in SOFA scores between day −2 and day +2 or day −2 and day +7 when additional regressions controlled for covariates such as baseline SOFA score (data not shown). Similarly, additional regressions on the change in SOFA score from baseline to day 0, day +2, or day +7 as the dependent variable were largely unrevealing for baseline predictor variables (data not shown).
Figure 3.
Time to active therapy among the cohort of 95 patients with PABSI. Time is measured from the day of the diagnostic blood culture.
Predictors of Morbidity and Mortality
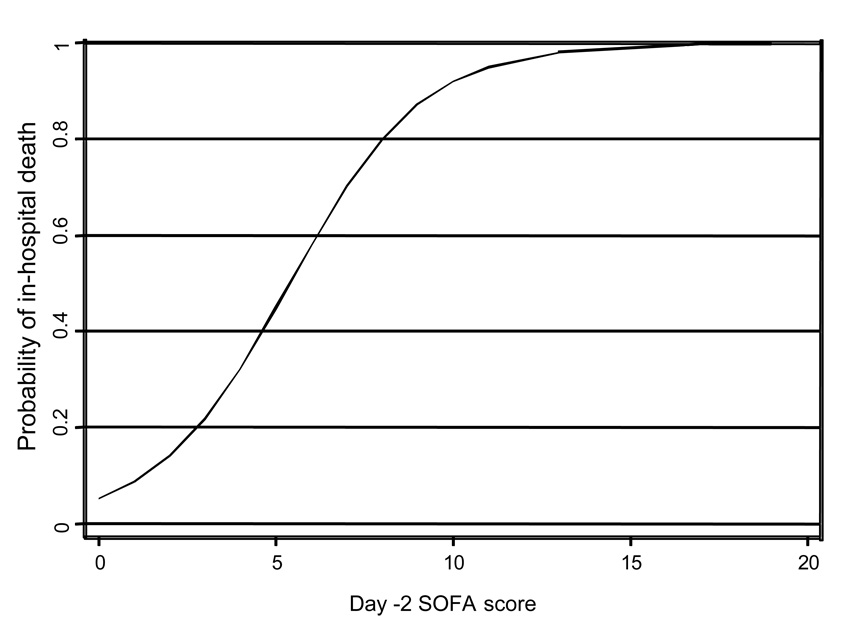
We identified factors associated with mortality and length of hospital stay in our cohort of patients with PABSI. Patients who ultimately survived or died were similar in demographic variables but differed markedly in baseline morbidity (Table 2). Bivariate analysis indicated that patients who eventually died had higher respiratory, coagulation, liver, and cardiovascular SOFA scores at baseline than did patients who survived (Table 2). Likewise patients who died had higher baseline composite SOFA scores than patients who survived (6.5 vs. 3.0, respectively; p < 0.001). The probability of hospital death escalated as a log-linear function across increasing values of baseline (day −2) SOFA scores (Fig. 4). Length of hospital stay prior to bacteremia was also associated with mortality, and an intravascular catheter as the source of bacteremia showed a trend towards increased survival. Multivariate analysis was next performed to identify factors independently (after controlling for confounders) associated with increased mortality with PABSI. Of the factors listed in Table 2, only increased baseline severity of illness (p<0.001) and advanced age (p=0.01) were independently predictive of mortality during the hospital stay (Table 3). Only nosocomial bacteremia predicted length of hospital stay after PABSI (p=0.005).
Table 2.
Baseline Variables Stratified by Mortality
| Variable | Survived (n=60) |
Died (n=35) |
Significance |
|---|---|---|---|
| Age in Years, mean (SD) | 55.6 (17.8) | 59.4 (20.9) | 0.341 |
| Ethnicity (n, %) | |||
| Caucasian | 28 (46.7) | 22 (62.9) | 0.13 |
| African-American | 12 (20.0) | 3(8.6) | 0.24 |
| Asian | 0 (0) | 2 (5.7) | * |
| Hispanic | 3 (5.0) | 3 (8.6) | 0.67 |
| Other or Unknown | 17 (28.3) | 5 (14.3) | 0.14 |
| Gender, n (%) | |||
| Male | 36 (60.0) | 15 (42.9) | 0.11 |
| SOFA score | |||
| Day −2, mean (SD) | |||
| Respiratory | 0.2 (0.5) | 1.1 (1.5) | <0.001 |
| Coagulation | 1.1 (1.4) | 1.8 (1.3) | 0.01 |
| Liver | 0.5 (0.9) | 1.3 (1.3) | <0.001 |
| Cardiovascular | 0.1 (0.3) | 0.6 (1.3) | 0.003 |
| Central Nervous System | 0.3 (0.8) | 0.5 (1.0) | 0.24 |
| Renal | 1.0 (1.2) | 1.2 (0.2) | 0.38 |
| Composite | 3.0 (2.2) | 6.5 (4.2) | <0.001 |
| Day 0, mean (SD) | |||
| Respiratory | 0.8 (1.2) | 2.2 (1.4) | <0.001 |
| Coagulation | 1.2 (1.5) | 1.9 (1.5) | 0.34 |
| Liver | 0.5 (1.0) | 1.4 (1.4) | <0.001 |
| Cardiovascular | 0.2 (0.6) | 1.7 (1.7) | <0.001 |
| Central Nervous System | 0.3 (0.8) | 0.8 (1.4) | 0.04 |
| Renal | 1.0 (1.2) | 1.5 (1.3) | 0.08 |
| Composite | 4.1 (2.5) | 9.5 (4.9) | <0.001 |
| Length of Hospital Stay Prior to Bacteremia, mean (SD) |
5.7 (11.0) | 15.6 (19.4) | 0.002 |
| Neutropenia, n (%) | 8 (13.6) | 9 (25.7) | 0.14 |
| Polymicrobial Bacteremia, n (%) | 22 (36.7) | 12 (34.3) | 0.82 |
| Polymicrobial Bacteremia excluding CNS**, n (%) |
18 (30.0) | 8 (22.9) | 0.45 |
| Catheter-Related Bacteremia, n (%) | 10 (16.7) | 1 (2.9) | 0.05 |
| Complex Bacteremia, n (%) | 28 (46.7) | 20 (57.1) | 0.32 |
unable to calculate due to a zero value
coagulase-negative staphylococci
Figure 4.
Probability of in-hospital death (y-axis) across values of SOFA scores (x-axis) at day −2. Data are modeled as a log-linear function after controlling for age and active therapy.
Table 3.
Multivariate Analyses of Patient Outcomes
| Linear Regression Model Predicting Day 0 to Day +2 Change in SOFA Score | ||||
|---|---|---|---|---|
| B | Std. Error |
t | Sig. | |
| Day −2 SOFA | 0.375 | 0.124 | 3.014 | 0.003 |
| Neutropenia | 2.409 | 1.086 | 2.218 | 0.029 |
| Age | 0.049 | 0.023 | 2.165 | 0.033 |
| Adjusted R-square=0.129, p=0.001 | ||||
| Linear Regression Model Predicting Day 0 to Day +7 Change in SOFA Score | ||||
| B | Std. Error | t | Sig. | |
| Age | 0.091 | 0.030 | 2.983 | 0.004 |
| Day −2 SOFA | 0.667 | 0.166 | 4.009 | <0.001 |
| Adjusted R-square=0.164, p=0.001 | ||||
| Linear Regression Model Predicting Length of Hospital Stay Post Culture | ||||
| B | Std. Error | t | Sig | |
| Healthcare bacteremia | 12.238 | 4.203 | 2.912 | 0.005 |
| Adjusted R-square=0.136, p=0.005 | ||||
| Logistic Regression Model Predicting Death during Hospitalization | ||||
| Odds Ratio (OR) |
95% CI for OR | Sig. | ||
| Day −2 SOFA | 1.704 | 1.320 to 2.199 | <0.001 | |
| Age | 1.040 | 1.008 to 1.072 | 0.011 | |
Multivariate analysis was also performed to determine factors associated with increased morbidity. Advanced age, neutropenia, and baseline severity of illness were each associated with increased SOFA scores at day +2 relative to day 0 (p=0.033, p=0.029, and p=0.003, respectively) (Table 3). Age and baseline severity of illness were associated with increased SOFA scores between day 0 and day +7 (p=0.004 and p<0.001, respectively). Only nosocomial bacteremia predicted length of hospital stay after PABSI (p=0.005). Variables affecting change in SOFA from day −2 (baseline) to day 0 were assessed. After controlling for relevant covariates, no variables significantly predicted change in SOFA score between baseline and the day of the diagnostic blood culture (data not shown).
Discussion
Numerous studies have demonstrated that PABSI is associated with high mortality rates, documenting the virulence of P. aeruginosa in this setting. Mortality, however, is a crude measure of a micro-organism’s ability to harm its host, since the majority of patients recover from their illnesses. In this regard, it has been suggested that morbidity is a more sensitive measure of disease severity (Petros et al., 1995). We used serial SOFA scores to measure the morbidity associated with PABSI over time. SOFA scores have emerged as a useful tool to quantify morbidity, to predict mortality, and have been validated in a variety of patient populations, including bacteremia (Vincent et al., 1998; Ferreira et al., 2001; Pettila et al., 2002; Routsi et al., 2007). In its ability to predict mortality, SOFA scoring has performed comparably to other morbidity quantification methods such as the Acute Physiology and Chronic Health Evaluation (APACHE) III score, Logistic Organ Dysfunction score (LODS), and the Multiple Organ Dysfunction Score (MODS) (Pettila et al., 2002). Furthermore, SOFA scoring can be applied sequentially to assess changes in morbidity over time (Ferreira et al., 2001). We calculated SOFA scores at day −2 to quantify baseline patient morbidity rather than assess a variable in the causal pathway of sepsis (McGregor et al., 2007). The impact of therapy was measured from the earliest feasible time that most patients could receive antibiotics, the day of the diagnostic blood culture (day 0). Subsequent changes in SOFA scores were then followed to measure the impact of PABSI on morbidity.
SOFA scores in surviving PABSI patients rose from baseline to their highest levels on day 0 of bacteremia, decreased somewhat on day +2, and returned to baseline by day +7. The increase in SOFA scores at day 0 and day +2 were largely driven by the respiratory and the cardiovascular components, indicating that these organ systems are most affected when P. aeruginosa is present in the bloodstream. These results indicate that in treated patients the morbidity of PABSI is largely incurred during the first few days following infection. Understanding the changing morbidity of patients relative to the course of the infection may help future studies target appropriate endpoints.
Overall mortality among our patients was 37%. This relatively high proportion is especially impressive given that most patients received prompt and active antimicrobial therapy. Nevertheless, our mortality rate was similar to those reported in the literature for PABSI. For example, Micek and colleagues reported an in-hospital PABSI mortality rate of 21% (Micek et al., 2005). Likewise Kang et al., Chamot et al., and Lodise et al. reported 30-day mortality rates of 39%, 39%, and 31%, respectively, associated with PABSI (Chamot et al., 2003; Kang et al., 2003; Lodise et al., 2007). While a large number of deaths occurred during the first few days following the diagnostic blood culture (Fig. 2) and likely were the result of the P. aeruginosa infection, mortality remained high over the subsequent 40 days. It is less clear whether these deaths are attributable to PABSI or underlying comorbid conditions. Our results suggest that the largest driver of outcomes in PABSI is baseline morbidity as defined by day −2 SOFA. As the baseline SOFA score approaches a value of 10, mortality is almost universal regardless of age and active antimicrobial treatment (Fig.4). Our length of hospital stay following PABSI for surviving patients was 16 days, which was also similar to previously reported values of 10.5 to 23.9 days (Blot et al., 2003; Micek et al., 2005; Osih et al., 2007). These findings underscore the continued poor outcomes associated with PABSI despite the development of more potent antimicrobial agents and advances in clinical care.
Surprisingly the timing and activity of antimicrobial therapy did not affect morbidity or mortality in our study. This finding was true in our bivariate comparisons even without controlling for the higher baseline morbidity in patients who did not receive active therapy. The concept of the importance of prompt administration of appropriate antimicrobial therapy is not unique to PABSI; multiple studies have addressed the timing of antibiotic therapy for various pathogens and disease states (Harbarth et al., 2007). Likewise most (Chamot et al., 2003; Kang et al., 2003; Kang et al., 2005; Micek et al., 2005; Lodise et al., 2007), although not all (Vidal et al., 1996; Osih et al., 2007), investigations of PABSI support the need for early active treatment. Contributing to our inability to detect an effect of antimicrobial therapy was the fact that most of our cohort of patients received prompt and active antibiotics. Only 12 (12.6%) of the 95 patients failed to receive active therapy. More than half (52%) of patients received active therapy within the first calendar day, and 77% of patients had active therapy started by the second calendar day (Fig. 3). Since active therapy by 52 hours is an important predictor of 30-day mortality in bacteremia (Lodise et al., 2007), most patients in our study received optimal therapy. Hence, we had relatively constrained power to show a morbidity or mortality difference between promptly given active therapies and delayed or inappropriate therapies. For instance, the power of our study to detect even a large difference in mortality between two groups (e.g. 20% difference between active therapy vs. inactive therapy) was approximately 20%.
Multivariate analysis indicated that age and baseline severity of illness were associated with mortality, suggesting that activity of initial antibiotics or time to active therapy for PABSI is less important than these factors (Bryan et al., 1983; Marra et al., 2006; Scarsi et al., 2006; Osih et al., 2007). However, it must be noted that the majority of evidence to date emphasizes the importance of timely therapy and this remains a modifiable variable (Chamot et al., 2003; Kang et al., 2003; Kang et al., 2005; Micek et al., 2005; Lodise et al., 2007). Our study was limited by a relative lack of power in regards to assessing the importance of time to therapy on outcomes. One must exercise caution in interpreting time to therapy results from our study unless it is supported by future well designed studies.
Patients with pre-existing liver and respiratory compromise were particularly prone to death from PABSI (Table 2). This is consistent with prior reports that acute and chronic liver failure are associated with poor outcomes in bacteremia (Graudal et al., 1986; Rolando et al., 1990), presumably because of the important role played by Kupffer cells in sterilizing the blood. In a mouse model of P. aeruginosa bacteremia, ablation of Kupffer cells resulted in the loss of bacterial clearance and subsequent organ failure and mortality (Ashare et al., 2006). Baseline respiratory compromise was likely associated with mortality because P. aeruginosa bacteremia caused a dramatic deterioration in pulmonary function (Fig. 2), and patients with pre-existing pulmonary injury lacked sufficient respiratory reserve to withstand this insult.
Increased morbidity in PABSI was associated with age, baseline severity of illness, and neutropenia in a multivariate analysis (Table 3). Other studies have also found that neutropenia at onset of bacteremia or progression to neutropenia during therapy for bacteremia was predictive of poorer outcomes in PABSI (Bodey et al., 1985; Hilf et al., 1989). These results are consistent with animal studies demonstrating that neutrophils play a predominant role in protecting the host from P. aeruginosa infection (Rehm et al., 1980; Terashima et al., 1995). Thus factors beyond the control of the treating practitioner may predominantly impact the outcomes of patients with PABSI and may explain the persistent morbidity and mortality associated with these infections.
This study has several important limitations. First, it is a retrospective analysis of the experience at a single center. Second, most patients promptly received active therapy, limiting our power to detect associations between antimicrobial therapy and outcomes. Third, our analysis required the many assumptions detailed in the manuscript. For instance, calculating morbidity scores for patients who have died is problematic. Our main analysis assigned maximum morbidity scores to these patients as a form of censorship for subsequent times after death, although in one of our analyses we only assessed morbidity for those that survived. To validate the appropriateness of our numerous assumptions, we conducted sensitivity analyses by varying the assumptions (e.g. assumed that the last documented SOFA score carried forward for all time points for deceased patients, assumed that monotherapy with an active aminoglycoside was considered sufficient treatment, etc.) and found similar results. This indicates that the assumptions did not substantially influence our findings. Despite these limitations, our study was conducted in a manner consistent with recent suggestions designed to standardize analyses of associations between appropriate antibiotics and outcomes in bacteremic patients (McGregor et al., 2007).
Morbidity associated with PABSI increases during the first few days of bacteremia and returns to baseline by seven days. Morbidity and mortality are primarily influenced by baseline severity of illness and age. Even with optimal antibiotic therapy, PABSI is associated with high mortality, underscoring the need for appropriate preventative infection control measures.
Acknowledgements
We thank Susan Collins and members of the Microbiology Laboratory at Northwestern Memorial Hospital for assistance in obtaining the strains and Jana Lichtenfield and Courtney Ramirez for help with collecting clinical data and Michael Postelnick for advice. Portions of this work were previously presented at the 2007 Interscience Conference on Antimicrobial Agents and Chemotherapy in Chicago, IL. (K-475).
Funding: This study was funded by the NIH (AI053674 and AI065615, A.R.H.)
Footnotes
Transparency declarations: All authors report no relevant conflicts.
References
- Ashare A, Monick MM, Powers LS, Yarovinsky T, Hunninghake GW. Severe bacteremia results in a loss of hepatic bacterial clearance. Am J Respir Crit Care Med. 2006;173:644–652. doi: 10.1164/rccm.200509-1470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisbe J, Gatell JM, Puig J, Mallolas J, Martinez JA, Jimenez de Anta MT, Soriano E. Pseudomonas aeruginosa bacteremia: univariate and multivariate analyses of factors influencing the prognosis in 133 episodes. Rev Infect Dis. 1988;10:629–635. doi: 10.1093/clinids/10.3.629. [DOI] [PubMed] [Google Scholar]
- Blot S, Vandewoude K, Hoste E, Colardyn F. Reappraisal of attributable mortality in critically ill patients with nosocomial bacteraemia involving Pseudomonas aeruginosa. J Hosp Infect. 2003;53:18–24. doi: 10.1053/jhin.2002.1329. [DOI] [PubMed] [Google Scholar]
- Bodey GP, Jadeja L, Elting L. Pseudomonas bacteremia. Retrospective analysis of 410 episodes. Arch Intern Med. 1985;145:1621–1629. doi: 10.1001/archinte.145.9.1621. [DOI] [PubMed] [Google Scholar]
- Bryan CS, Reynolds KL, Brenner ER. Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effects of antimicrobial therapy. Rev Infect Dis. 1983;5:629–638. doi: 10.1093/clinids/5.4.629. [DOI] [PubMed] [Google Scholar]
- Chamot E, Boffi El Amari El, Rohner P, Van Delden C. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2003;47:2756–2764. doi: 10.1128/AAC.47.9.2756-2764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzinikolaou I, Abi-Said D, Bodey GP, Rolston KV, Tarrand JJ, Samonis G. Recent experience with Pseudomonas aeruginosa bacteremia in patients with cancer: Retrospective analysis of 245 episodes. Arch Intern Med. 2000;160:501–509. doi: 10.1001/archinte.160.4.501. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute. CLSI document M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute; 2007. Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement. [Google Scholar]
- Edwards JR, Peterson KD, Andrus ML, Tolson JS, Goulding JS, Dudeck MA, Mincey RB, Pollock DA, Horan TC. National Healthcare Safety Network (NHSN) Report, data summary for 2006, issued June 2007. Am J Infect Control. 2007;35:290–301. doi: 10.1016/j.ajic.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Elting LS, Rubenstein EB, Rolston KV, Bodey GP. Outcomes of bacteremia in patients with cancer and neutropenia: observations from two decades of epidemiological and clinical trials. Clin Infect Dis. 1997;25:247–259. doi: 10.1086/514550. [DOI] [PubMed] [Google Scholar]
- Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. Jama. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- Graudal N, Milman N, Kirkegaard E, Korner B, Thomsen AC. Bacteremia in cirrhosis of the liver. Liver. 1986;6:297–301. doi: 10.1111/j.1600-0676.1986.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Harbarth S, Nobre V, Pittet D. Does antibiotic selection impact patient outcome? Clin Infect Dis. 2007;44:87–93. doi: 10.1086/510075. [DOI] [PubMed] [Google Scholar]
- Hauser AR, Cobb E, Bodi M, Mariscal D, Valles J, Engel JN, Rello J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30:521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med. 1989;87:540–546. doi: 10.1016/s0002-9343(89)80611-4. [DOI] [PubMed] [Google Scholar]
- Hocquet D, Berthelot P, Roussel-Delvallez M, Favre R, Jeannot K, Bajolet O, Marty N, Grattard F, Mariani-Kurkdjian P, Bingen E, Husson MO, Couetdic G, Plesiat P. Pseudomonas aeruginosa may accumulate drug resistance mechanisms without losing its ability to cause bloodstream infections. Antimicrob Agents Chemother. 2007;51:3531–3536. doi: 10.1128/AAC.00503-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KV, Shenep JL, Young LS. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730–751. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- Kang CI, Kim SH, Kim HB, Park SW, Choe YJ, Oh MD, Kim EC, Choe KW. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis. 2003;37:745–751. doi: 10.1086/377200. [DOI] [PubMed] [Google Scholar]
- Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49:760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuikka A, Valtonen VV. Factors associated with improved outcome of Pseudomonas aeruginosa bacteremia in a Finnish university hospital. Eur J Clin Microbiol Infect Dis. 1998;17:701–708. doi: 10.1007/s100960050164. [DOI] [PubMed] [Google Scholar]
- Lodise TP, Jr, Patel N, Kwa A, Graves J, Furuno JP, Graffunder E, Lomaestro B, McGregor JC. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother. 2007;51:3510–3515. doi: 10.1128/AAC.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis. 2003;36:1418–1423. doi: 10.1086/375057. [DOI] [PubMed] [Google Scholar]
- Mallolas J, Gatell JM, Miro JM, Marco F, Soriano E. Epidemiologic characteristics and factors influencing the outcome of Pseudomonas aeruginosa bacteremia. Rev Infect Dis. 1990;12:718–719. doi: 10.1093/clinids/12.4.718. [DOI] [PubMed] [Google Scholar]
- Marra AR, Bearman GM, Wenzel RP, Edmond MB. Comparison of severity of illness scoring systems for patients with nosocomial bloodstream infection due to Pseudomonas aeruginosa. BMC Infect Dis. 2006;6:132. doi: 10.1186/1471-2334-6-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor JC, Rich SE, Harris AD, Perencevich EN, Osih R, Lodise TP, Jr, Miller RR, Furuno JP. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin Infect Dis. 2007;45:329–337. doi: 10.1086/519283. [DOI] [PubMed] [Google Scholar]
- Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother. 2005;49:1306–1311. doi: 10.1128/AAC.49.4.1306-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- Osih RB, McGregor JC, Rich SE, Moore AC, Furuno JP, Perencevich EN, Harris AD. Impact of empiric antibiotic therapy on outcomes in patients with Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2007;51:839–844. doi: 10.1128/AAC.00901-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros AJ, Marshall JC, van Saene HK. Should morbidity replace mortality as an endpoint for clinical trials in intensive care? Lancet. 1995;345:369–371. doi: 10.1016/s0140-6736(95)90347-x. [DOI] [PubMed] [Google Scholar]
- Pettila V, Pettila M, Sarna S, Voutilainen P, Takkunen O. Comparison of multiple organ dysfunction scores in the prediction of hospital mortality in the critically ill. Crit Care Med. 2002;30:1705–1711. doi: 10.1097/00003246-200208000-00005. [DOI] [PubMed] [Google Scholar]
- Pittet D, Li N, Woolson RF, Wenzel RP. Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin Infect Dis. 1997;24:1068–1078. doi: 10.1086/513640. [DOI] [PubMed] [Google Scholar]
- Rehm SR, Gross GN, Pierce AK. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J Clin Invest. 1980;66:194–199. doi: 10.1172/JCI109844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolando N, Harvey F, Brahm J, Philpott-Howard J, Alexander G, Gimson A, Casewell M, Fagan E, Williams R. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11:49–53. doi: 10.1002/hep.1840110110. [DOI] [PubMed] [Google Scholar]
- Routsi C, Pratikaki M, Sotiropoulou C, Platsouka E, Markaki V, Paniara O, Vincent JL, Roussoss C. Application of the sequential organ failure assessment (SOFA) score to bacteremic ICU patients. Infection. 2007;35:240–244. doi: 10.1007/s15010-007-6217-6. [DOI] [PubMed] [Google Scholar]
- Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis. 2001;183:1767–1774. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
- Scarsi KK, Feinglass JM, Scheetz MH, Postelnick MJ, Bolon MK, Noskin GA. Impact of inactive empiric antimicrobial therapy on inpatient mortality and length of stay. Antimicrob Agents Chemother. 2006;50:3355–3360. doi: 10.1128/AAC.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima T, Kanazawa M, Sayama K, Urano T, Sakamaki F, Nakamura H, Waki Y, Soejima K, Tasaka S, Ishizaka A. Neutrophil-induced lung protection and injury are dependent on the amount of Pseudomonas aeruginosa administered via airways in guinea pigs. Am J Respir Crit Care Med. 1995;152:2150–2156. doi: 10.1164/ajrccm.152.6.8520789. [DOI] [PubMed] [Google Scholar]
- Vidal F, Mensa J, Almela M, Martinez JA, Marco F, Casals C, Gatell JM, Soriano E, Jimenez de Anta MT. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch Intern Med. 1996;156:2121–2126. [PubMed] [Google Scholar]
- Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]