Abstract
Background
Folic acid is known to be associated with inflammatory diseases, but the relationship between folic acid and allergic diseases is unclear.
Objectives
The purpose of the study was to examine the relationship between serum folate levels and markers of atopy, wheeze, and asthma.
Methods
Data were obtained from the 2005–2006 National Health and Nutrition Examination Survey (NHANES) in which serum folate and total IgE levels were measured in 8,083 subjects 2 years of age and older. A high total IgE level was defined as >100kU/L. Allergen-specific IgE levels were measured for a panel of 5 common aeroallergens. Atopy was defined as at least 1 positive allergen-specific IgE level. Doctor-diagnosed asthma and wheeze in the previous 12 months were assessed by questionnaire.
Results
Serum folate levels were inversely associated with total IgE levels (p<.001). The odds of a high total IgE, atopy, and wheeze decreased across quintiles of serum folate, indicating a dose-response relationship between serum folate levels and these outcomes. Each of these associations remained statistically significant after adjusting for age, sex, race/ethnicity and poverty index ratio. Adjusted odds ratios[95% confidence intervals] associated with the fifth quintile (Q5) of folate relative to the first quintile (Q1) were as follows: High IgE: 0.70[0.53–0.92]; atopy: 0.69[0.57–0.85]; and wheeze: 0.60[0.44–0.82]. Higher folate levels were also associated with a lower risk of doctor-diagnosed asthma, but this finding was not statistically significant (OR[95% CI] for Q5 vs. Q1: 0.84 [0.70–1.02]).
Conclusions
Serum folate levels are inversely associated with high total IgE, atopy, and wheeze.
Clinical Implications
Folic acid status may influence the development and/or progression of atopy and wheeze.
Keywords: asthma, allergy, atopy, folate, NHANES, Centers for Disease Control
Introduction
The prevalence of asthma and allergy has risen dramatically over the past 20–30 years in developed countries and the reasons behind this striking trend remain unclear. This increase in prevalence cannot be attributed to changes in the genetic makeup of the population because this trend has occurred over a relatively short period of time. As such, there has been active investigation in changes in environmental exposures as a potential cause of the recent rise in allergic diseases.
Allergen exposure(1–7), pollutant exposure(8–12), endotoxin exposure(13–15), immunizations(16–19), diet(20–23), and exposure to parasitic(24–26) and viral(27–29) infections have all been implicated in the epidemic. These exposures could act directly on the immune system or end-organs to elicit or attenuate sensitization and disease, but they may also result in epigenetic changes that could tilt the immunophenotypic balance in favor of allergic disease. One particular dietary component that could directly influence the propensity for epigenetic modifications is folic acid which serves as a source for methyl donors for DNA methylation. In fact, a methyl donor-enriched diet that included folic acid enhanced allergic humoral responses and lung inflammation in a mouse model(30), suggesting that epigenetic changes can indeed promote the development of allergic disease.
Although the findings from this mouse model are intriguing, it is unclear how they might translate to humans. On the one hand, the concomitant rise in allergic diseases(31;32) and serum folate levels in the United States(33) suggests that the relatively recent enrichment of the US diet with folic acid may be a risk factor for allergic disease, an observation that would be consistent with the findings in mice. On the other hand, lower folic acid levels have been implicated in a variety of inflammation-mediated diseases such as cardiovascular disease(34–37) and rheumatoid arthritis(38;39), so it is possible that folate may mitigate against, rather than promote, allergic diseases, which are also inflammation-mediated.
In order to gain insight into the role of folate in the development of allergic disease, we examined relationships between serum folate levels and measures of atopy and airways disease in the 2005–6 NHANES population.
Methods
The 2005–6 NHANES dataset(40) was used to examine relationships between serum folate levels and measures of atopy, wheeze, and asthma. The NHANES is a nationally representative survey of the non-institutionalized United States civilian population. NHANES uses a complex multistage probability design to select a representative population and oversamples certain underserved groups to increase the precision of estimates generated during analysis. The NHANES was approved by the IRB of the National Center for Health Statistics, Centers for Disease Control and Prevention, and informed consent was obtained from all participants.
All participants who had serum folate and total IgE levels measured were included in the analyses, which resulted in a final sample size of 8,083. Ages ranged from 2 years to greater than 85 years. Sociodemographic, serum folate, total and specific IgE levels, the respiratory disease questionnaire and medical conditions questionnaire data were included in the dataset. A participant was considered to have had wheeze in the preceding 12 months if he/she responded affirmatively to the following question: “In the past 12 months, have you had wheezing or whistling in your chest?” A participant was considered to have doctor-diagnosed asthma if he/she responded affirmatively to the following question: “Has a doctor or other health professional ever told you that you have asthma?”
Laboratory Evaluations
Serum total IgE levels were measured using the ImmunoCAP system (Phadia, Uppsala, Sweden). Cat, dog (e5), Dermatophagoides farinae, Dermatophagoides pteronyssinus, Alternaria, and cockroach-specific IgE levels were also measured using the ImmunoCAP system. A level ≥ 0.35 kU/L was considered positive and atopy was defined as at least one positive allergen-specific IgE. Serum folate levels were measured by radioassay with the Quantaphase II Folate kit (Bio-Rad Laboratories).
Statistical Analyses
Statistical analyses were performed with StataSE 8.0 (StataCorp, College Station, TX). The primary sampling units and strata were taken into account using the variables provided in the NHANES dataset to account for the complex survey design. Sampling weights provided by NHANES were used to generate estimates that are representative of the United States non-institutionalized civilian population.
Relationships between variables of interest were examined using logistic or linear regression methods that accounted for the sampling design and the weighting of the observations in the NHANES dataset. Bivariate analyses of folate and the outcome variables of interest were used to examine trends in the outcomes across a continuous measure of serum folate as well as quintiles of folate. A test for trend was performed by using either the continuous folate variable or the variable for quintiles of folate, without inclusion of dummy variables. Multivariate models were adjusted for age, sex, race/ethnicity, and poverty income ratio which is the ratio of family income to the poverty threshold. Analysis of variance (ANOVA) was used to compare serum folate levels among race/ethnicity subgroups. A two-tailed p < .05 was considered statistically significant.
Results
The study population reflected the national representation of the survey design and consisted of approximately equal proportions of males and females, with a mean age of 38 years. (Table I.) The population was predominantly Non-hispanic white, with 13% self-identified as Mexican American or another Hispanic ethnicity, and 12% Non-hispanic Black. Approximately 14% of the population had doctor-diagnosed asthma, with 15.7% reporting wheeze in the previous 12 months. Twenty-seven percent had a total IgE level above 100kU/L and 32% were atopic, having at least one positive allergen-specific IgE level.
Table I.
Sociodemographic Characteristics
| Characteristic | % |
|---|---|
| Sex | |
| Male | 48.9 |
| Female | 51.1 |
| Age, mean±SE | 38.3±0.8 |
| Race/ethnicity | |
| Non-Hispanic White | 69.7 |
| Hispanic | 12.9 |
| Non-Hispanic Black | 11.8 |
| Other | 5.6 |
| Income (n=8004) | |
| <20,000 | 15.9 |
| ≥20,000 | 83.9 |
| Refused/don’t know | 1.2 |
| Asthma, doctor diagnosed (8074)* | 14.4 |
| Wheezing, past 12 months (8080) | 15.7 |
| High Total IgE(>100 kU/L) (8083) | 27.4 |
| Allergen-specific IgE, positive | |
| Dust mite (8082) | 20.0 |
| Cat (8082) | 11.6 |
| Dog (8079) | 11.4 |
| Cockroach (8072) | 10.0 |
| Alternaria (8053) | 8.4 |
| Atopy (≥ 1 positive) (8052) | 32.4 |
number of participants with complete data is indicated in parentheses after each variable
Serum folate levels ranged from 0.7–171.0 ng/ml, and were stratified by quintile. The quintile cutpoints were as follows: Q1: 0.7–8.1; Q2: 8.2–10.9; Q3: 11.0–13.8; Q4: 13.9–17.9; and Q5: 18.0–171.0 ng/ml. Non-hispanic Blacks and Hispanics had lower serum folate levels than Non-hispanic whites (mean serum folate levels: 12.0, 12.5, and 15.0 ng/ml, respectively; p < .001). Adjustment for income did not alter these racial/ethnic associations with serum folate levels, but higher income tended to be associated with higher serum folate levels. Specifically, serum folate levels were estimated to be 2.8 ng/ml lower among Non-hispanic Blacks than Non-hispanic Whites and 2.1 ng/ml lower among Hispanics than Non-hispanic Whites. (Table II.)
Table II.
Race/ethnicity, Poverty and Serum Folate Levels
| Characteristic | Differences in Serum Folate Levels (ng/ml) (95% CI) | p value |
|---|---|---|
| Race/ethnicity (reference=Non-hispanic white) | ||
| Non-hispanic Black | −2.8 (−3.5–−2.0) | <.001 |
| Hispanic | −2.1 (−3.1–−1.1) | .006 |
| Other | −1.4 (−3.1–0.4) | .10 |
| Poverty Index Ratio† | 0.2 (−0.1–0.5) | .04 |
Ratio of family income to poverty threshold
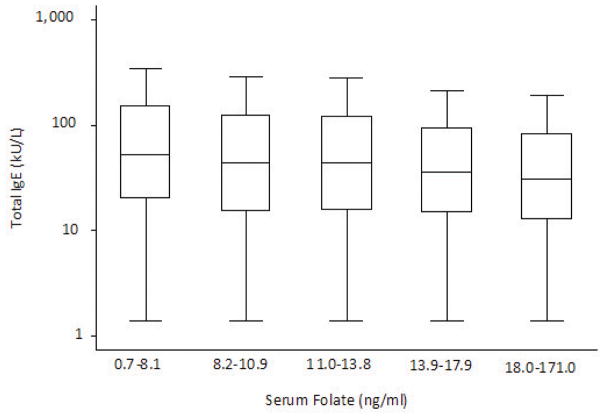
Total IgE levels decreased across quintiles of serum folate levels (p < .001 for trend) (Figure 1). After adjusting for age, sex, race/ethnicity and poverty index ratio, higher serum folate levels remained statistically significantly associated with lower total IgE levels (data not shown). Relationships between serum folate levels and high total IgE (>100 kU/L) and atopy (≥1 positive allergen-specific IgE level) were also examined. The odds of both a high total IgE level and atopy decreased across quintiles of serum folate in unadjusted analyses (p < .001 for trend) as well as multivariate analyses that adjusted for age, sex, race/ethnicity, and poverty index ratio. (Table III.)
Figure 1.
Distribution of serum total IgE levels across quintiles of serum folate. Outside values are not shown. Geometric mean total IgE levels for each quintile of serum folate are as follows: 53.6, 44.9, 45.1, 38.7, and 34.5 kU/L, respectively. p< .001 for trend.
Table III.
Relationships Between Serum Folate Levels and Atopy, Asthma, and Wheeze
| High Total IgE (>100kU/L) | Atopy† | Asthma, doctor diagnosed | Wheeze, past 12 months | |||||
|---|---|---|---|---|---|---|---|---|
| Serum Folate§ (ng/ml), Quintiles | Crude OR | Adjusted* OR (95% CI) | Crude OR | Adjusted* OR | Crude OR | Adjusted* OR | Crude OR | Adjusted* OR |
| Q1 (0.7–8.1) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 (8.2–10.9) | 0.87 (0.69–1.09) | 0.94 (0.73–1.21) | 0.87 (0.72–1.05) | 0.89 (0.71–1.12) | 1.03 (0.77–1.39) | 1.01 (0.76–1.33) | 0.83 (0.59–1.17) | 0.81 (0.57–1.15) |
| Q3 (11.0–13.8) | 0.82 (0.71–0.94) | 0.89 (0.77–1.03) | 0.74 (0.65–0.85) | 0.76 (0.65–0.88) | 0.85 (0.66–1.09) | 0.82 (0.63–1.08) | 0.82 (0.63–1.06) | 0.82 (0.64–1.04) |
| Q4 (13.9–17.9) | 0.62 (0.48–0.79) | 0.73 (0.58–0.93) | 0.72 (0.57–0.92) | 0.77 (0.59–0.99) | 0.89 (0.68–1.16) | 0.83 (0.64–1.07) | 0.67 (0.48–0.94) | 0.64 (0.46–0.89) |
| Q5 (18.0–171.0) | 0.54 (0.42–0.70) | 0.70 (0.53–0.92) | 0.60 (0.49–0.73) | 0.69 (0.57–0.85) | 0.84 (0.71–1.01) | 0.84 (0.70–1.02) | 0.65 (0.48–0.88) | 0.60 (0.44–0.82) |
Range of normal serum folate: 3–26.5ng/ml
at least one positive specific IgE test for dust mite, cat, dog, cockroach, or Alternaria
adjusted for age, sex, race/ethnicity, and poverty index ratio
To determine if increasing folate levels were associated with a decreasing number of positive specific IgE tests, an ordinal logistic regression model that accounted for the survey design of NHANES was constructed. The outcome variable was a categorical variable that indicated the number of positive specific IgE tests as follows: 0, 1, 2–3, and 4 or more positives allergen-specific IgE tests. Sixty-eight percent had no positive specific IgE tests, 9% had one, 16% had two or three, and 7% had four or more positive specific IgE tests. For every one unit increase in log10(folate), there was approximately a 50% decrease in the odds of having a greater number of positive specific IgE tests (OR[95%CI]: 0.51[0.37–0.69]). This marked protective effect of log10(folate) on number of positive specific IgE tests was also seen between log10(folate) and atopy (OR[95%CI]: 0.27[0.15–0.47]), while the effects of log10(folate) on asthma and wheeze were less strong. (Table E1, online supplement)
Higher folate levels were also associated with a lower odds of doctor-diagnosed asthma and wheeze in the previous 12 months in unadjusted analyses (p = .02 and p < .001 for trend, respectively). Higher serum folate levels remained significantly associated with a lower odds of wheeze in the previous 12 months after adjusting for age, sex, race/ethnicity, and poverty index ratio, but the association with doctor-diagnosed asthma was not statistically significant. (Table III.)
To determine if the effects of serum folate levels on atopy and high total IgE were independent of the effect of folate on wheeze, wheeze was included as a covariate in the final multivariate models. (Table IV.) In models with high total IgE or atopy as the outcomes, the addition of wheeze as a covariate did not alter the relationship between serum folate levels and these outcomes as there continued to be a decreasing risk of these outcomes across quintiles of serum folate. Similarly, in the models with wheeze as the outcome, the addition of either high total IgE or atopy as covariates did not alter the relationship between serum folate levels and wheeze. These findings suggest that the relationships between serum folate level and measures of atopic status (high total IgE, atopy) are independent of wheeze, and vice versa.
Table IV.
Multivariate Models: Wheeze, Adjusted for Atopy and High IgE and Atopy and High IgE, Adjusted for Wheeze
| Outcome | ||||
|---|---|---|---|---|
| Predictors | Atopy§ OR (95%CI) | High IgE§ (>100kU/L) | Wheeze§ OR (95%CI) | Wheeze§ OR (95%CI) |
| Folate Quintiles* (ng/ml) | ||||
| Q2(8.2–10.9) | 0.89 (0.72–1.09) | 0.91 (0.71–1.16) | 0.84 (0.61–1.17) | 0.84 (0.60–1.17) |
| Q3(11.0–13.8) | 0.75 (0.65–0.86) | 0.86 (0.75–0.99) | 0.85 (0.66–1.09) | 0.83 (0.65–1.07) |
| Q4(13.9–17.9) | 0.76 (0.59–0.98) | 0.70 (0.64–0.90) | 0.68 (0.49–0.94) | 0.69 (0.49–0.97) |
| Q5(18.0–171.0) | 0.68 (0.56–0.84) | 0.66 (0.50–0.86) | 0.64 (0.47–0.86) | 0.64 (0.47–0.87) |
| Atopy | -- | -- | 1.62 (1.28–2.06) | -- |
| High IgE | -- | -- | -- | 1.84 (1.50–2.26) |
| Wheeze | 1.61 (1.27–2.05) | 1.84 (1.50–2.26) | -- | -- |
ORs are relative to folate Q1
All models are adjusted for age, sex, race/ethnicity, and poverty income ratio
It is also possible that the effect of serum folate levels on either total IgE levels or atopic status could vary depending on wheezing status or that the effect of serum folate on wheezing could vary depending on atopic status. To address these questions, final multivariate models were stratified by wheezing status, atopic status, and total IgE status, depending on the outcome that was being evaluated. Overall, the risk of the outcomes of interest was lowest at the highest serum folate levels, regardless of atopic status, wheeze status, or total IgE status, suggesting that neither atopic status nor wheeze modified the relationships between serum folate levels and these outcomes (data not shown).
The effects of age on the relationships between serum folate levels and atopy, high IgE, and wheeze were also examined. Across all age groups, higher serum folate levels were associated with a decreased odds of high IgE levels and wheeze, with a trend towards a decreased odds of asthma. Across all age groups, except for those 60 years and older, higher serum folate levels were associated with a decreased odds of atopy. For those 60 years and older, there was no relationship between serum folate levels and atopy. (Tables E1 and E2, online repository)
To determine if income or race/ethnicity modified the relationship between serum folate levels and our outcomes of interest, we conducted analyses stratified by tertiles of poverty income ratio and by race/ethnicity (Table V.). The inverse relationship between serum folate levels and odds of high total IgE level, atopy, and wheeze persisted across all tertiles of poverty income ratio, although some of the point estimates lost statistical significance with stratification.
Table V.
Relationships between log10 (folate) and Outcomes, Stratified by Income and Race/ethnicity
| High IgE OR (95%CI) | Atopy OR (95%CI) | Wheeze OR (95%CI) | |
|---|---|---|---|
| Poverty Income Ratio§ | |||
| First Tertile (0–1.27) | 0.42(0.25–0.69) | 0.55(0.29–1.06) | 0.85(0.31–2.35) |
| Second Tertile (1.28–3.09) | 0.45(0.24–0.86) | 0.42(0.22–0.79) | 0.53(0.24–1.19) |
| Third Tertile (3.10–5.00) | 0.51(0.26–0.97) | 0.54(0.34–0.88) | 0.35(0.17–0.75) |
|
| |||
| Race/ethnicity | |||
| White | 0.48(0.29–0.78) | 0.58(0.39–0.85) | 0.33(0.20–0.54) |
| Black | 0.84(0.53–1.35) | 0.39(0.20–0.76) | 0.83(0.45–1.54) |
| Hispanic | 0.74(0.35–1.60) | 1.03(0.43–2.49) | 2.87(0.63–13.12) |
All models adjusted for age and sex
Ratio of family income to poverty threshold
When analyses were stratified by race/ethnicity, the inverse relationships between serum folate and high total IgE, atopy, and wheeze were present among Non-hispanic Whites and Blacks (Table V.). The relationships were somewhat stronger among Non-hispanic Whites than Blacks, but these differences were not statistically significant. Among Hispanics, there was no association between serum folate levels and atopy or wheeze, and some suggestion that this group may have a higher risk of wheeze with higher serum folate levels.
Discussion
In a representative US population, a higher serum folate level was associated with lower total IgE levels and a lower risk of atopy and wheeze. Although higher serum folate levels have been linked to lower risk of other inflammatory conditions such as cardiovascular disease(35–37) and rheumatoid arthritis(38), this is the first report, to our knowledge, that links higher serum folate levels to a lower risk of atopy and wheeze. These findings suggest that dietary folic acid and factors affecting its metabolism may play an important role in the development and perpetuation of allergy and asthma.
Two previous epidemiologic studies examined polymorphisms in a folate metabolism gene, methylenetetrahydrofolate reductase (MTHFR), and atopy and/or asthma. In a cross-sectional population-based Danish study(41), the TT allele of the MTHFR gene which results in impaired folate metabolism and reduces the intracellular methyl donor pool was associated with a higher prevalence of atopy.(42) Using a dietary questionnaire, that study also found that lower intake of dietary factors known to influence methyl donor metabolism (folic acid, methionine, and B vitamins) was associated with a higher risk of atopy in study participants with the TT allele of the MTHFR gene. Findings from this study are consistent conceptually with our study’s findings, as both studies suggest that a lower methyl donor pool – as evidenced by either lower serum folate levels, impaired folate metabolism, or lower dietary intake of cofactors of methyl donor metabolism – are associated with a higher risk of atopy. On the other hand, in a United Kingdom population-based cohort of mothers and children(43), there was no association between MTHFR polymorphisms and atopy or asthma. The reasons for the inconsistent findings of these two studies of MTHFR polymorphisms are unclear, but neither study examined relationships between directly measured serum folate levels, which may be a more accurate measure of the methyl donor pool, and asthma or atopy.
Although we examined serum folate levels and not dietary intake of folic acid, greater oral intake of folic acid is associated with higher serum folate levels(33;44). However, it is important to note that different dietary sources of folic acid impact serum folate levels to varying degrees. For example, supplements appear to have the greatest impact on serum folate levels followed by enriched ready-to-eat cereals and enriched cereal grain products(44). The lower limit for a “normal” serum folate level is generally considered to be in the range of 3–4.5 ng/ml(33;45), and approximately 1% of the 2005–6 NHANES population meets this criterion for a low serum folate level. There is greater debate about the definition of a high serum folate level, although a recent paper suggested that a level above 26.5 ng/ml should be considered to be high(46). In this NHANES population, approximately 5% had a serum folate level above this threshold. Thus, the findings from our study are relevant for a range of serum folate levels that would be considered to be “normal.” Whether future studies provide sufficient data to warrant a revision of the current definition of a “normal” serum folate level remains to be seen.
We found a lower prevalence of atopy in this NHANES population than reported in NHANES III(47). Skin testing was performed in NHANES III, but specific IgE testing was performed in NHANES 2005–6. In addition, in the NHANES III study, the population ranged in age from 20–59y, and in our study the age ranges from 2–85+ years. A combination of a younger study population and utilization of specific IgE testing on a fewer number of allergens is the most likely explanation for the differences in atopy prevalence between the two study populations.
In direct contrast to the two epidemiologic studies, a recently published study using a mouse model found that a diet enriched for methyl donors, including folic acid, was a risk factor, rather than a protective factor, for allergy and asthma(30). This positive association between higher dietary folate intake and enhanced allergic immune and inflammatory responses was thought to be mediated by epigenetic modification that was inherited transgenerationally since animals exposed in utero to increased methyl donors as well as their progeny who were provided normal feed exhibited increased severity of allergic airway disease. There are several possible explanations for the differences between this mouse study and the epidemiologic studies – aside from the obvious differences between mice and humans. In this study, mice were fed a cocktail of methyl donors and cofactors, including vitamin B12, choline, L-methionine, zinc, and betaine in addition to folic acid. It is also not clear how this methyl donor-enriched diet impacted serum folate levels, so it is possible that the diet resulted in supra-physiologic levels that would rarely, if ever, be observed in human populations. While our study’s findings suggest that low levels of folate may be a risk factor for allergic disease, the mouse study may indicate that very high levels of methyl donors, such as folate, could also be a risk factor for allergic disease and that optimal levels of folate (or methyl donors) fall somewhere in between.
In contrast to gestational exposure, the mice that were provided high methyl donor diets during lactation or early adulthood did not demonstrate a similar increase in disease severity, suggesting that the timing of methyl donor supplementation may play a pivotal role in determining its impact on the development of allergic disease. As the NHANES is a cross-sectional survey, our study does not address this question of the impact of in utero or early post-natal serum folate levels on the development of allergic sensitization and disease. Rather, the serum folate levels in our study reflect “current” serum folate levels, which are likely the end result of a combination of current or recent dietary folic acid intake and underlying polymorphisms in genes that are involved in folate metabolism.
Although our study does not address the effects of in utero folate exposure on asthma and atopy, one recent prospective birth cohort study examined the impact of in utero exposure to folic acid supplementation on risk of respiratory outcomes in the first 18 months of life(48). The investigators found a very small increase in the risk of lower respiratory tract infection and wheeze associated with folic acid supplementation in the first trimester only and atopic outcomes were not evaluated. More prospective studies are clearly needed and current birth cohort studies are well-positioned to evaluate the impact of in utero or very early life serum folate levels on subsequent risk of allergic sensitization and disease.
A number of epidemiologic studies have described an inverse relationship between folate levels and inflammatory states(36;37), in keeping with our study’s findings. Although evidence exists that folic acid supplementation may change serum cytokine levels via non-epigenetic mechanisms(49), changes in DNA methylation have been associated with immune modulation, including the aforementioned mouse study. The precise role of epigenetics in asthma and atopy are poorly understood, but recent studies suggest potential effects on both immunity(50–53) and inflammation(54;55). In fact, we also found that the relationship between serum folate levels and measures of atopy were independent of wheeze, and vice versa. These findings suggest that serum folate levels may indeed have dual and independent functions, potentially reducing the risk of atopy through immunomodulation and also potentially reducing the risk of wheeze through modulation of inflammation.
This study also confirmed previous observations of an association between Non-hispanic Black or Hispanic race/ethnicity and lower serum folate levels(33). We also found that the relationships between serum folate levels and risk of high total IgE, atopy, and wheeze were similar across all income levels and among Non-hispanic Whites and Blacks, suggesting that low serum folate levels may contribute to risk of high total IgE, atopy, and wheeze in Black and Non-hispanic White populations of all income levels. However, relationships between folate and these outcomes among the Hispanic population are less clear, with folate having little impact on risk of atopy and wheeze, perhaps even increasing risk of wheeze in this racial/ethnic group. The reasons behind these differences across racial/ethnic groups are unclear and could be related to dietary, socio-cultural, or genetic factors.
Although the inverse association between serum folate levels and atopy and wheeze in this NHANES population is intriguing, the cross-sectional study design limits our ability to conclude that there is a causal relationship between serum folate levels and atopy and wheeze. Because the temporal relationships between serum folate levels and the development of atopy or wheeze are unknown, it is not clear whether high serum folate levels might protect against the development of allergic sensitization or elevated total IgE and/or protect against wheeze in a previously sensitized individual. Additional prospective studies will be required to lend insight into the potential role of folic acid supplementation in the primary prevention and/or treatment of allergic diseases. Ultimately, clinical trials will be needed to draw any firm conclusions about the preventive or therapeutic potential for folic acid in allergic diseases as folic acid supplementation in patients with cardiovascular disease may be related to worse outcomes in some cases(56).
In summary, higher serum folate levels are associated with lower total IgE levels, and a lower risk of allergic sensitization and wheeze. Future studies are needed to define the temporal relationships among serum folate levels and allergy and asthma and to determine whether these associations, if causal, are mediated by epigenetic changes or by other mechanisms.
Acknowledgments
Supported by grants from the NIAID (R01AI070630) and the NIEHS (DISCOVER Award: 5P50ES015903).
Abbreviations
- CI
confidence interval
- IRB
Institutional Review Board
- MTHFR
methylenetetrahydrofolate reductase
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
- Q
quintile
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323(8):502–7. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 2.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336(19):1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 3.Matsui EC, Eggleston PA, Buckley TJ, Krishnan JA, Breysse P, Rand C, et al. Household mouse allergen exposure and asthma morbidity in inner-city pre-school children. Ann Allergy Asthma Immunol. 2006 doi: 10.1016/S1081-1206(10)60943-X. In Press. Ref Type: Generic. [DOI] [PubMed] [Google Scholar]
- 4.Lewis SA, Weiss ST, Platts-Mills TA, Burge H, Gold DR. The role of indoor allergen sensitization and exposure in causing morbidity in women with asthma. Am J Respir Crit Care Med. 2002;165(7):961–6. doi: 10.1164/ajrccm.165.7.2103044. [DOI] [PubMed] [Google Scholar]
- 5.Squillace SP, Sporik RB, Rakes G, Couture N, Lawrence A, Merriam S, et al. Sensitization to dust mites as a dominant risk factor for asthma among adolescents living in central Virginia. Multiple regression analysis of a population-based study. Am J Respir Crit Care Med. 1997;156(6):1760–4. doi: 10.1164/ajrccm.156.6.9704026. [DOI] [PubMed] [Google Scholar]
- 6.Perzanowski MS, Ronmark E, Platts-Mills TA, Lundback B. Effect of cat and dog ownership on sensitization and development of asthma among preteenage children. Am J Respir Crit Care Med. 2002;166(5):696–702. doi: 10.1164/rccm.2201035. [DOI] [PubMed] [Google Scholar]
- 7.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, III, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 8.Kattan M, Gergen PJ, Eggleston P, Visness CM, Mitchell HE. Health effects of indoor nitrogen dioxide and passive smoking on urban asthmatic children. J Allergy Clin Immunol. 2007;120(3):618–24. doi: 10.1016/j.jaci.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Eggleston PA, Butz A, Rand C, Curtin-Brosnan J, Kanchanaraksa S, Swartz L, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol. 2005;95(6):518–24. doi: 10.1016/S1081-1206(10)61012-5. [DOI] [PubMed] [Google Scholar]
- 10.Moore K, Neugebauer R, Lurmann F, Hall J, Brajer V, Alcorn S, et al. Ambient ozone concentrations cause increased hospitalizations for asthma in children: an 18-year study in Southern California. Environ Health Perspect. 2008;116(8):1063–70. doi: 10.1289/ehp.10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortimer K, Neugebauer R, Lurmann F, Alcorn S, Balmes J, Tager I. Air pollution and pulmonary function in asthmatic children: effects of prenatal and lifetime exposures. Epidemiology. 2008;19(4):550–7. doi: 10.1097/EDE.0b013e31816a9dcb. [DOI] [PubMed] [Google Scholar]
- 12.Hansel NN, Breysse PN, McCormack MC, Matsui EC, Curtin-Brosnan J, Williams DL, et al. A longitudinal study of indoor nitrogen dioxide levels and respiratory symptoms in inner-city children with asthma. Environ Health Perspect. 2008;116(10):1428–32. doi: 10.1289/ehp.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ, Jr, Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med. 2005;172(11):1371–7. doi: 10.1164/rccm.200505-758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perzanowski MS, Miller RL, Thorne PS, Barr RG, Divjan A, Sheares BJ, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol. 2006;117(5):1082–9. doi: 10.1016/j.jaci.2005.12.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347(12):869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 16.Spycher BD, Silverman M, Kuehni CE. Timing of routine vaccinations and the risk of childhood asthma. J Allergy Clin Immunol. 2008;122(3):656–8. doi: 10.1016/j.jaci.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 17.McDonald KL, Huq SI, Lix LM, Becker AB, Kozyrskyj AL. Delay in diphtheria, pertussis, tetanus vaccination is associated with a reduced risk of childhood asthma. J Allergy Clin Immunol. 2008;121(3):626–31. doi: 10.1016/j.jaci.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Zhou Z, An J, Zhang C, Sun B, Zhong N. Absence of relationships between tuberculin responses and development of adult asthma with rhinitis and atopy. Chest. 2008;133(1):100–6. doi: 10.1378/chest.07-1467. [DOI] [PubMed] [Google Scholar]
- 19.Linehan MF, Frank TL, Hazell ML, Francis HC, Morris JA, Baxter DN, et al. Is the prevalence of wheeze in children altered by neonatal BCG vaccination? J Allergy Clin Immunol. 2007;119(5):1079–85. doi: 10.1016/j.jaci.2006.12.672. [DOI] [PubMed] [Google Scholar]
- 20.Castro-Rodriguez JA, Garcia-Marcos L, fonseda Rojas JD, Valverde-Molina J, Sanchez-Solis M. Mediterranean diet as a protective factor for wheezing in preschool children. J Pediatr. 2008;152(6):823–8. 828. doi: 10.1016/j.jpeds.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Willers SM, Wijga AH, Brunekreef B, Kerkhof M, Gerritsen J, Hoekstra MO, et al. Maternal food consumption during pregnancy and the longitudinal development of childhood asthma. Am J Respir Crit Care Med. 2008;178(2):124–31. doi: 10.1164/rccm.200710-1544OC. [DOI] [PubMed] [Google Scholar]
- 22.Burns JS, Dockery DW, Neas LM, Schwartz J, Coull BA, Raizenne M, et al. Low dietary nutrient intakes and respiratory health in adolescents. Chest. 2007;132(1):238–45. doi: 10.1378/chest.07-0038. [DOI] [PubMed] [Google Scholar]
- 23.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85(3):788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonardi-Bee J, Pritchard D, Britton J. Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am J Respir Crit Care Med. 2006;174(5):514–23. doi: 10.1164/rccm.200603-331OC. [DOI] [PubMed] [Google Scholar]
- 25.Dagoye D, Bekele Z, Woldemichael K, Nida H, Yimam M, Hall A, et al. Wheezing, allergy, and parasite infection in children in urban and rural Ethiopia. Am J Respir Crit Care Med. 2003;167(10):1369–73. doi: 10.1164/rccm.200210-1204OC. [DOI] [PubMed] [Google Scholar]
- 26.Scrivener S, Yemaneberhan H, Zebenigus M, Tilahun D, Girma S, Ali S, et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. Lancet. 2001;358(9292):1493–9. doi: 10.1016/S0140-6736(01)06579-5. [DOI] [PubMed] [Google Scholar]
- 27.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston NW, Johnston SL, Norman GR, Dai J, Sears MR. The September epidemic of asthma hospitalization: school children as disease vectors. J Allergy Clin Immunol. 2006;117(3):557–62. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 29.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119(5):1105–10. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118(10):3462–9. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Linneberg A, Gislum M, Johansen N, Husemoen LL, Jorgensen T. Temporal trends of aeroallergen sensitization over twenty-five years. Clin Exp Allergy. 2007;37(8):1137–42. doi: 10.1111/j.1365-2222.2007.02760.x. [DOI] [PubMed] [Google Scholar]
- 32.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma--United States, 1980–1999. MMWR Surveill Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 33.McDowell MA, Lacher DA, Pfeiffer CM, Mulinare J, Picciano MF, Rader JI, et al. Blood folate levels: The latest NHANES results. NCHS data briefs no. 6. 2008. Hyattsville, MD: National Center for Health Statistics; Ref Type: Generic. [PubMed] [Google Scholar]
- 34.Moens AL, Claeys MJ, Wuyts FL, Goovaerts I, Van HE, Wendelen LC, et al. Effect of folic acid on endothelial function following acute myocardial infarction. Am J Cardiol. 2007;99(4):476–81. doi: 10.1016/j.amjcard.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 35.Forman JP, Rimm EB, Stampfer MJ, Curhan GC. Folate intake and the risk of incident hypertension among US women. JAMA. 2005;293(3):320–9. doi: 10.1001/jama.293.3.320. [DOI] [PubMed] [Google Scholar]
- 36.Connor SL, Ojeda LS, Sexton G, Weidner G, Connor WE. Diets lower in folic acid and carotenoids are associated with the coronary disease epidemic in Central and Eastern Europe. J Am Diet Assoc. 2004;104(12):1793–9. doi: 10.1016/j.jada.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 37.Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288(16):2023–31. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- 38.Schroecksnadel K, Frick B, Kaser S, Wirleitner B, Ledochowski M, Mur E, et al. Moderate hyperhomocysteinaemia and immune activation in patients with rheumatoid arthritis. Clin Chim Acta. 2003;338(1–2):157–64. doi: 10.1016/j.cccn.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Kurzawski M, Pawlik A, Safranow K, Herczynska M, Drozdzik M. 677C>T and 1298A>C MTHFR polymorphisms affect methotrexate treatment outcome in rheumatoid arthritis. Pharmacogenomics. 2007;8(11):1551–9. doi: 10.2217/14622416.8.11.1551. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. www.cdc.gov/nchs/about/major/nhanes/nhanes2005-6/nhanes05_06.htm. Ref Type: Generic. [Google Scholar]
- 41.Husemoen LL, Toft U, Fenger M, Jorgensen T, Johansen N, Linneberg A. The association between atopy and factors influencing folate metabolism: is low folate status causally related to the development of atopy? Int J Epidemiol. 2006;35(4):954–61. doi: 10.1093/ije/dyl094. [DOI] [PubMed] [Google Scholar]
- 42.Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A. 2002;99(8):5606–11. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granell R, Heron J, Lewis S, Davey SG, Sterne JA, Henderson J. The association between mother and child MTHFR C677T polymorphisms, dietary folate intake and childhood atopy in a population-based, longitudinal birth cohort. Clin Exp Allergy. 2008;38(2):320–8. doi: 10.1111/j.1365-2222.2007.02902.x. [DOI] [PubMed] [Google Scholar]
- 44.Yeung L, Yang Q, Berry RJ. Contributions of total daily intake of folic acid to serum folate concentrations. JAMA. 2008;300(21):2486–7. doi: 10.1001/jama.2008.742. [DOI] [PubMed] [Google Scholar]
- 45.Selhub J, Jacques PF, Dallal G, Choumenkovitch S, Rogers G. The use of blood concentrations of vitamins and their respective functional indicators to define folate and vitamin B12 status. Food Nutr Bull. 2008;29(2 Suppl):S67–S73. doi: 10.1177/15648265080292S110. [DOI] [PubMed] [Google Scholar]
- 46.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85(1):193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116(2):377–83. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 48.Haberg SE, London SD, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child. 2008 doi: 10.1136/adc.2008.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solini A, Santini E, Ferrannini E. Effect of short-term folic acid supplementation on insulin sensitivity and inflammatory markers in overweight subjects. Int J Obes(Lond) 2006;30(8):1197–202. doi: 10.1038/sj.ijo.0803265. [DOI] [PubMed] [Google Scholar]
- 50.Bruniquel D, Schwartz RH. Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat Immunol. 2003;4(3):235–40. doi: 10.1038/ni887. [DOI] [PubMed] [Google Scholar]
- 51.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6(8):597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 52.Oelke K, Lu Q, Richardson D, Wu A, Deng C, Hanash S, et al. Overexpression of CD70 and overstimulation of IgG synthesis by lupus T cells and T cells treated with DNA methylation inhibitors. Arthritis Rheum. 2004;50(6):1850–60. doi: 10.1002/art.20255. [DOI] [PubMed] [Google Scholar]
- 53.Lu Q, Wu A, Richardson BC. Demethylation of the same promoter sequence increases CD70 expression in lupus T cells and T cells treated with lupus-inducing drugs. J Immunol. 2005;174(10):6212–9. doi: 10.4049/jimmunol.174.10.6212. [DOI] [PubMed] [Google Scholar]
- 54.Wang R, An J, Ji F, Jiao H, Sun H, Zhou D. Hypermethylation of the Keap1 gene in human lung cancer cell lines and lung cancer tissues. Biochem Biophys Res Commun. 2008;373(1):151–4. doi: 10.1016/j.bbrc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Lee KS, Kim SR, Park HS, Park SJ, Min KH, Lee KY, et al. A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-kappaB and hypoxia-inducible factor-1alpha. Exp Mol Med. 2007;39(6):756–68. doi: 10.1038/emm.2007.82. [DOI] [PubMed] [Google Scholar]
- 56.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]