Abstract
Lipid body accumulation within leukocytes is a common feature in both clinical and experimental infectious, neoplasic and other inflammatory conditions. Here, we will review the contemporary evidence related to the biogenesis and structure of leukocyte lipid bodies (also known as lipid droplets) as inflammatory organelles. Studies of leukocyte lipid bodies are providing functional, ultrastructural and protein compositional evidences that lipid bodies are not solely storage depots of neutral lipid. Over the past years substantial progresses have been made to demonstrate that lipid body biogenesis is a highly regulated process, that culminate in the compartmentalization of a specific set of proteins and lipids, that place leukocyte lipid bodies as inducible cytoplasmic organelles with roles in cell signaling and activation, regulation of lipid metabolism, membrane trafficking and control of the synthesis and secretion of inflammatory mediators. Pertinent to the roles of lipid bodies in inflammation and cell signaling, enzymes involved in eicosanoid synthesis are localized at lipid bodies and lipid bodies are sites for eicosanoid generation. Collectively, lipid bodies in leukocytes are emerging as critical regulators of different inflammatory diseases, key markers of leukocyte activation and attractive targets for novel anti-inflammatory therapies.
Keywords: Lipid droplets, inflammation, foam cell, eicosanoids, leukocytes, eosinophils, neutrophils
1.0 Introduction
Lipid bodies, also named lipid droplets or adiposomes, are lipid-rich organelles present in virtually all organisms, including plants, yeast, prokaryotes and both non-mammalian and mammalian animal cells. Although resting mammalian leukocytes contain few lipid bodies, lipid bodies characteristically increase in numbers and prominence in cells associated with diverse inflammatory responses, including leukocytes (e.g., macrophages, neutrophils, and eosinophils) and other cell types (e.g., endothelial cells). Lipid body accumulation within leukocytes is observed in both clinical and experimental infectious, neoplasic and other inflammatory conditions, including in atherosclerosis [1, 2], bacterial sepsis [3, 4], acute respiratory distress syndrome [5, 6], allergic lung inflammation [7–9], arthritis [10–13], and in mycobacterial infections [14–16]. Lipid bodies are bound not by a classic bilayer membrane but rather by an outer monolayer of phospholipids, which at least in some cells may have a unique fatty acid composition [17, 18]. The internal core of lipid bodies is rich in neutral lipids; and it is likely that more complex membranous domains, often obscured by overlying neutral lipids, are present within lipid bodies. Indeed, studies of leukocyte lipid bodies are providing functional, ultrastructural and protein compositional evidences that lipid bodies are not solely “bags” of neutral lipid. The presence of membranous domains within leukocyte lipid bodies is also consonant with findings pertinent to lipid bodies in adipocytes, steroidogenic cells and other cells, as we consider below. Although in the past the presence of lipid bodies in cells has been largely associated with neutral lipid storage and transport, it has become clear that lipid bodies are highly regulated, dynamic and functionally active organelles.
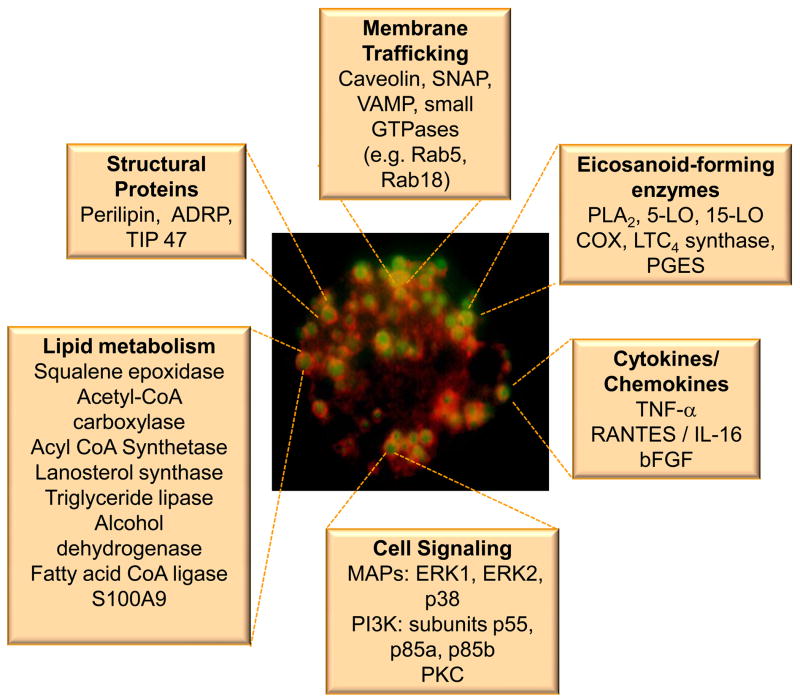
Lipid bodies in leukocytes and other cells compartmentalize a diverse set of proteins (Figure 1). The major structural proteins present at the surface of lipid bodies are the proteins from the PAT family [19], including perilipin [20], adipose differentiation related protein (ADRP) [21, 22], and TIP 47 (tail-interacting protein of 47 kDa) [23]. These proteins have been implicated in lipid body assembly and biogenesis [21, 22, 24, 25]. By using techniques of protein identification in subcellular lipid body-enriched fractionation combined with immunodetection of proteins by EM or light microscopy it has been shown that lipid bodies compartmentalize enzymes involved in the biosynthesis, transport and catabolism of lipids [1] [26–31], caveolin, proteins of Rab family and proteins involved in vesicular transport includingVAT-1, SNAP and VAMP [29–37], eicosanoid-forming enzymes [3, 38–43], protein kinases as phosphatidylinositide 3 kinase (PI3 kinase), mitogen-activated protein (MAP) kinases, and protein kinase C (PKC) [26, 44, 45]. The regulated formation of lipid bodies, their protein and lipid content, and their association with other intracellular organelles have established leukocyte lipid bodies as specialized, inducible cytoplasmic domains that function as organelles with roles in cell signaling and activation, regulation of lipid metabolism, membrane trafficking and control of the synthesis and secretion of inflammatory mediators. Here, we will review the contemporary evidence related to the biogenesis and structure of leukocyte lipid bodies as inflammatory organelles. Collectively, lipid bodies in leukocytes are critical regulators of different inflammatory diseases, key markers of leukocyte activation and attractive targets for novel anti-inflammatory therapies.
Figure 1.
Lipid body-associated proteins
2.0 Leukocyte lipid body biogenesis
Different from neutral lipid storing cells, leukocytes contain few lipid bodies under resting conditions (e.g., human blood neutrophils and eosinophils contain ~ 1 and ~ 5 lipid bodies/cell [46, 47]. Of note, lipid bodies in leukocytes have often not been recognized since lipid bodies are dissolved by common alcohol-based hematologic stains. However, rapid and well-regulated lipid body biogenesis is triggered upon leukocyte activation by different stimuli and pathological conditions including obesity-induced inflammation, ox-LDL- and LPS-induced inflammation or bacterial infection. Thus increased numbers of cytoplasmic lipid bodies within leukocytes are often associated with infectious, atherosclerotic and other inflammatory conditions.
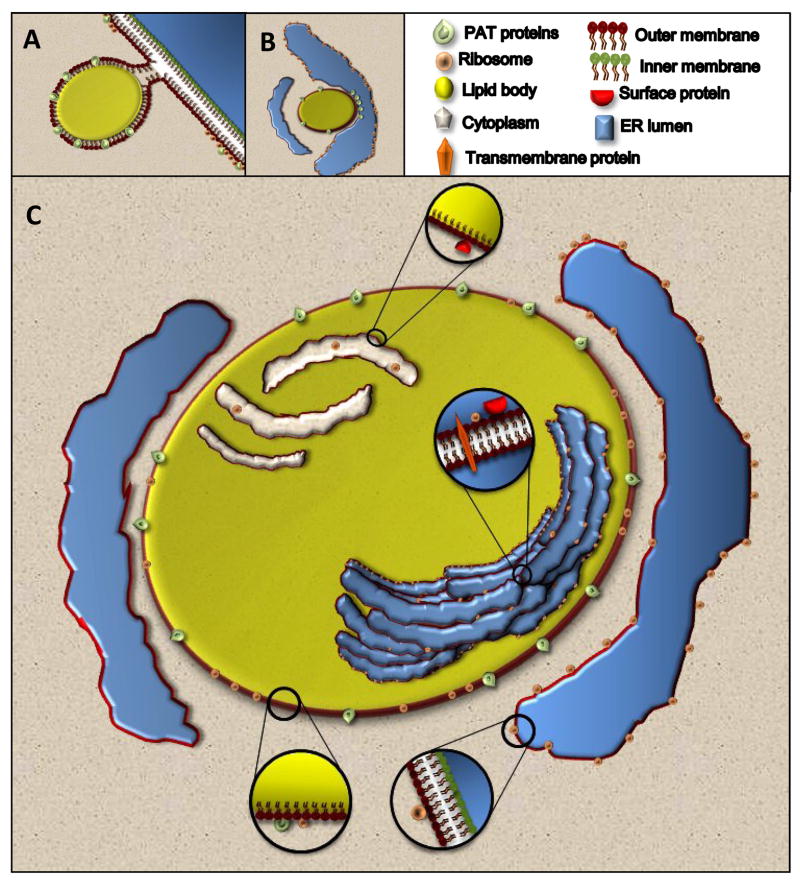
Hypothesized mechanisms for the biogenesis of lipid bodies in varied cells types recognize that lipid bodies are intimately related with and likely derived from the endoplasmic reticulum (ER)(Figure 2). The proposed models of the biogenesis of lipid bodies involve ER transfer of lipids and proteins; however the precise mechanisms involved remain to be ascertained. Three main models have been proposed to explain the formation of lipid bodies and their interaction with the ER. One of the first models proposed was a “budding model” where enzymes involved in lipid metabolism accumulate in specific domains of the ER, thus favoring neutral lipid synthesis in these regions forming a hydrophobic neutral lipid mass between the two leaflets of the ER bilayer (Figure 2 A). After reaching a certain size, nascent lipid bodies loaded with proteins lacking trans-membrane spanning domains bud off the ER into the cytoplasm that ends up surrounded by a half-unit membrane of phospholipids directly derived from the cytoplasmic leaflet of the ER [48–51].
Figure 2. Biogenesis and structure of lipid bodies.
Lipid body biogenesis involves ER-dependent mechanisms of lipid and protein transference. Three main models have been proposed: (A) formation of a neutral lipid mass synthesized by ER enzymes that is deposited in a hydrophobic domain between the two leaflets of the ER membrane; followed by budding off of this lipid structure into the cytoplasm that ends up surrounded by a half-unit membrane of phospholipids directly derived from the cytoplasmic leaflet of the ER; (B) formation of lipid bodies at ADRP-enriched clusters in the cytoplasmic leaflet of ER with the transference of lipids from ER to nascent lipid bodies within ER cups, rather than between ER leaflets; (C) formation of lipid bodies by incorporation of multiple loops of ER membranous domains, with accumulation of neutral lipids among the membranes within lipid bodies. As detailed in C, ultrastructural and proteomic studies of lipid bodies isolated from leukocytes revealed internal membranes within these leukocyte lipid bodies and identified diverse proteins, about half of which had predicted membrane insertions [31]. In addition, ribosome and ribosomal-associated proteins have also been identified within lipid bodies.
An alternative second model has been proposed. Based on freeze-fracture immunogold electron microscopy observations, Robenek and colleagues have recently suggested that formation of lipid bodies occurs preferentially alongside, not within, the ER; and an “egg cup model” of lipid body formation was proposed (Figure 2 B). According to this model, lipid bodies would form within ER cups particularly enriched in ADRP clusters in the cytoplasmic leaflet of ER, with ADRP clusters functioning to transfer lipids from ER to nascent lipid bodies [52].
Based both on studies of leukocyte lipid bodies and on findings related to lipid bodies in other cell types, we have proposed a novel “enfolding model” of lipid body biogenesis that accommodates membrane-associated and transmembrane spanning proteins within lipid body cores by incorporation of multiple loops of ER membranous domains [31] (Figure 2 C).
Just as PAT proteins can localize at the peripheral delimiting membrane of lipid bodies, an initially attractive hypothetical mechanism for cytosolic proteins (e.g., 5-lipoxygenase (5-LO), cytosolic phospholipase A2 (cPLA2) involved in eicosanoid metabolism to function at lipid bodies was to have these membrane-active proteins “translocate” to the peripheral membrane of lipid bodies. Of note, however, immunogold electron microscopic localization of 5-LO revealed 5-LO to be distributed throughout lipid bodies, which were shown to have a “honeycomb” internal structure [39], never suggesting a ”translocated” membrane association solely at the peripheral delimiting monolayer membrane of lipid bodies. The cyclooxygenase (COX, prostaglandin H synthase) enzymes are integral membrane proteins [53] and have been localized at lipid bodies in different cells and by the use of different techniques including EM immunogold, immunolocalization in intact cells and western blotting from subcellular fractions [3, 38–40, 54, 55] [41–43]. Similar to the described for 5-LO, EM immunogold analysis of COX enzymes localized these proteins throughout lipid bodies in diverse cells and not restricted to the periphery of the lipid body. Analogously, the transmembrane spanning enzyme, leukotriene C4 (LTC4) synthase, was localized at lipid bodies [39]. As described in detailed below, eicosanoid-forming enzymes localized at lipid bodies retain their functional enzymatic capacity since newly generated arachidonate-derived eicosanoids are formed at lipid bodies in inflammatory cells [8, 15, 56–58]. Collectively, these findings indicated that since membrane-associated enzymes localized and functioned at leukocyte lipid bodies there would likely be internal membranes present within these lipid bodies.
Proteomic studies of lipid bodies isolated from the U937 macrophage cell line identified diverse proteins, about half of which had predicted membrane insertions [31], combined with ultrastructural observations of membranous structures within lipid bodies of U937 macrophage cell line and human eosinophils, strongly suggested the presence of internal membranes within leukocyte lipid bodies. Accordingly, lamellar concentrically arranged membranes have been previously described to occur on the margins of lipid droplets that penetrated the matrix of the droplets in acetylated LDL-stimulated macrophages [1]. The presence of membranotubular structures have also been recognized in adipocyte lipid bodies recently by embedment-free electron microscopy [59]. The existence of membranous structures within lipid bodies of non-leukocytic cells could also explain how stanniocalcin and its membrane receptor are present at lipid bodies of ovarian steroidogenic cells and adipocytes [60]. Membranes within lipid bodies would account for the freeze-fracture immunogold electron microscopic findings that revealed caveolin-1, an integral membrane protein, as well as TIP47 and ADRP were localized to freeze-fractured lamellae not only at the periphery of lipid bodies but also pervading lipid body cores [61].
Although stores of neutral lipids that accumulate at lipid bodies can obscure the internal ER-derived components of lipid bodies, ribosomal structures and ribosomal associated proteins have been described in leukocyte lipid bodies [31] [62, 63]. Moreover, protein analyses of Drosophila, yeast and hepatoma lipid bodies also identified ribosomal and RNA-interacting proteins [64–68]. Future studies will be necessary to confirm and characterize the presence of membranous structures in the lipid body core.
The triggering process and detailed molecular mechanisms involved in lipid body biogenesis have been intensely investigated, demonstrating that leukocyte lipid body biogenesis is a highly regulated phenomena that has been characterized as a cell and stimuli dependent event [69] (Table 1).
Table 1.
Leukocyte lipid body formation is cell- and stimuli-dependent
| Stimuli | Cell Type | References |
|---|---|---|
| Fatty Acids | ||
| Unsaturated fatty acids, but not saturated fatty acids | Neutrophils, eosinophils, monocyte/macrophages | 13, 71,131 |
| Lipoproteins | ||
| Ac-LDL/E-LDL/AGE-LDL/Ox-LDL, but not native LDL | Macrophages, but not neutrophils | 1, 2, 73, 79–82, 102 |
| Pathogen-derived molecules | ||
| LPS/LAM | Macrophages, neutrophils | 3, 15 |
| Lipid mediators | ||
| PAF, but not lysoPAF | Neutrophils, eosinophils, macrophages | 39, 40, 70, 73 |
| 5-HETE | Neutrophils | 70 |
| PGD2 | Eosinophils but not macrophages | 9 |
| Hormones | ||
| Leptin, resistin | Macrophages | 92, 93 |
| Cytokines/Growth factors | ||
| IL-5, GM-csf, IL-16 | Eosinophils | 40, 75, 76 |
| Chemokines | ||
| CCL5/CCL11/CCL24/CCL26 | Eosinophils | 56, 74 |
| CCL2 | Macrophages | 58 |
Upon stimulation the genesis of new cytoplasmic lipid bodies can be induced within neutrophils, eosinophils, and monocytes/macrophages, as well as in other inflammation-related cell types including endothelial and epithelial cells. Studies investigating the mechanisms of lipid body formation in leukocytes, using physiological amounts of fatty acids or other relevant inflammatory stimuli, demonstrated that a complex biogenic process rather than unregulated lipid incorporation takes place in leukocytes to form new lipid bodies. Among the observation that indicate the existence of regulated production of lipid bodies in leukocyte are: (i) while cis-unsaturated fatty acids are potent inducers of lipid bodies, saturated fatty acids do not elicit lipid body assembly [70, 71]; (ii) non-esterifiable cis-fatty acids such as the arachidonate analog arachidonyl trifluoromethyl ketone are able to induce formation of new lipid bodies [72]; and (iii) stimulation with cytokines/chemokines and hormones induces receptor-mediated lipid body biogenesis not only in vivo but even in vitro in the absence of exogenous lipids (Table 1).
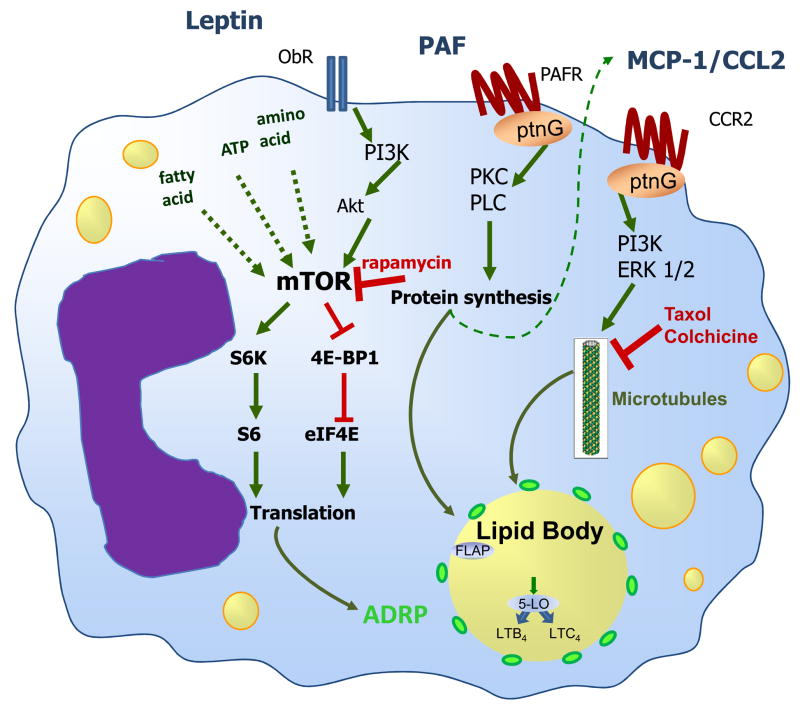
Attempts to characterize the signaling pathways committed to lipid body biogenesis in leukocytes revealed that different pathways in a stimulus-dependent fashion are activated to trigger leukocyte lipid body biogenesis. For instance, platelet-activating factor (PAF) and PAF-like molecules, but not lyso-PAF, acting via its G-protein-linked receptor induces lipid body formation via downstream signaling that requires 5-LO, PKC and phospholipase C (PLC) activation [39, 40, 70, 73], in a mechanism that may involve an autocrine loop on CCL2 generation (Figure 3). Prostaglandin (PG)D2 – another potent leukocyte chemoattractant acting through a G-protein-linked receptor – also directly induces biogenesis of lipid bodies in eosinophils, but not in macrophages, even though both cell types express functional PGD2 receptors demonstrating stimulus/signaling specificity according to the cell type [9]. Of note, other G-protein-coupled receptor agonists, including IL-8, C5a and LTB4, did not induce leukocyte lipid body formation demonstrating the requirement of specific intracellular signaling mechanisms in the process of lipid body biogenesis [70].
Figure 3. Schematic representation of the molecular mechanisms regulating leukocyte lipid body biogenesis and function.
Lipid body formation in leukocytes is a highly regulated process that involves receptor-mediated signaling. Lipid body formed during inflammatory stimulation culminates in the compartmentalization of lipids and proteins and function as specialized domains involved in eicosanoid synthesis.
Leukocytes incubated with cytokines and chemokines even in the absence of exogenous lipids rapidly form new cytoplasmic lipid bodies by receptor-mediated processes [40, 56, 58, 74, 75]. In human eosinophils, IL-5 alone or combined with GM-CSF, as well as immobilized IgG lead to significant increases in lipid body numbers [40, 75]. In addition, RANTES (CCL5) and eotaxin (CCL11), eotaxin 2 (CCL24) and eotaxin 3 (CCL26) acting via their CCR3 receptors, initiate intracellular signaling in eosinophils and basophils, but not neutrophils, to rapidly form lipid bodies [8, 56, 76]. CCR3-driven lipid body biogenesis, in contrast to PAF’s effect, was mediated by activation of MAP kinases, PI3K and tyrosine kinases, but not PKC or PLC [56]. Likewise, in vivo administration of RANTES, eotaxin or PGD2 also induced significant influx of eosinophils loaded with lipid bodies through their specific receptors [8, 9]. Eosinophils attracted to the site of allergic inflammatory reaction exhibited increased numbers of lipid bodies [8, 9]. Allergic inflammation induces in vivo biogenesis of lipid bodies within recruited eosinophils in a selective manner, since it failed to activate resident mononuclear cells to form lipid bodies. Neutralizing antibodies to eotaxin, RANTES or the eotaxin and RANTES receptor, CCR3, as well as a specific inhibitor of PGD2 synthesis inhibited lipid body formation within recruited eosinophils during experimental allergic inflammatory reactions [8, 9]. Therefore, allergic inflammation triggers in vivo formation of new lipid bodies within infiltrating eosinophils, a phenomenon largely mediated by a cross-talk of eotaxin/RANTES acting via CCR3 receptors and PGD2 acting via a receptor that has yet to be identified. A role for eotaxin/CCR3 in lipid body biogenesis was also demonstrated to occur in infection-driven lipid body formation in eosinophils, but not in macrophages; a mechanism that was largely dependent on TLR2-dependent macrophage-derived eotaxin-mediated CCR3 activation [77].
Accumulation of lipid body enriched macrophage foam cells in atherosclerotic blood vessel intima is a critical component of atherogenesis. The formation of foam cells involves complex and multi-step mechanisms that depend on different signaling pathways regulating lipid influx, metabolization, storage and mobilization [2, 78, 79]. Uncontrolled modified LDL uptake by macrophages through scavenger receptors causes triglyceride and cholesterol loading, followed by cholesterol esterification mediated by acyl coenzyme A:acylcholesterol transferase and storage of cholesteryl esters (CEs) in cytoplasmic lipid bodies [2, 78, 79]. Different modifications of LDL, including enzymatic modification (E-LDL), acetylation (Ac-LDL), oxidation (Ox-LDL), and glycation (AGE-LDL) have been associated to foam cell formation in atherosclerosis [2, 79–82]. Recognition and activation of scavenger receptors, mostly CD36, by modified LDL play major roles in lipid accumulation in macrophages [83–87]. In addition, different lipid-derived molecules generated in the process of LDL oxidation are involved in lipid body formation including PAF-like molecules [73, 88], sterol ester [89], oxysterols [90], 1-palmitoyl-2-(5′-oxovaleroyl)-sn-glycero-3-phosphocholine [91], azelaoyl-phosphatidylcholine [73].
Adipocytokines including leptin and resistin were also shown to modulate lipid body formation in macrophages and may participate in the mechanisms of foam cell formation [92–94]. In addition, current studies have demonstrated that monocyte chemoattractant protein (MCP-1/CCL2), a key endogenous mediator involved in the pathogenesis of macrophage-driven inflammation such as atherosclerosis and sepsis, is centrally involved in the regulation of macrophage lipid body biogenesis in oxidized LDL-and LPS-induced inflammation as well as in experimental sepsis [58, 88], Silva, submitted). MCP-1-driven lipid body accumulation is a highly regulated phenomenon, requisitely dependent of MCP-1 receptor, CCR2, and downstream signaling through MAP-and PI3-kinases [58]. Moreover, MCP-1-elicited lipid body assembly and protein compartmentalization was demonstrated to depend on a functional microtubule network. Accordingly, lipid bodies are enmeshed in a cytoskeleton network in several cell types [31, 95–97], and lipid body-cytoskeleton interaction were shown also to have roles in lipid body motility [36], rapid relocation upon cell activation with chemotactic agents [56], and lipid body fusion and growth [98] (Figure 3).
Whether inflammation-driven lipid body formation depends on either a direct effect of endogenous mediators present at the site of inflammatory reaction or is triggered by cellular migration and/or phagocytosis dependent mechanisms at the inflammatory site has been investigated. Attempts to answer the role of cell migration – a multi-step process that involves rolling, adhesion, transmigration and chemotaxis – to lipid body biogenesis indicate that migration may modulate, but is not requisitely involved in leukocyte lipid body formation during inflammation. Increased lipid body biogenesis may be dissociated from cell recruitment as (i) resident macrophages have significantly increased lipid body numbers within 6 hours of LPS stimulation when there is no concurrent increase in the number of macrophages at the inflammatory site [3]; (ii) while in vivo infection with Mycobacterium bovis BCG induced both migration and lipid body formation within recruited leukocytes, infection with non-pathogenic Mycobacterium smegmatis although inducing an intense leukocyte recruitment, failed to trigger the process of lipid body biogenesis [15, 69]; and (iii) lipid body numbers are drastically increased in blood leukocytes from septic patients or rats with Chagas disease when compared to blood leukocytes from healthy subjects [3, 99], indicating that leukocytes that did not undergo migration can also form new lipid bodies in vivo during a systemic inflammatory disease. The lack of correlation between phagocytosis and lipid body formation was also experimentally demonstrated. By comparisons of percentages of infected cells and of cells with increased lipid body numbers after BCG infection, it was demonstrated that more than 95% of cells had highly increased lipid body numbers, while less than 30% of the cells were infected. In addition, macrophage uptake of latex beads, Bacillus subtillis, and nonpathogenic mycobacteria M. smegmatis was not able to trigger lipid body formation [15]. Together, these findings indicate that phagocytosis is neither sufficient nor essential for pathogen-induced lipid body formation, and may suggest that transfer of bacterial lipids to non- infected bystander cells and/or cytokines and other inflammatory factors generated at infection foci might contribute to lipid body formations in leukocytes. Accordingly, components from bacterial cell walls, including E. coli LPS and M. bovis BCG- or M. tuberculosis-derived lipoarabinomannan (LAM) can mimic the pathogen and induce lipid body accumulation in a time- and dose-dependent manner [3, 15, 58]. Pattern recognition receptors play major roles in regulating lipid body biogenesis during infection [100]. LPS-induced lipid body formation in macrophages was demonstrated to occur through a mechanism largely dependent on TLR-4 in cooperation with CD14 and CD11b/CD18 [3]. Both BCG and the purified cell component, LAM, failed to induce lipid body formation in macrophages and other leukocytes from TLR2-deficient mice, although lipid body formation was not modified in TLR4 deficient animals, suggesting an important role for TLR2 in this phenomenon [15, 77]. Similarly, Chlamydia pneumonia, a pathogen that has been implicated in human and murine macrophage foam cell formation, a hallmark of early atherosclerosis, express a variety of ligands that could serve as potential TLR ligands and chlamydial infection induced macrophage lipid body formation in the presence of LDL was shown to occur through TLR2-, but not TLR4-, dependent mechanisms [101]. Interestingly, stimulation of macrophages in vitro with zymosan, a potent TLR2 activating agent, failed to induce lipid body formation, thus suggesting that although TLR2 activation is essential for mycobacteria-induced lipid body formation, it is not sufficient to trigger pathways of lipid body formation [15] and may involve TLR2 co-factors.
The biogenesis of lipid bodies in leukocytes is a rapidly regulated phenomenon therefore, raising the question whether lipid body formation depends on new protein synthesis. Of note, although maximum PAF-induced lipid body formation occurs within 1 hour, pretreatment of leukocytes with protein synthesis inhibitors partially inhibited PAF-, but not eotaxin- and RANTES-induced lipid body formation, thus indicating that, depending on the stimulus, induction of lipid bodies requires protein synthesis and it is likely that the transcription of early response genes is activated [3, 13, 39, 56, 70]. A role for Peroxisome Proliferator-Activated Receptors (PPARs), members of the nuclear receptor gene family that function in ligand-activated transcription, on macrophage differentiation on lipid accumulating cells has been demonstrated [102, 103]. Indeed, specific PPARγ ligands, significantly potentiate lipid body formation induced by ox-LDL, PAF-like agonists and G-CSF, suggesting that PPARs have a role in regulating leukocyte lipid body formation [73, 104]. Accordingly, PPAR are capable of modulating ADRP gene transcription in different cells including macrophages [105, 106].
Interestingly, leptin-induced lipid body accumulation in macrophages in vivo or in vitro is accompanied by increased levels of ADRP [93], demonstrating that leptin directly regulates the increase in ADRP cell content and accumulation of lipids within ADRP-enriched lipid bodies in macrophages and may have a role in foam cell formation. Increased ADRP expression by itself has been shown to be directly related to the enhanced capacity of neutral lipid storage, as ADRP promotes triglycerides and cholesterol storage and reduces cholesterol efflux [107]. ADRP may act also as a nucleation center for the assembly of lipids to form nascent lipid bodies and to enhance droplet stability upon lipolytic conditions [108, 109]. New findings are starting to unveil an important role for translational control by mTOR in the biogenic mechanisms of lipid bodies. We have recently established that mTOR activity is an important intracellular player in the regulation of macrophage lipid metabolism and inflammatory mediator production induced by leptin [93]. Leptin-induced ADRP-enriched lipid bodies were drastically reduced by the treatment with the mTOR inhibitor rapamycin. Taken together with the ability of leptin to induce the time- and dose-dependent P70S6K and 4EBP1 phosphorylation in a rapamycin-sensitive way, these data strongly suggest that leptin-induced increased cellular levels of ADRP depend on translational control through mTOR-dependent activation [93, 94] (Figure 3). Similarly, mTOR-dependent translational control of ADRP expression has also been observed in adipocytes stimulated with conjugated linoleic acid [110]. Of note, mTOR pathway integrates signals from nutrients, energy status and growth factors to regulate many processes, including cell growth, proliferation, autophagy and metabolism [111]. Indeed, rapamycin treatment is a potent inducer of autophagy. The interplay between lipid bodies and autophagy are starting to be investigated [112, 113]. Those studies suggest that lipid bodies are in close contact and act in concert with the proteasomal and autophagic pathways of protein degradation [112, 113]. Intriguingly, starvation-induced autophagy in Balb-c mouse macrophages is accompanied by increases in lipid body numbers and facilitation of Leishmania parasite growth [114].
3.0 Lipid Body Functions in Inflammation
The compartmentalization of signaling components within discrete and dynamic sites in the cell is critical for specificity and efficiency of enzymatic reactions of phosphorylation, enzyme activation and function [115]. Spatio-temporal regulation of the different enzymes involved in cell signaling is an area of increasingly interest. Accumulating evidence has placed lipid bodies as key organelles involved in the regulation of cell signaling in inflammatory cells [69, 116]. Over the past years substantial progresses have been made demonstrating that enzymes involved in eicosanoid syntheses localize at lipid bodies and lipid bodies are major sites for eicosanoid generation. Moreover, other inflammatory-relevant functions for lipid bodies have been hypothesized based on their composition and will be also discussed below.
3.1 Lipid bodies are specialized locales of eicosanoid synthesis
Eicosanoids are a family of arachidonic acid-derived signaling lipids that control important cellular processes, including cell proliferation, apoptosis, metabolism and migration [117, 118]. Thus, eicosanoids have key roles in physiological and pathological conditions such as tissue homeostasis, inflammation and cancer [117, 118]. Analyses of lipid bodies in different cell types and different stimulatory conditions have demonstrated that lipid bodies are particularly active sites for the metabolism of arachidonyl lipids. Electron microscopic autoradiographic observations demonstrated that exogenous radiolabbelled arachidonate was incorporated prominently in lipid bodies of eosinophils, neutrophils, mast cells, macrophages and epithelial cells [46, 119–122]. Lipid bodies obtained by subcellular fractionation provided direct evidence that these organelles are stores of esterified arachidonate. In eosinophils, arachidonate was incorporated predominantly in the phospholipid pool [120]; whereas arachidonic acid-containing neutral lipids appear to be the major store of arachidonic acid in monocyte/macrophages [5, 26]. Free arachidonic acid is an extremely reactive molecule that functions in cell signaling acting as an intracellular second messenger, as a paracrine mediator of cell activation and as a substrate for enzymatic conversion into eicosanoids [117, 123]. Although, negligible amounts of free AA were identified in lipid bodies, different enzymes involved in arachidonic acid metabolism as well proteins involved in arachidonic acid transport were characterized in lipid bodies, thus providing strong evidence for a major role for lipid bodies in arachidonic acid metabolism. If lipid bodies are indeed involved in arachidonic acid signaling and eicosanoid mediator formation, then the arachidonic acid present in these lipid-rich organelles must be released by phospholipases; and the free arachidonate must have access to eicosanoid-forming enzymes. cPLA2 specifically hydrolyzes arachidonic acid from the sn-2 position of glycerophospholipids and thus serves as the rate-limiting enzyme in the formation of eicosanoids and platelet-activating factor [124]. cPLA2 and its activating protein kinases, ERK1 and ERK2, was demonstrated to co-localize at lipid bodies [26]. cPLA2α localizes to lipid bodies in cells responding to a wide range of stimuli, including arachidonic acid [125], Moreira, submitted). Moreover, high cPLA2 specific activity was present in the lipid body fraction detected by measuring the release of radiolabeled arachidonic acid from the sn-2 position of 1-palmitoyl-2-[14C] arachidonyl phosphatidylcholine [26]. A large proportion of leukocyte arachidonate is stored in triglyceride pools in lipid bodies [5, 26, 126]. However the function and utilization of this arachidonic acid triglyceride pool has been elusive. The identification of triglyceride lipase and its activator CGI-58 within lipid bodies [29–31] opens the perspective that arachidonate from triglycerides within lipid bodies could be used as a storage pool to replenish, upon transfer, lipid body arachidonyl-phospholipids, from which regulated activation of cPLA2 would provide arachidonate for local eicosanoid synthesis.
Proteins potentially involved in arachidonic acid transport were also shown to localize within lipid bodies. S100A9, a protein involved in the transport of arachidonate and in the shuttle of unsaturated fatty acids to membranes [127, 128], was recently identified in the proteomic analysis of leukocyte lipid bodies [31]. S100A9 might participate in arachidonate-derived eicosanoid formation within leukocyte lipid bodies. Lipid bodies in neutrophils have also been shown to rapidly move and interact with phagosomes, potentially delivering lipid body-derived arachidonate to activate phagosomal NADPH oxidase [129, 130].
Intracellular compartmentalization of eicosanoid synthesis within leukocytes has emerged as a key feature that regulates the amount and may also regulate the eicosanoid produced. In support of this, significant correlations between lipid body formation and enhanced generation of both 5-LO- and COX-derived eicosanoids (LTC4, LTB4 and PGE2) were observed, thus indicating that lipid body numbers in leukocytes would result in enhanced capacity of eicosanoid production by leukocytes (Table 2). Indeed, stimuli known to prime leukocytes to induce eicosanoid generation, including PKC activators, arachidonate and PAF, are also active in stimulating lipid body formation [3, 13, 39, 70, 71]. Accordingly, others and we observed a significant correlation between lipid body formation and enhanced generation of both LO- and COX-derived eicosanoids in vitro [13, 39, 40, 58, 70, 75, 131] as well as in vivo [3, 8, 73, 88, 93, 99].
Table 2.
Increased leukocyte lipid body formation is involved in priming for eicosanoid production
| Cell | Stimulus | LTC4 | LTB4 | PGE2 | Ref |
|---|---|---|---|---|---|
| Human PMNs | Oleic acid | + | + | 13, 131 | |
| Arachidonic acid | + | + | 13, 131, 145 | ||
| Platelet Activating Factor (PAF) | + | + | 70, 131 | ||
| Phorbol ester (PMA) | + | 131 | |||
| Human Monocytes | Lipopolysaccharide (LPS) | + | + | 3 | |
| Human Eosinophils | Oleic acid | + | 13 | ||
| Arachidonic acid | + | 13 | |||
| Oleyl-acetyl-glycerol (OAG) | + | 131 | |||
| PAF | + | + | 39, 40 | ||
| Eotaxin/CCL11 | + | 56, 74 | |||
| CCL24/CCL26 | + | 74 | |||
| RANTES/CCL5 | + | 59 | |||
| IL-5-IL-5R (via PAF) | + | 40, 75 | |||
| IgG-FcgR (via PAF) | + | 75 | |||
| PGD2 | + | 9 | |||
| IL-16 (via CCL11/CCL5) | + | 74 | |||
| Human Eosinophil cytoplasts | PAF | + | + | 39 | |
| Mouse macrophages | Arachidonic acid | + | 13 | ||
| Leptin | + | + | 93 | ||
| CCL2 | + | 58 | |||
| Mouse PMN + macrophages | LPS | + | + | 3,58 | |
| PAF and PAF-like | + | + | 70, 73 |
To support a role for lipid bodies in AA metabolism the location of key eicosanoid-forming enzymes were investigated by using a variety of techniques, cells and stimulatory conditions. The major enzymes, 5-LO, 15-LO, FLAP and COX, involved in the enzymatic conversion of AA into eicosanoids were shown by immunocytochemistry/immunofluorescence, ultrastructural postembedding immunogold EM and/or western blotting from subcellular fractions to localize within lipid bodies stimulated in vitro [38–40, 54, 57] or obtained from in vivo inflammatory responses [3, 8, 15, 93] (Figure 4). Moreover, even the down-stream membrane spanning enzymes involved in LTC4 production – LTC4 synthase –, and PGE2 production –PGE2 synthase- have been found at lipid bodies [39, 42, 57].
Figure 4. Leukocyte lipid bodies are intracellular locales for eicosanoid synthesis in inflammation.
Leukotriene forming enzymes 5-LO (A) and FLAP (B) are localized at leukocyte lipid bodies obtained from in vivo LPS-stimulated animals. In A, colocalization of 5-LO at ADRP-labeled In C and D, Newly formed eicosanoids were immobilized at its site of synthesis by cross-linking of the lipids to adjacent proteins using 1-ethyl-3-(3-dimethylamino-propyl) carbodiimide (EDAC). LTB4 were identified by using anti-LTB4 and revealed with Cy2-labeled anti-rabbit IgG. In C, Images show LTB4 immuno-reactive lipid bodies (as identified by anti-ADRP) of peritoneal macrophages. In D, Leukocytes from in vivo vehicle- (right panel) or LPS-stimulated animals (middle panel) were immunolabeled for newly formed LTB4 after LPS stimulation. LPS-stimulated animals were treated with the 5-LO inhibitor zileuton before incubation with EDAC (left panel), demonstrating that eicosanoid production at lipid bodies is a regulated and enzymatic-dependent process. Images show that LTB4 immunolabeling has a punctate cytoplasmic pattern that is absent in nonstimulated cells, inducible by LPS, and sensitive to zileuton (Part C and D were reproduced with permission from [58] Copyright 2007 The American Association of Immunologists, Inc.).
Overall, lipid bodies compartmentalize the substrate and the entire enzymatic machinery for eicosanoid synthesis. It was recently established that successful eicosanoid production is not merely determined by AA and eicosanoid-forming enzymes availability, but requires sequential interactions between over 6 specific biosynthetic proteins acting in cascade, and may involve very unique spatial interactions. Therefore, just by detecting eicosanoid-forming enzymes within lipid bodies one cannot assure that these organelles are indeed accountable for the efficient and enhanced eicosanoid synthesis observed during inflammatory responses. That lipid bodies can properly arrange enzymatic complexes with successful eicosanoid-forming properties and, therefore, function as specialized domains for focal eicosanoid generation has been documented by the direct intracellular localizations of newly formed eicosanoids. Direct assessment of specific intracellular sites of eicosanoid synthesis has been elusive, as those lipid mediators are newly formed, not stored and often rapidly released upon cell stimulation. By means of a strategy to covalently cross-link, capture and localize newly formed eicosanoids at their sites of synthesis, we demonstrated that lipid bodies are major intracellular locales for the activation-elicited formation of LTC4 in eosinophils [8, 56, 132], LTB4 in neutrophils and macrophages [58] and PGE2 in macrophages and epithelial cells [57, 122, 133] (Figure 4).
Importantly, eicosanoid formation within lipid bodies is not restricted to leukocytes or to inflammatory conditions. Cells that produce high quantities of eicosanoids under physiological conditions, including granulosa cells of periovulatory follicles involved in the production of PGE2 which is necessary for normal ovulation [134], luteal steroid-producing and interstitial cells involved in regression of the corpus luteum [43], and fetal membranes with advancing gestation and labor [42, 135], were demonstrated to exhibit high numbers of lipid bodies containing eicosanoid synthetizing enzymes. Moreover, endothelial and epithelial cells involved in pathological conditions such as in cancer, hypoxia and during infections were shown to contain increased numbers of eicosanoid-synthesizing lipid bodies [38, 54, 55, 57, 122, 136].
The roles of lipid body-derived eicosanoids may vary depending on leukocyte type, stimulus and inflammatory conditions controlling the eicosanoid synthesis. Eicosanoids synthesized at lipid body sites may function as intracellular and extracellular mediators. For instance, LTC4 synthesized at lipid bodies within eosinophils following in vitro eotaxin stimulation were shown to have a novel function as an intracrine mediator regulating IL-4 secretion from eosinophils [137]. On the other hand, LTC4 synthesized within eosinophil lipid bodies during in vivo allergic inflammation has paracrine activities, inasmuch as LTC4 was released from eosinophils and was able to activate other cells present in the inflammatory site [8, 9].
3.2 Storage of Cytokines
Another group of inflammatory mediators that were identified to localize within lipid bodies are cytokines — a family of glycoproteins with diverse biological activities involved in cell growth, inflammation, immunity, differentiation and repair. Cytokines and chemokines are produced and secreted by a variety of activated leukocytes including macrophages, neutrophils, mast cells, basophils and eosinophils. In addition to their ability to synthesize new cytokine proteins, leukocytes also contain cytokines as stored, preformed pools that can be found within a variety of cellular compartments, including granules, vesicles and lipid bodies [138]. Tumor necrosis factor-alpha (TNF-α) was the first cytokine found within lipid bodies, detected throughout cytoplasmic lipid bodies cores by immune gold EM of different cell types present in colonic Crohn’s disease biopsies, including infiltrating neutrophils, macrophages and eosinophils [139]. TNF-α was also found at lipid bodies of circulating monocytes and neutrophils of septic patients and murine macrophages and neutrophils of experimentally induced sepsis [3]. Cytoplasmic lipid bodies in isolated human lung mast cells contain the basic fibroblast growth factor (bFGF), which also is present in the mast cell secretory granules [140]. Close associations of bFGF-containing lipid bodies and smooth ER, ribosomes and nuclei were observed in human lung mast cells, which may suggest a non-classical synthetic/secretory- storage pathway for bFGF in human mast cells [140].
In human eosinophils, TNF-α [139] and the lymphocyte chemoattractants RANTES and IL-16 [141] have been detected at lipid bodies. Notably, suggestive interactions between lipid bodies and secretory vesicles involved in cytokine release in these cells termed eosinophil sombrero vesicles (EoSVs) [142, 143], are frequently identified in the cytoplasm and raises a potential role for lipid bodies in vesicular trafficking (Melo, Dvorak and Weller, unpublished data; [69].
If lipid bodies represent additional subcellular storage compartments for cytokines within leukocytes, routes for cytokine-mediated secretion may exist and still need to be characterized. Alternatively, lipid body-stored cytokines may function as intracrine signaling mediators. In leukocytes, stimulus-coupled release of cytokine containing granule and/or vesicles to the cell surface for release is largely dependent on fusion mediated by the SNAREs that are present on granules/secretory vesicles and on the plasma membrane [144]. The internal membranes and cytoplasmic domains within lipid bodies may have candidate roles in the processes of vesicular transport, membrane fusion and protein secretion. Interestingly, a number of proteins likely involved in vesicular trafficking were shown to localize at lipid bodies in macrophages, including VAT-1 (synaptic vesicle membrane protein) homologue, SNAP29 (vesicle membrane fusion protein), transmembrane traffic protein, GTP binding protein SAR 1a, and Rap-1a [31]. The presence of members of the vesicular trafficking system within lipid bodies was recently confirmed by Bostrom et al, who showed the presence of NSF (N-ethylmaleimide-sensitivefactor), α-SNAP (soluble NSF attachment protein) and the SNARE s (SNAP receptors), SNAP23 (synaptosomal-associated protein of 23 kDa), syntaxin-5 and VAMP4 (vesicle-associated membrane protein 4) at lipid bodies and demonstrated that SNARE proteins have roles in lipid body fusion processes in the cell [37]. Future studies will be necessary to characterize the regulation and function of cytokines within lipid bodies.
3.3 Compartmentalization of cell signaling
The presence of a variety of kinases within leukocyte lipid bodies has implicated this organelle as a cytoplasmic domain with compartmentalizing roles in intracellular signaling. The MAP kinases (also known as extracellular signal-regulated kinases – ERKs), as well as PKC and PI3K are key enzymes implicated in intracellular signaling of diverse cellular responses that can be found within leukocyte lipid bodies [26, 44, 45]. MAP kinase ERK1, ERK2 and p38 are key enzymes in the activation of cPLA2, the enzyme that specifically hydrolyzes arachidonic acid from the sn-2 position of glycerophospholipids. Yu et al. demonstrated the co-compartmentalization of several MAP kinases and cPLA2 at arachidonate enriched-lipid bodies [26]. The substantial association of cPLA2 activators ERK1 and ERK2 with lipid bodies may serve to ensure rapid phosphorylation of cPLA2 on lipid bodies in response to extracellular stimuli.
PI3K regulatory and catalytic subunits were also localized to lipid bodies by immunocytochemistry and/or immunoblotting and enzyme assays of subcellular fractions of isolated lipid bodies from monocyte/macrophage and polymorphonuclear leukocytes [44]. In addition, co-immunoprecipitation studies demonstrated PI3K to be physically associated with phosphorylated Lyn kinase in lipid bodies induced to form in human polymorphonuclear leukocytes [44]. Although functional studies still need to be carried out to characterize the actual roles of lipid body-resident kinases, accumulating evidence indicate that kinase-mediated signaling is active within cytoplasmic lipid bodies in leukocytes.
Novel findings are starting to reveal functions of leukocyte lipid bodies as sites of ribosomal translation and de novo protein synthesis with potential implications for the regulation of inflammation-related proteins. EM analyses of leukocyte lipid bodies demonstrated the presence of ribosomes or particles resembling ribosomal subunits. Moreover, ribosomes or ribosome subunit-like particles were present within the lipid-rich cores and/or attached to LB borders of LBs in monocyte cell line U937 and in human neutrophils and eosinophils [31]. That lipid bodies may be sites of ribosomal function is supported by the demonstration of 3H-uridine accumulation in lipid bodies and poly (A) mRNA detection in lipid bodies by in situ hybridization [62, 63]. Moreover, proteomic analyses of purified lipid body fractions of U937 identified several ribosomal subunit proteins as well as translation initiation factors in isolated lipid bodies [31]. Likewise, proteomic analyses of lipid bodies from hepatitis C virus core protein expressing hepatoma cell have identified ribosomal and RNA-interacting (DEAD box) proteins [66]. Does ribosomal localization within lipid bodies translate into compartmentalized protein synthesis at lipid bodies? Are there specific transcripts that are regulated within lipid bodies? Future investigations are necessary to characterize the roles of lipid bodies in the regulation of local protein synthesis during inflammation.
4.0 Involvement of lipid bodies in inflammatory disorders and potential as targets for therapeutic intervention
Lipid bodies may function as specialized intracellular sites of signaling within leukocytes engaged in inflammatory process ranging from allergy, to infections, to cancer and atherosclerosis. Inducible mechanisms that enhance eicosanoid production are attractive targets for anti-inflammatory pharmacological intervention. Although no specific lipid body inhibitor has been described so far, different classes of drugs as well as gene knockdown of PAT proteins have been demonstrated to inhibit lipid body formation. The hypothesis of lipid body inhibition as target for anti-inflammatory therapy has been tested in different model systems.
Aspirin and selected other non-steroidal anti-inflammatory drugs (NSAIDs) inhibited lipid body formation in vivo and in vitro [13, 145, 146]. This inhibition occurs through COX-independent mechanisms, because cis-unsaturated fatty acid-induced formation of new lipid bodies in macrophages from COX-1- and COX-2-deficient mice was not impaired; and NSAIDs, including aspirin, sodium salicylate, indomethacin, and NS-398, inhibited lipid body formation equally in macrophages from wild-type and COX-1- or COX-2-deficient mice [13, 145]. Pertinent to understanding the anti-inflammatory activities of aspirin, aspirin and NSAIDs, by a COX-independent mechanism, inhibited the early induction by cis-fatty acids of both lipid body formation and priming for enhanced eicosanoid formation in leukocytes, including notably inhibiting enhanced 5-LO pathway-mediated leukotriene generation [13]. These findings extend the anti-inflammatory capacities of aspirin and NSAIDs to include suppression of more than COX pathway-derived eicosanoids. On the other hand, in more complex stimulatory conditions, such as allergic inflammatory reactions, both COX independent and dependent processes appear to intermediate activation of lipid body-regulated lipoxygenase pathway [9].
Lipoxygenase pathway-derived cys-LTs (LTC4, LTD4, LTE4) have key roles in the pathogenesis of allergic inflammatory diseases, such as asthma [147]. In fact, pharmacological blockage of cys-LTs synthesis/effects has been proved to be beneficial in controlling aspects of allergic pulmonary inflammation [148–150]. Therefore, one can hypothesize that the inhibitory impact on the biogenesis or function of allergen-driven lipid bodies - the cysLTs-synthesizing compartments in vivo [8, 9] - may have additional therapeutic effects.
Approaches to inhibit lipid accumulation in macrophage foam cells may be of therapeutic value in preventing atherosclerosis and has been recently reviewed elsewhere [78]. Different strategies to inhibit lipid body formation have been tested to address the role of macrophage lipid bodies as targets for therapeutic intervention in atherosclerosis. ADRP expression facilitates foam cell formation induced by modified lipoproteins in mouse macrophages in vitro, conversely ADRP gene inactivation in apolipoprotein E-deficient mice reduces the number of lipid bodies in foam cells in atherosclerotic lesions and protects the mice against atherosclerosis [151]. ACAT inhibitors including the fungal-derived cyclodepsipeptides showed potent inhibitory activity of lipid body accumulation in mouse peritoneal macrophages and exerted antiatherogenic activity in both low-density lipoprotein receptor- and apolipoprotein E-knockout mice [152].
The enhanced capacity of macrophages to generate PGE2 in the course of mycobacterial infection due to increased lipid body formation and compartmentalization of stimulated local eicosanoid production within lipid bodies may contribute to the mechanisms that intracellular pathogens have evolved to survive in host cells and suggest inhibition of lipid body function as a target for pharmacological intervention in intracellular pathogen infection. Indeed, PGE2 inhibits the Th1 type response and TNF–α and NO production [153, 154]. In order to characterize the roles of NSAID-induced inhibition of formation of lipid bodies and PGE2 on mycobacterial host response, the levels of one pro-inflammatory (TNF-α) and one anti-inflammatory cytokine (IL-10) were investigated. Accordingly, treatment with aspirin or NS 398 lead to an enhancement of TNF-α production and a drastic reduction in IL-10 generation induced by BCG-infection, that paralleled the inhibitory effect of these NSAIDs on PGE2 and lipid body formation [15]. Similar immunomodulation of mycobacterial infection was obtained with lipid body inhibition by C75, a fatty acid synthase inhibitor [155]. Interestingly, although C75 is not a COX inhibitor, it significantly inhibited mycobacterial induced lipid body-derived PGE2 and enhanced TNF-α production. These data suggest that lipid body induction and lipid body-derived PGE2 down-modulate the macrophage response by inhibiting BCG-induced TNF-α production (key cytokine in mediating macrophage-induced mycobacterial killing) and increasing the levels of the anti-inflammatory cytokine IL-10 which may favor intracellular pathogen growth. Thus, inhibition of lipid body formation may be of value as a co-treatment in intracellular pathogen infections.
A role for lipid bodies as a potential target to generate new drugs for cancer treatment has been recently suggested [57]. Colonic adenocarcinoma cells contain increased numbers of lipid bodies with documented PGE2 synthase localization and focal PGE2 synthesis.. Inhibition of lipid body formation by either aspirin or a fatty acid synthase inhibitor correlated with both inhibition of PGE2 generation and cell proliferation in CACO-2 and IEC-6 H-rasV12 cells [57]. The inhibition of lipid body generation may affect the subcellular compartmentalization of COX-2 and in consequence inhibit the enhanced prostaglandin synthesis that is related to the pathogenesis of colon cancer.
Future studies will be necessary to characterize the role of lipid bodies as targets for therapeutic intervention in diseases that progress with increased lipid body accumulation as in atherosclerosis, hepatic steatosis, cancer and inflammation. That to include further safety characterization of lipid body inhibition as lipid accumulation within lipid bodies may act as protective mechanisms in lipid homeostasis against cellular lipotoxicity. Moreover, the development of selective lipid body inhibitors is in need.
6.0 Concluding Remarks
Major advances in the understanding of the cellular and molecular mechanisms regulating leukocyte lipid body biogenesis and functions were achieved in recent years. Our contemporary view of lipid bodies places this organelle as critical regulators of different inflammatory and infectious diseases and key markers of leukocyte activation. Notably, leukocyte lipid body biogenesis is highly regulated and is cell and stimuli specific. Studies of lipid body structural features have revealed a much more complex structure then initially anticipated that beside lipids includes a diverse array of proteins that may vary according to the cell type and cellular activation state and thus may determine different cellular functions for lipid bodies. Moreover, internal membranes and ribosomes were identified within leukocyte lipid bodies adding to the system complexity. In leukocytes and other cells, it have been established that lipid bodies are specialized, inducible cytoplasmic domains that have central roles to control the synthesis and secretion of inflammatory mediators. However, for an organelle centrally involved in cellular lipid balance and cellular signaling, our current understanding of its biogenesis, dynamics, heterogeneity and function in different cellular systems are still very limited. Critical open questions remain about the formation and functions of lipid bodies not only to understand normal leukocyte function but also in several inflammatory-related diseases. In conclusion, recent studies place lipid bodies as multifunctional organelles with key function in lipid storage and cell signaling in inflammation and as such are emerging as attractive target candidate for therapeutic intervention.
Acknowledgments
The work of the authors is supported by PRONEX-MCT, Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq, Brazil), PAPES-FIOCRUZ, Fundação de Amparo à Pesquisa do Rio de Janeiro (FAPERJ, Brazil) (to PTB) and NIH grants (AI022571, AI020241, AI051645) (to PFW).
Abbreviations
- 5-LO
5- lipoxygenase
- AA
arachidonic acid
- ACAT
acyl-coenzyme A cholesterol acyltransferase
- ADRP
adipose differentiation related protein
- BCG
Bacillus Calmett Guerrin
- COX
cyclooxygenase
- cPLA2
cytosolic phospholipase A2
- EDAC
1 – ethyl – 3 (3 – dimethylamino – propyl) carbodiimide
- ER
endoplasmic reticulum
- EM
electron microscopy
- FLAP
5- lipoxygenase activating protein
- IL
interleukin
- LDL
low density lipoprotein
- LPS
lipopolysaccharide
- LT
leukotriene
- MAP
mitogen-activated protein
- MCP-1
monocyte chemotactic protein-1
- NSAID
non-steroidal anti-inflammatory drug
- PI3K
phosphatidylinositide 3 kinase
- PG
prostaglandin
- PKC
protein kinase C
- PPAR
Peroxisome Proliferator-Activated Receptor
- TIP 47
tail-interacting protein of 47 kDa
- TLR
toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McGookey DJ, Anderson RG. Morphological characterization of the cholesteryl ester cycle in cultured mouse macrophage foam cells. J Cell Biol. 1983;97:1156–1168. doi: 10.1083/jcb.97.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 3.Pacheco P, Bozza FA, Gomes RN, Bozza M, Weller PF, Castro-Faria-Neto HC, Bozza PT. Lipopolysaccharide-induced leukocyte lipid body formation in vivo: innate immunity elicited intracellular Loci involved in eicosanoid metabolism. J Immunol. 2002;169:6498–6506. doi: 10.4049/jimmunol.169.11.6498. [DOI] [PubMed] [Google Scholar]
- 4.Leite MS, Pacheco P, Gomes RN, Guedes AT, Castro-Faria-Neto HC, Bozza PT, Koatz VLG. Mechanisms of increased survival after lipopolysaccharide-induced endotoxic shock in mice consuming olive oil-enriched diet. Shock. 2005;23:173–178. doi: 10.1097/01.shk.0000148072.12094.77. [DOI] [PubMed] [Google Scholar]
- 5.Triggiani M, Oriente A, Seeds MC, Bass DA, Marone G, Chilton FH. Migration of human inflammatory cells into the lung results in the remodeling of arachidonic acid into a triglyceride pool. J Exp Med. 1995;182:1181–1190. doi: 10.1084/jem.182.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salluh JI, Pino AV, Silva AR, Gomes RN, Souza HS, e Silva JR, Jandre FC, Giannella-Neto A, Zimmerman GA, Stafforini DM, Prescott SM, Castro-Faria-Neto HC, Bozza PT, Bozza FA. Lung production of platelet-activating factor acetylhydrolase in oleic acid-induced acute lung injury. Prostaglandins Leukot Essent Fatty Acids. 2007;77:1–8. doi: 10.1016/j.plefa.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Dvorak AM, Hammel I, Schulman ES, Peters SP, MacGlashan DW, Jr, Schleimer RP, Newball HH, Pyne K, Dvorak HF, Lichtenstein LM, et al. Differences in the behavior of cytoplasmic granules and lipid bodies during human lung mast cell degranulation. J Cell Biol. 1984;99:1678–1687. doi: 10.1083/jcb.99.5.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira-de-Abreu A, Assis EF, Gomes GS, Castro-Faria-Neto HC, Weller PF, Bandeira-Melo C, Bozza PT. Allergic Challenge-Elicited Lipid Bodies Compartmentalize In Vivo Leukotriene C4 Synthesis within Eosinophils. Am J Respir Cell Mol Biol. 2005;33:254–261. doi: 10.1165/rcmb.2005-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesquita-Santos FP, Vieira-de-Abreu A, Calheiros AS, Figueiredo IH, Castro-Faria-Neto HC, Weller PF, Bozza PT, Diaz BL, Bandeira-Melo C. Cutting Edge: Prostaglandin D2 Enhances Leukotriene C4 Synthesis by Eosinophils during Allergic Inflammation: Synergistic In Vivo Role of Endogenous Eotaxin. J Immunol. 2006;176:1326–1330. doi: 10.4049/jimmunol.176.3.1326. [DOI] [PubMed] [Google Scholar]
- 10.Schlesinger PA, Stillman MT, Peterson L. Polyarthritis with birefringent lipid within synovial fluid macrophages: case report and ultrastructural study. Arthritis Rheum. 1982;25:1365–1368. doi: 10.1002/art.1780251114. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein J. Synovial fluid leukocytosis associated with intracellular lipid inclusions. Arch Intern Med. 1980;140:560–561. [PubMed] [Google Scholar]
- 12.Reginato AJ, Schumacher HR, Allan DA, Rabinowitz JL. Acute monoarthritis associated with lipid liquid crystals. Ann Rheum Dis. 1985;44:537–543. doi: 10.1136/ard.44.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozza PT, Payne JL, Morham SG, Langenbach R, Smithies O, Weller PF. Leukocyte lipid body formation and eicosanoid generation: cyclooxygenase-independent inhibition by aspirin. Proc Natl Acad Sci U S A. 1996;93:11091–11096. doi: 10.1073/pnas.93.20.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherjee A, Misra RS, Meyers WM. An electron microscopic study of lymphatics in the dermal lesions of human leprosy. Int J Lepr Other Mycobact Dis. 1989;57:506–510. [PubMed] [Google Scholar]
- 15.D’Avila H, Melo RC, Parreira GG, Werneck-Barroso E, Castro-Faria-Neto HC, Bozza PT. Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J Immunol. 2006;176:3087–3097. doi: 10.4049/jimmunol.176.5.3087. [DOI] [PubMed] [Google Scholar]
- 16.Cardona PJ, Llatjos R, Gordillo S, Diaz J, Ojanguren I, Ariza A, Ausina V. Evolution of granulomas in lungs of mice infected aerogenically with Mycobacterium tuberculosis. Scand J Immunol. 2000;52:156–163. doi: 10.1046/j.1365-3083.2000.00763.x. [DOI] [PubMed] [Google Scholar]
- 17.Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RG, Liu P, Chapman KD. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res. 2007;48:837–847. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, Fujimoto T. The surface of lipid droplets is a phospholipid monolayer with a unique Fatty Acid composition. J Biol Chem. 2002;277:44507–44512. doi: 10.1074/jbc.M207712200. [DOI] [PubMed] [Google Scholar]
- 19.Miura S, Gan JW, Brzostowski J, Parisi MJ, Schultz CJ, Londos C, Oliver B, Kimmel AR. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem. 2002;277:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–11346. [PubMed] [Google Scholar]
- 21.Heid HW, Moll R, Schwetlick I, Rackwitz HR, Keenan TW. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res. 1998;294:309–321. doi: 10.1007/s004410051181. [DOI] [PubMed] [Google Scholar]
- 22.Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249–2263. [PubMed] [Google Scholar]
- 23.Wolins NE, Rubin B, Brasaemle DL. TIP47 associates with lipid droplets. J Biol Chem. 2001;276:5101–5108. doi: 10.1074/jbc.M006775200. [DOI] [PubMed] [Google Scholar]
- 24.Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–49. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Brasaemle DL. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Yu W, Bozza PT, Tzizik DM, Gray JP, Cassara J, Dvorak AM, Weller PF. Co-compartmentalization of MAP kinases and cytosolic phospholipase A2 at cytoplasmic arachidonate-rich lipid bodies. Am J Pathol. 1998;152:759–769. [PMC free article] [PubMed] [Google Scholar]
- 27.Umlauf E, Csaszar E, Moertelmaier M, Schuetz GJ, Parton RG, Prohaska R. Association of stomatin with lipid bodies. J Biol Chem. 2004;279:23699–23709. doi: 10.1074/jbc.M310546200. [DOI] [PubMed] [Google Scholar]
- 28.Fujimoto Y, Itabe H, Sakai J, Makita M, Noda J, Mori M, Higashi Y, Kojima S, Takano T. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim Biophys Acta. 2004;1644:47–59. doi: 10.1016/j.bbamcr.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem. 2004;279:3787–3792. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 30.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 31.Wan HC, Melo RC, Jin Z, Dvorak AM, Weller PF. Roles and origins of leukocyte lipid bodies: proteomic and ultrastructural studies. Faseb J. 2007;21:167–178. doi: 10.1096/fj.06-6711com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin S, Driessen K, Nixon SJ, Zerial M, Parton RG. Regulated localization of Rab18 to lipid droplets: effects of lipolytic stimulation and inhibition of lipid droplet catabolism. J Biol Chem. 2005;280:42325–42335. doi: 10.1074/jbc.M506651200. [DOI] [PubMed] [Google Scholar]
- 33.Ozeki S, Cheng J, Tauchi-Sato K, Hatano N, Taniguchi H, Fujimoto T. Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum-derived membrane. J Cell Sci. 2005;118:2601–2611. doi: 10.1242/jcs.02401. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto T, Kogo H, Ishiguro K, Tauchi K, Nomura R. Caveolin-2 is targeted to lipid droplets, a new “membrane domain” in the cell. J Cell Biol. 2001;152:1079–1085. doi: 10.1083/jcb.152.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pol A, Luetterforst R, Lindsay M, Heino S, Ikonen E, Parton RG. A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance. J Cell Biol. 2001;152:1057–1070. doi: 10.1083/jcb.152.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pol A, Martin S, Fernandez MA, Ferguson C, Carozzi A, Luetterforst R, Enrich C, Parton RG. Dynamic and regulated association of caveolin with lipid bodies: modulation of lipid body motility and function by a dominant negative mutant. Mol Biol Cell. 2004;15:99–110. doi: 10.1091/mbc.E03-06-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bostrom P, Andersson L, Rutberg M, Perman J, Lidberg U, Johansson BR, Fernandez-Rodriguez J, Ericson J, Nilsson T, Boren J, Olofsson SO. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat Cell Biol. 2007;9:1286–1293. doi: 10.1038/ncb1648. [DOI] [PubMed] [Google Scholar]
- 38.Dvorak AM, Weller PF, Harvey VS, Morgan ES, Dvorak HF. Ultrastructural localization of prostaglandin endoperoxide synthase (cyclooxygenase) to isolated, purified fractions of guinea pig peritoneal macrophage and line 10 hepatocarcinoma cell lipid bodies. Int Arch Allergy Immunol. 1993;101:136–142. doi: 10.1159/000236511. [DOI] [PubMed] [Google Scholar]
- 39.Bozza PT, Yu W, Penrose JF, Morgan ES, Dvorak AM, Weller PF. Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. J Exp Med. 1997;186:909–920. doi: 10.1084/jem.186.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bozza PT, Yu W, Cassara J, Weller PF. Pathways for eosinophil lipid body induction: differing signal transduction in cells from normal and hypereosinophilic subjects. J Leukoc Biol. 1998;64:563–569. doi: 10.1002/jlb.64.4.563. [DOI] [PubMed] [Google Scholar]
- 41.Thore CR, Beasley TC, Busija DW. In vitro and in vivo localization of prostaglandin H synthase in fetal sheep neurons. Neurosci Lett. 1998;242:29–32. doi: 10.1016/s0304-3940(98)00040-8. [DOI] [PubMed] [Google Scholar]
- 42.Meadows JW, Pitzer B, Brockman DE, Myatt L. Expression and localization of adipophilin and perilipin in human fetal membranes: association with lipid bodies and enzymes involved in prostaglandin synthesis. J Clin Endocrinol Metab. 2005;90:2344–2350. doi: 10.1210/jc.2004-1199. [DOI] [PubMed] [Google Scholar]
- 43.Arend A, Masso R, Masso M, Selstam G. Electron microscope immunocytochemical localization of cyclooxygenase-1 and -2 in pseudopregnant rat corpus luteum during luteolysis. Prostaglandins Other Lipid Mediat. 2004;74:1–10. doi: 10.1016/j.prostaglandins.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Yu W, Cassara J, Weller PF. Phosphatidylinositide 3-kinase localizes to cytoplasmic lipid bodies in human polymorphonuclear leukocytes and other myeloid-derived cells. Blood. 2000;95:1078–1085. [PubMed] [Google Scholar]
- 45.Chen JS, Greenberg AS, Wang SM. Oleic acid-induced PKC isozyme translocation in RAW 264.7 macrophages. J Cell Biochem. 2002;86:784–791. doi: 10.1002/jcb.10266. [DOI] [PubMed] [Google Scholar]
- 46.Weller PF, Ackerman SJ, Nicholson-Weller A, Dvorak AM. Cytoplasmic lipid bodies of human neutrophilic leukocytes. Am J Pathol. 1989;135:947–959. [PMC free article] [PubMed] [Google Scholar]
- 47.Weller PF, Dvorak AM. Arachidonic acid incorporation by cytoplasmic lipid bodies of human eosinophils. Blood. 1985;65:1269–1274. [PubMed] [Google Scholar]
- 48.Murphy DJ. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res. 2001;40:325–438. doi: 10.1016/s0163-7827(01)00013-3. [DOI] [PubMed] [Google Scholar]
- 49.Robenek MJ, Severs NJ, Schlattmann K, Plenz G, Zimmer KP, Troyer D, Robenek H. Lipids partition caveolin-1 from ER membranes into lipid droplets: updating the model of lipid droplet biogenesis. Faseb J. 2004;18:866–868. doi: 10.1096/fj.03-0782fje. [DOI] [PubMed] [Google Scholar]
- 50.Brown DA. Lipid droplets: proteins floating on a pool of fat. Curr Biol. 2001;11:R446–449. doi: 10.1016/s0960-9822(01)00257-3. [DOI] [PubMed] [Google Scholar]
- 51.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 52.Robenek H, Hofnagel O, Buers I, Robenek MJ, Troyer D, Severs NJ. Adipophilin-enriched domains in the ER membrane are sites of lipid droplet biogenesis. J Cell Sci. 2006;119:4215–4224. doi: 10.1242/jcs.03191. [DOI] [PubMed] [Google Scholar]
- 53.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 54.Dvorak AM, Morgan E, Schleimer RP, Ryeom SW, Lichtenstein LM, Weller PF. Ultrastructural immunogold localization of prostaglandin endoperoxide synthase (cyclooxygenase) to non-membrane-bound cytoplasmic lipid bodies in human lung mast cells, alveolar macrophages, type II pneumocytes, and neutrophils. J Histochem Cytochem. 1992;40:759–769. doi: 10.1177/40.6.1316915. [DOI] [PubMed] [Google Scholar]
- 55.Dvorak AM, Morgan ES, Tzizik DM, Weller PF. Prostaglandin endoperoxide synthase (cyclooxygenase): ultrastructural localization to nonmembrane-bound cytoplasmic lipid bodies in human eosinophils and 3T3 fibroblasts. Int Arch Allergy Immunol. 1994;105:245–250. doi: 10.1159/000236764. [DOI] [PubMed] [Google Scholar]
- 56.Bandeira-Melo C, Phoofolo M, Weller PF. Extranuclear lipid bodies, elicited by CCR3-mediated signaling pathways, are the sites of chemokine-enhanced leukotriene C4 production in eosinophils and basophils. J Biol Chem. 2001;276:22779–22787. doi: 10.1074/jbc.M101436200. [DOI] [PubMed] [Google Scholar]
- 57.Accioly MT, Pacheco P, Maya-Monteiro CM, Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA, Bozza PT, Viola JP. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 2008;68:1732–1740. doi: 10.1158/0008-5472.CAN-07-1999. [DOI] [PubMed] [Google Scholar]
- 58.Pacheco P, Vieira-de-Abreu A, Gomes RN, Barbosa-Lima G, Wermelinger LB, Maya-Monteiro CM, Silva AR, Bozza MT, Castro-Faria-Neto HC, Bandeira-Melo C, Bozza PT. Monocyte chemoattractant protein-1/CC chemokine ligand 2 controls microtubule-driven biogenesis and leukotriene B4-synthesizing function of macrophage lipid bodies elicited by innate immune response. J Immunol. 2007;179:8500–8508. doi: 10.4049/jimmunol.179.12.8500. [DOI] [PubMed] [Google Scholar]
- 59.Kondo H, Iwasa H, Saino-Saito S. First disclosure of lipid droplet substructure and myelin translucency in embedment-free section electron microscopy. Tohoku J Exp Med. 2008;214:167–174. doi: 10.1620/tjem.214.167. [DOI] [PubMed] [Google Scholar]
- 60.Paciga M, Hirvi ER, James K, Wagner GF. Characterization of big stanniocalcin variants in mammalian adipocytes and adrenocortical cells. Am J Physiol Endocrinol Metab. 2005;289:E197–205. doi: 10.1152/ajpendo.00581.2004. [DOI] [PubMed] [Google Scholar]
- 61.Robenek H, Robenek MJ, Troyer D. PAT family proteins pervade lipid droplet cores. J Lipid Res. 2005;46:1331–1338. doi: 10.1194/jlr.M400323-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Dvorak AM. Mast cell secretory granules and lipid bodies contain the necessary machinery important for the in situ synthesis of proteins. Chem Immunol Allergy. 2005;85:252–315. doi: 10.1159/000086520. [DOI] [PubMed] [Google Scholar]
- 63.Dvorak AM, Morgan ES, Weller PF. RNA is closely associated with human mast cell lipid bodies. Histol Histopathol. 2003;18:943–968. doi: 10.14670/HH-18.943. [DOI] [PubMed] [Google Scholar]
- 64.Beller M, Riedel D, Jansch L, Dieterich G, Wehland J, Jackle H, Kuhnlein RP. Characterization of the Drosophila lipid droplet subproteome. Mol Cell Proteomics. 2006;5:1082–1094. doi: 10.1074/mcp.M600011-MCP200. [DOI] [PubMed] [Google Scholar]
- 65.Gronke S, Beller M, Fellert S, Ramakrishnan H, Jackle H, Kuhnlein RP. Control of fat storage by a Drosophila PAT domain protein. Curr Biol. 2003;13:603–606. doi: 10.1016/s0960-9822(03)00175-1. [DOI] [PubMed] [Google Scholar]
- 66.Sato S, Fukasawa M, Yamakawa Y, Natsume T, Suzuki T, Shoji I, Aizaki H, Miyamura T, Nishijima M. Proteomic profiling of lipid droplet proteins in hepatoma cell lines expressing hepatitis C virus core protein. J Biochem. 2006;139:921–930. doi: 10.1093/jb/mvj104. [DOI] [PubMed] [Google Scholar]
- 67.Cermelli S, Guo Y, Gross SP, Welte MA. The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr Biol. 2006;16:1783–1795. doi: 10.1016/j.cub.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 68.Binns D, Januszewski T, Chen Y, Hill J, Markin VS, Zhao Y, Gilpin C, Chapman KD, Anderson RG, Goodman JM. An intimate collaboration between peroxisomes and lipid bodies. J Cell Biol. 2006;173:719–731. doi: 10.1083/jcb.200511125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bozza PT, Melo RC, Bandeira-Melo C. Leukocyte lipid bodies regulation and function: Contribution to allergy and host defense. Pharmacol Ther. 2007;113:30–49. doi: 10.1016/j.pharmthera.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 70.Bozza PT, Payne JL, Goulet JL, Weller PF. Mechanisms of platelet-activating factor-induced lipid body formation: requisite roles for 5-lipoxygenase and de novo protein synthesis in the compartmentalization of neutrophil lipids. J Exp Med. 1996;183:1515–1525. doi: 10.1084/jem.183.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weller PF, Ryeom SW, Picard ST, Ackerman SJ, Dvorak AM. Cytoplasmic lipid bodies of neutrophils: formation induced by cis-unsaturated fatty acids and mediated by protein kinase C. J Cell Biol. 1991;113:137–146. doi: 10.1083/jcb.113.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bozza PT, Weller PF. Arachidonyl trifluoromethyl ketone induces lipid body formation in leukocytes. Prostaglandins Leukot Essent Fatty Acids. 2001;64:227–230. doi: 10.1054/plef.2001.0264. [DOI] [PubMed] [Google Scholar]
- 73.de Assis EF, Silva AR, Caiado LF, Marathe GK, Zimmerman GA, Prescott SM, McIntyre TM, Bozza PT, de Castro-Faria-Neto HC. Synergism between platelet-activating factor-like phospholipids and peroxisome proliferator-activated receptor gamma agonists generated during low density lipoprotein oxidation that induces lipid body formation in leukocytes. J Immunol. 2003;171:2090–2098. doi: 10.4049/jimmunol.171.4.2090. [DOI] [PubMed] [Google Scholar]
- 74.Bandeira-Melo C, Sugiyama K, Woods LJ, Phoofolo M, Center DM, Cruikshank WW, Weller PF. IL-16 promotes leukotriene C(4) and IL-4 release from human eosinophils via CD4- and autocrine CCR3-chemokine-mediated signaling. J Immunol. 2002;168:4756–4763. doi: 10.4049/jimmunol.168.9.4756. [DOI] [PubMed] [Google Scholar]
- 75.Bartemes KR, McKinney S, Gleich GJ, Kita H. Endogenous platelet-activating factor is critically involved in effector functions of eosinophils stimulated with IL-5 or IgG. J Immunol. 1999;162:2982–2989. [PubMed] [Google Scholar]
- 76.Bandeira-Melo C, Herbst A, Weller PF. Eotaxins. Contributing to the diversity of eosinophil recruitment and activation. Am J Respir Cell Mol Biol. 2001;24:653–657. doi: 10.1165/ajrcmb.24.6.f209. [DOI] [PubMed] [Google Scholar]
- 77.D’Avila H, Almeida PE, Roque NR, Castro-Faria-Neto HC, Bozza PT. Toll-like receptor-2-mediated C-C chemokine receptor 3 and eotaxin-driven eosinophil influx induced by Mycobacterium bovis BCG pleurisy. Infect Immun. 2007;75:1507–1511. doi: 10.1128/IAI.01326-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 79.Schmitz G, Grandl M. Lipid homeostasis in macrophages - implications for atherosclerosis. Rev Physiol Biochem Pharmacol. 2008;160:93–125. doi: 10.1007/112_2008_802. [DOI] [PubMed] [Google Scholar]
- 80.Buechler C, Ritter M, Duong CQ, Orso E, Kapinsky M, Schmitz G. Adipophilin is a sensitive marker for lipid loading in human blood monocytes. Biochim Biophys Acta. 2001;1532:97–104. doi: 10.1016/s1388-1981(01)00121-4. [DOI] [PubMed] [Google Scholar]
- 81.Kapinsky M, Torzewski M, Buchler C, Duong CQ, Rothe G, Schmitz G. Enzymatically degraded LDL preferentially binds to CD14(high) CD16(+) monocytes and induces foam cell formation mediated only in part by the class B scavenger-receptor CD36. Arterioscler Thromb Vasc Biol. 2001;21:1004–1010. doi: 10.1161/01.atv.21.6.1004. [DOI] [PubMed] [Google Scholar]
- 82.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 83.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Villiers WJ, Smart EJ. Macrophage scavenger receptors and foam cell formation. J Leukoc Biol. 1999;66:740–746. doi: 10.1002/jlb.66.5.740. [DOI] [PubMed] [Google Scholar]
- 85.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hodgkinson CP, Laxton RC, Patel K, Ye S. Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:2275–2281. doi: 10.1161/ATVBAHA.108.175992. [DOI] [PubMed] [Google Scholar]
- 87.Zhao Z, de Beer MC, Cai L, Asmis R, de Beer FC, de Villiers WJ, van der Westhuyzen DR. Low-density lipoprotein from apolipoprotein E-deficient mice induces macrophage lipid accumulation in a CD36 and scavenger receptor class A-dependent manner. Arterioscler Thromb Vasc Biol. 2005;25:168–173. doi: 10.1161/01.ATV.0000149145.00865.d9. [DOI] [PubMed] [Google Scholar]
- 88.Silva AR, de Assis EF, Caiado LF, Marathe GK, Bozza MT, McIntyre TM, Zimmerman GA, Prescott SM, Bozza PT, Castro-Faria-Neto HC. Monocyte chemoattractant protein-1 and 5-lipoxygenase products recruit leukocytes in response to platelet-activating factor-like lipids in oxidized low-density lipoprotein. J Immunol. 2002;168:4112–4120. doi: 10.4049/jimmunol.168.8.4112. [DOI] [PubMed] [Google Scholar]
- 89.Chen JS, Greenberg AS, Tseng YZ, Wang SM. Possible involvement of protein kinase C in the induction of adipose differentiation-related protein by Sterol ester in RAW 264.7 macrophages. J Cell Biochem. 2001;83:187–199. doi: 10.1002/jcb.1225. [DOI] [PubMed] [Google Scholar]
- 90.Leonarduzzi G, Chiarpotto E, Biasi F, Poli G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol Nutr Food Res. 2005;49:1044–1049. doi: 10.1002/mnfr.200500090. [DOI] [PubMed] [Google Scholar]
- 91.Boullier A, Friedman P, Harkewicz R, Hartvigsen K, Green SR, Almazan F, Dennis EA, Steinberg D, Witztum JL, Quehenberger O. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res. 2005;46:969–976. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- 92.Xu W, Yu L, Zhou W, Luo M. Resistin increases lipid accumulation and CD36 expression in human macrophages. Biochem Biophys Res Commun. 2006;351:376–382. doi: 10.1016/j.bbrc.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 93.Maya-Monteiro CM, Almeida PE, D’Avila H, Martins AS, Rezende AP, Castro-Faria-Neto H, Bozza PT. Leptin induces macrophage lipid body formation by a phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent mechanism. J Biol Chem. 2008;283:2203–2210. doi: 10.1074/jbc.M706706200. [DOI] [PubMed] [Google Scholar]
- 94.Maya-Monteiro CM, Bozza PT. Leptin and mTOR: partners in metabolism and inflammation. Cell Cycle. 2008;7:1713–1717. doi: 10.4161/cc.7.12.6157. [DOI] [PubMed] [Google Scholar]
- 95.Dvorak AM. In Basophil and mast cell degranulation and recovery. Plenum Press; New York: 1991. Biochemical contents of granules and lipid bodies - Two distinctive organelles found in basophils and mast cells. [Google Scholar]
- 96.Franke WW, Hergt M, Grund C. Rearrangement of the vimentin cytoskeleton during adipose conversion: formation of an intermediate filament cage around lipid globules. Cell. 1987;49:131–141. doi: 10.1016/0092-8674(87)90763-x. [DOI] [PubMed] [Google Scholar]
- 97.Mermelstein CS, Guma FC, Mello TG, Fortuna VA, Guaragna RM, Costa ML, Borojevic R. Induction of the lipocyte phenotype in murine hepatic stellate cells: reorganisation of the actin cytoskeleton. Cell Tissue Res. 2001;306:75–83. doi: 10.1007/s004410100428. [DOI] [PubMed] [Google Scholar]
- 98.Bostrom P, Rutberg M, Ericsson J, Holmdahl P, Andersson L, Frohman MA, Boren J, Olofsson SO. Cytosolic lipid droplets increase in size by microtubule-dependent complex formation. Arterioscler Thromb Vasc Biol. 2005;25:1945–1951. doi: 10.1161/01.ATV.0000179676.41064.d4. [DOI] [PubMed] [Google Scholar]
- 99.Melo RC, D’Avila H, Fabrino DL, Almeida PE, Bozza PT. Macrophage lipid body induction by Chagas disease in vivo: putative intracellular domains for eicosanoid formation during infection. Tissue Cell. 2003;35:59–67. doi: 10.1016/s0040-8166(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 100.D’Avila H, Maya-Monteiro CM, Bozza PT. Lipid bodies in innate immune response to bacterial and parasite infections. Int Immunopharmacol. 2008;8:1308–1315. doi: 10.1016/j.intimp.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 101.Cao F, Castrillo A, Tontonoz P, Re F, Byrne GI. Chlamydia pneumoniae--induced macrophage foam cell formation is mediated by Toll-like receptor 2. Infect Immun. 2007;75:753–759. doi: 10.1128/IAI.01386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 103.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 104.Inazawa Y, Nakatsu M, Yasugi E, Saeki K, Yuo A. Lipid droplet formation in human myeloid NB4 cells stimulated by all trans retinoic acid and granulocyte colony-stimulating factor: possible involvement of peroxisome proliferator-activated receptor gamma. Cell Struct Funct. 2003;28:487–493. doi: 10.1247/csf.28.487. [DOI] [PubMed] [Google Scholar]
- 105.Targett-Adams P, McElwee MJ, Ehrenborg E, Gustafsson MC, Palmer CN, McLauchlan J. A PPAR response element regulates transcription of the gene for human adipose differentiation-related protein. Biochim Biophys Acta. 2005;1728:95–104. doi: 10.1016/j.bbaexp.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 106.Wei P, Taniguchi S, Sakai Y, Imamura M, Inoguchi T, Nawata H, Oda S, Nakabeppu Y, Nishimura J, Ikuyama S. Expression of adipose differentiation-related protein (ADRP) is conjointly regulated by PU.1 and AP-1 in macrophages. J Biochem. 2005;138:399–412. doi: 10.1093/jb/mvi136. [DOI] [PubMed] [Google Scholar]
- 107.Larigauderie G, Furman C, Jaye M, Lasselin C, Copin C, Fruchart JC, Castro G, Rouis M. Adipophilin enhances lipid accumulation and prevents lipid efflux from THP-1 macrophages: potential role in atherogenesis. Arterioscler Thromb Vasc Biol. 2004;24:504–510. doi: 10.1161/01.ATV.0000115638.27381.97. [DOI] [PubMed] [Google Scholar]
- 108.Wang SM, Hwang RD, Greenberg AS, Yeo HL. Temporal and spatial assembly of lipid droplet-associated proteins in 3T3-L1 preadipocytes. Histochem Cell Biol. 2003;120:285–292. doi: 10.1007/s00418-003-0575-7. [DOI] [PubMed] [Google Scholar]
- 109.Gross DN, Miyoshi H, Hosaka T, Zhang HH, Pino EC, Souza S, Obin M, Greenberg AS, Pilch PF. Dynamics of lipid droplet-associated proteins during hormonally stimulated lipolysis in engineered adipocytes: stabilization and lipid droplet binding of adipocyte differentiation-related protein/adipophilin. Mol Endocrinol. 2006;20:459–466. doi: 10.1210/me.2005-0323. [DOI] [PubMed] [Google Scholar]
- 110.Chung S, Brown JM, Sandberg MB, McIntosh M. Trans-10,cis-12 CLA increases adipocyte lipolysis and alters lipid droplet-associated proteins: role of mTOR and ERK signaling. J Lipid Res. 2005;46:885–895. doi: 10.1194/jlr.M400476-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 112.Fujimoto T, Ohsaki Y. Proteasomal and autophagic pathways converge on lipid droplets. Autophagy. 2006;2:299–301. doi: 10.4161/auto.2904. [DOI] [PubMed] [Google Scholar]
- 113.Ohsaki Y, Cheng J, Fujita A, Tokumoto T, Fujimoto T. Cytoplasmic lipid droplets are sites of convergence of proteasomal and autophagic degradation of apolipoprotein B. Mol Biol Cell. 2006;17:2674–2683. doi: 10.1091/mbc.E05-07-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pinheiro RO, Nunes MP, Pinheiro CS, D’Avila H, Bozza PT, Takiya CM, Corte-Real S, Freire-de-Lima CG, Dosreis GA. Induction of autophagy correlates with increased parasite load of Leishmania amazonensis in BALB/c but not C57BL/6 macrophages. Microbes Infect. 2008 doi: 10.1016/j.micinf.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 115.White MA, Anderson RG. Signaling networks in living cells. Annu Rev Pharmacol Toxicol. 2005;45:587–603. doi: 10.1146/annurev.pharmtox.45.120403.095807. [DOI] [PubMed] [Google Scholar]
- 116.Bozza PT, Bandeira-Melo C. Mechanisms of Leukocyte Lipid Body Formation and Function in Inflammation. Mem Inst Oswaldo Cruz. 2005;100:113–120. doi: 10.1590/s0074-02762005000900020. [DOI] [PubMed] [Google Scholar]
- 117.Yaqoob P. Fatty acids as gatekeepers of immune cell regulation. Trends Immunol. 2003;24:639–645. doi: 10.1016/j.it.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 118.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 119.Dvorak AM, Dvorak HF, Peters SP, Shulman ES, MacGlashan DW, Jr, Pyne K, Harvey VS, Galli SJ, Lichtenstein LM. Lipid bodies: cytoplasmic organelles important to arachidonate metabolism in macrophages and mast cells. J Immunol. 1983;131:2965–2976. [PubMed] [Google Scholar]
- 120.Weller PF, Monahan-Earley RA, Dvorak HF, Dvorak AM. Cytoplasmic lipid bodies of human eosinophils. Subcellular isolation and analysis of arachidonate incorporation. Am J Pathol. 1991;138:141–148. [PMC free article] [PubMed] [Google Scholar]
- 121.Galli SJ, Dvorak AM, Peters SP, Shulman ES, MacGlashan DW, Jr, Isomura T, Pyne K, Harvey VS, Hammel I, Lichtenstein LM, Dvorak HF. Lipid bodies: widely distributed cytoplasmic structures that represent preferential nonmembrane repositories of exogenous [3H] arachidonic acid incorporeted by mast cells, macrophages and other cell types. In: Bailey JM, editor. Prostaglandins, leukotrienes, and lipoxins. Plenum Publishing; New York: 1985. pp. 221–239. [Google Scholar]
- 122.Plotkowski MC, Brandao BA, de Assis MC, Feliciano LF, Raymond B, Freitas C, Saliba AM, Zahm JM, Touqui L, Bozza PT. Lipid body mobilization in the ExoU-induced release of inflammatory mediators by airway epithelial cells. Microb Pathog. 2008;45:30–37. doi: 10.1016/j.micpath.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 123.Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 124.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A2 family. Prog Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 125.Wooten RE, Willingham MC, Daniel LW, Leslie CC, Rogers LC, Sergeant S, O’Flaherty JT. Novel translocation responses of cytosolic phospholipase A(2)alpha fluorescent proteins. Biochim Biophys Acta. 2008;1783:1544–1550. doi: 10.1016/j.bbamcr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johnson MM, Vaughn B, Triggiani M, Swan DD, Fonteh AN, Chilton FH. Role of arachidonyl triglycerides within lipid bodies in eicosanoid formation by human polymorphonuclear cells. Am J Respir Cell Mol Biol. 1999;21:253–258. doi: 10.1165/ajrcmb.21.2.3489. [DOI] [PubMed] [Google Scholar]
- 127.Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999;274:32672–32679. doi: 10.1074/jbc.274.46.32672. [DOI] [PubMed] [Google Scholar]
- 128.Roulin K, Hagens G, Hotz R, Saurat JH, Veerkamp JH, Siegenthaler G. The fatty acid-binding heterocomplex FA-p34 formed by S100A8 and S100A9 is the major fatty acid carrier in neutrophils and translocates from the cytosol to the membrane upon stimulation. Exp Cell Res. 1999;247:410–421. doi: 10.1006/excr.1998.4382. [DOI] [PubMed] [Google Scholar]
- 129.van Manen HJ, Kraan YM, Roos D, Otto C. Single-cell Raman and fluorescence microscopy reveal the association of lipid bodies with phagosomes in leukocytes. Proc Natl Acad Sci U S A. 2005;102:10159–10164. doi: 10.1073/pnas.0502746102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berthier S, Paclet MH, Lerouge S, Roux F, Vergnaud S, Coleman AW, Morel F. Changing the conformation state of cytochrome b558 initiates NADPH oxidase activation: MRP8/MRP14 regulation. J Biol Chem. 2003;278:25499–25508. doi: 10.1074/jbc.M209755200. [DOI] [PubMed] [Google Scholar]
- 131.Weller PF, Bozza PT, Yu W, Dvorak AM. Cytoplasmic lipid bodies in eosinophils: central roles in eicosanoid generation. Int Arch Allergy Immunol. 1999;118:450–452. doi: 10.1159/000024161. [DOI] [PubMed] [Google Scholar]
- 132.Bandeira-Melo C, Bozza PT, Weller PF. The cellular biology of eosinophil eicosanoid formation and function. J Allergy Clin Immunol. 2002;109:393–400. doi: 10.1067/mai.2002.121529. [DOI] [PubMed] [Google Scholar]
- 133.D’Avila H, Melo RC, Parreira GG, Werneck-Barroso E, Castro Faria Neto HC, Bozza PT. Mycobacterium bovis BCG induces TLR 2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J Immunol. 2006;176:3087–3097. doi: 10.4049/jimmunol.176.5.3087. [DOI] [PubMed] [Google Scholar]
- 134.Seachord CL, VandeVoort CA, Duffy DM. Adipose differentiation-related protein: a gonadotropin- and prostaglandin-regulated protein in primate periovulatory follicles. Biol Reprod. 2005;72:1305–1314. doi: 10.1095/biolreprod.104.037523. [DOI] [PubMed] [Google Scholar]
- 135.Meadows JW, Eis AL, Brockman DE, Myatt L. Expression and localization of prostaglandin E synthase isoforms in human fetal membranes in term and preterm labor. J Clin Endocrinol Metab. 2003;88:433–439. doi: 10.1210/jc.2002-021061. [DOI] [PubMed] [Google Scholar]
- 136.Scarfo LM, Weller PF, Farber HW. Induction of endothelial cell cytoplasmic lipid bodies during hypoxia. Am J Physiol Heart Circ Physiol. 2001;280:H294–301. doi: 10.1152/ajpheart.2001.280.1.H294. [DOI] [PubMed] [Google Scholar]
- 137.Bandeira-Melo C, Woods LJ, Phoofolo M, Weller PF. Intracrine cysteinyl leukotriene receptor-mediated signaling of eosinophil vesicular transport-mediated interleukin-4 secretion. J Exp Med. 2002;196:841–850. doi: 10.1084/jem.20020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bandeira-Melo C, Weller PF. Mechanisms of eosinophil cytokine release. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1) doi: 10.1590/s0074-02762005000900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beil WJ, Weller PF, Peppercorn MA, Galli SJ, Dvorak AM. Ultrastructural immunogold localization of subcellular sites of TNF-alpha in colonic Crohn’s disease. J Leukoc Biol. 1995;58:284–298. doi: 10.1002/jlb.58.3.284. [DOI] [PubMed] [Google Scholar]
- 140.Dvorak AM, Morgan ES, Weller PF. Ultrastructural immunolocalization of basic fibroblast growth factor to lipid bodies and secretory granules in human mast cells. Histochem J. 2001;33:397–402. doi: 10.1023/a:1013771827069. [DOI] [PubMed] [Google Scholar]
- 141.Lim KG, Wan HC, Bozza PT, Resnick MB, Wong DT, Cruikshank WW, Kornfeld H, Center DM, Weller PF. Human eosinophils elaborate the lymphocyte chemoattractants. IL-16 (lymphocyte chemoattractant factor) and RANTES. J Immunol. 1996;156:2566–2570. [PubMed] [Google Scholar]
- 142.Melo RC, Perez SA, Spencer LA, Dvorak AM, Weller PF. Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic. 2005;6:866–879. doi: 10.1111/j.1600-0854.2005.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Melo RC, Spencer LA, Perez SA, Ghiran I, Dvorak AM, Weller PF. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6:1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nat Rev Immunol. 2006;6:919–929. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- 145.Bozza PT, Pacheco P, Yu W, Weller PF. NS-398: cyclooxygenase-2 independent inhibition of leukocyte priming for lipid body formation and enhanced leukotriene generation. Prostaglandins Leukot Essent Fatty Acids. 2002;67:237–244. doi: 10.1054/plef.2002.0425. [DOI] [PubMed] [Google Scholar]
- 146.Vieira-de-Abreu A, Amendoeira FC, Gomes GS, Zanon C, Chedier LM, Figueiredo MR, Kaplan MA, Frutuoso VS, Castro-Faria-Neto HC, Weller PF, Bandeira-Melo C, Bozza PT. Anti-allergic properties of the bromeliaceae Nidularium procerum: inhibition of eosinophil activation and influx. Int Immunopharmacol. 2005;5:1966–1974. doi: 10.1016/j.intimp.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 147.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173:1503–1510. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- 148.Busse W, Kraft M. Cysteinyl leukotrienes in allergic inflammation: strategic target for therapy. Chest. 2005;127:1312–1326. doi: 10.1378/chest.127.4.1312. [DOI] [PubMed] [Google Scholar]
- 149.Arm JP. Leukotriene generation and clinical implications. Allergy Asthma Proc. 2004;25:37–42. [PubMed] [Google Scholar]
- 150.Henderson WR, Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med. 2002;165:108–116. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 151.Paul A, Chang BH, Li L, Yechoor VK, Chan L. Deficiency of adipose differentiation-related protein impairs foam cell formation and protects against atherosclerosis. Circ Res. 2008;102:1492–1501. doi: 10.1161/CIRCRESAHA.107.168070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Namatame I, Tomoda H, Ishibashi S, Omura S. Antiatherogenic activity of fungal beauveriolides, inhibitors of lipid droplet accumulation in macrophages. Proc Natl Acad Sci U S A. 2004;101:737–742. doi: 10.1073/pnas.0307757100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146:108–113. [PubMed] [Google Scholar]
- 154.Renz H, Gong JH, Schmidt A, Nain M, Gemsa D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J Immunol. 1988;141:2388–2393. [PubMed] [Google Scholar]
- 155.D’Avila H, Roque NR, Cardoso RM, Castro-Faria-Neto HC, Melo RC, Bozza PT. Neutrophils recruited to the site of Mycobacterium bovis BCG infection undergo apoptosis and modulate lipid body biogenesis and prostaglandin E production by macrophages. Cell Microbiol. 2008;10:2589–2604. doi: 10.1111/j.1462-5822.2008.01233.x. [DOI] [PubMed] [Google Scholar]