Abstract
Purpose
Tumor regression has been observed in some patients with metastatic renal cell carcinoma (RCC) after nonmyeloablative allogeneic hematopoietic cell transplantation (HCT). Cellular and molecular characterization of antigens recognized by tumor-reactive T cells isolated from responding patients could potentially provide insight into the mechanisms of tumor regression.
Experimental Design
CD8+ cytotoxic T lymphocyte (CTL) clones that recognized a novel RCC-associated minor histocompatibility (H) antigen presented by HLA-A*0201 were isolated from two patients with metastatic RCC who experienced tumor regression or stable disease following nonmyeloablative allogeneic HCT. These clones were used to screen a cDNA library and isolate the unique cDNA encoding the antigen.
Results
An alternative open reading frame in the C19orf48 gene located on chromosome 19q13 encodes the HLA-A*0201-restricted minor H antigen recognized by the RCC-reactive T cells. Differential T cell recognition of donor- and recipient-derived target cells is attributable to a nonsynonymous single nucleotide polymorphism within the nucleotide interval that encodes the antigenic peptide. Assays for gene expression and CTL recognition demonstrated that the C19orf48-encoded peptide is widely expressed in renal tumors and solid tumors of other histologies. The antigenic peptide can be processed for CTL recognition via both TAP-dependent and TAP-independent pathways.
Conclusions
Donor T cell responses against the HLA-A*0201-restricted minor H antigen encoded by C19orf48 may contribute to RCC regression after MHC-matched allogeneic HCT.
Keywords: Minor histocompatibility antigen, Cytotoxic T-lymphocyte, Graft-versus-tumor response, Transporter Associated with Antigen Processing, Renal Cell Carcinoma
Introduction
Allogeneic hematopoietic cell transplantation (HCT) accomplished with a nonmyeloablative conditioning regimen can result in graft-versus-tumor (GVT) as well as graft-versus-host (GVH) reactions thought to be mediated by an alloreactive T cell response (1). In recipients of allogeneic HCT from donors who are matched at the Major Histocompatibility Complex (MHC), donor-derived T cells can recognize recipient minor histocompatibility (H) antigens, MHC-associated peptides that are encoded by polymorphic genes (2). Most non-sex linked minor H antigens presented by MHC class I molecules and recognized by CD8+ CTL are created by nonsynonymous single nucleotide polymorphisms (SNPs) in the coding sequences of normal genes that cause amino acid changes within the CTL epitope sequence (3–11). More than 3.1 million validated human SNPs are catalogued in the HapMap Phase II database, including over 17,000 nonsynonymous SNPs mapped to coding sequences (12). These data therefore suggest that the total pool of polymorphic peptide sequences that may function as minor H antigens in the human population is extremely large. However, less than 20 genes encoding human minor H antigens have been identified to date (13).
Nonmyeloablative allogeneic HCT has been investigated as a novel form of adoptive immunotherapy for patients with metastatic renal cell carcinoma (RCC). A review summarizing results of nonmyeloablative HCT for RCC patients noted an overall response rate of 20%, comprising primarily partial responses, in over 200 total patients (14). Several features of the regression of RCC observed following reduced-intensity allogeneic HCT have suggested a critical role for donor T cells. The conditioning regimens employed in these trials included conditioning agents such as fludarabine that lacked activity against RCC. Tumor regression was typically delayed for months after transplantation, and correlated with the withdrawal of immune suppression and the development of full donor chimerism of CD3+ cells. Tumor regression was also associated with the development of clinically significant GVHD in the majority of responding patients (14). Studies from our laboratory have provided compelling support for the hypothesis that minor H antigen-specific CTL could contribute to RCC regression after allogeneic HCT by showing that CD8+ CTL specific for multiple distinct minor H antigens could be detected in the peripheral blood of five patients with metastatic RCC treated at our institution. A subset of these clones demonstrated MHC-restricted recognition of RCC tumor cells in vitro (15).
Further development of allogeneic cellular immunotherapy for RCC would benefit from the cellular and molecular dissection of GVT effects that may provide insight for selectively manipulating a GVT response. We previously described a novel minor H antigen presented by HLA-A*0201 that was expressed on both RCC tumor cells and Epstein-Barr virus-transformed lymphoblastoid cell lines (EBV-LCL), and recognized by the CTL clones 2B3 and 12B3. These CTL clones were isolated from two patients with metastatic RCC who underwent MHC-matched allogeneic HCT at our institution and experienced posttransplant GVT activity, with one patient achieving a near complete response of bulky metastatic disease, and the other exhibiting stable disease and survival 6 years posttransplant. Genetic linkage analysis mapped the location of the gene encoding the minor H antigen recognized by these two CTL clones to a marker at chromosome 19q13 (15). However, the 0.5 Mb region at 19q13 is particularly gene-dense. Thus, to identify the gene encoding the epitope recognized by these CTL clones, the alternate strategy of cDNA expression cloning was pursued.
Materials and Methods
Cell Lines
Isolation and maintenance of the CD8+ CTL clones 2B3 and 12B3 has been described (15). The COS-7 cell line was maintained as described (16). The T2 cell line and T2 stably transfected with plasmid vectors encoding TAP1 and TAP2 have been described (17). The RCC lines A-498, CAKI-2, and 786-0, the breast carcinoma lines HTB-21, MDA-231, and MCF7, and the colon carcinoma line SW480 were obtained from ATCC (Rockville, MD). The RCC lines STAR, FARP, and LYOG were a gift of Dr. Richard Childs (National Heart, Lung, and Blood Institute, NIH, Bethesda, MD). The RCC lines 1.11, 1.18, 1.24, and 1.26 were a gift of Dr. Elizabeth Jaffee (The Johns Hopkins University, Baltimore, MD). The RCC lines LB1828, BB65, BB64, LB1047, LB996, GERK, LE9211, and DOBSKI were isolated in the laboratory of Dr. B. Van den Eynde (Ludwig Institute for Cancer Research (LICR), Brussels, Belgium). The lines LB1828, BB65, BB64, and LB996 were identified as TREP, CAJE, JAUP, and LOBE RCC, respectively, in a previous report (15). Isolation of RCC lines SST125 and SST140 was previously described (15). The RCC line SST207 was isolated at the FHCRC from malignant ascites fluid. RCC tumor lines were maintained in culture as previously described (15). The ovarian carcinoma line H3729 was a gift of Dr. Ingegerd Hellstrom (University of Washington, Seattle, WA). The melanoma tumor line SK29.1 was a gift of Dr. Pierre G. Coulie, (Université Catholique de Louvain and LICR, Brussels, Belgium). The melanoma lines A375, 526mel and 624mel were a gift of Dr. Yutaka Kawakami (Keio University School of Medicine, Tokyo, Japan). Ovarian, breast, melanoma, and colon carcinoma lines were maintained in the same culture media as RCC tumor cells. Isolation and maintenance of dermal fibroblast and EBV-transformed LCL lines has been described (18).
Transient Transfection of COS-7 Cells and Cytokine Release Assays
A cDNA library was generated from the LB373-MEL melanoma tumor line and expressed in the pcDNAI/Amp plasmid vector, as previously described (19). Minigene expression vectors were generated by PCR, or, for short constructs, by annealing complementary oligonucleotides designed with 3′ overhanging deoxyadenosines, and then cloned into pcDNA3.1/V5-His-TOPO (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Plasmid transfection from cDNA library stocks was carried out with 50 ng plasmid DNA and 1–2 ×104 COS-7 cells per microtiter well using the DEAE-dextran-choloroquine method (20). One to two ×104 CTL were added to each well 48 hrs. after COS-7 transfection and cultured overnight. For library screening, supernatants were first assayed for TNF by WEHI 164 clone 13 cytotoxicity, as described (16, 21). Supernatants from cDNA library screening that were positive for TNF and supernatants from minigene transfection experiments were assayed for interferon (IFN)-γ by enzyme-linked immunosorbent assay (ELISA), (Endogen, Rockford, IL).
Quantitative Real-Time RT-PCR Analysis of C19orf48 Transcripts NM_199249 and NM_199250
Total RNA was prepared from cell culture samples of normal or malignant cells using Trizol (Invitrogen), or using an RNeasy Mini Kit (QIAGEN) following the manufacturer’s instructions. First-strand cDNA was synthesized from 1 to 4 μg DNase-treated total RNA using Oligo-dT and SuperScript II reverse transcriptase (all from Invitrogen). Additional normal human tissue and blood cDNA samples were obtained (human Multiple Tissue cDNA panels I and II, and Human Blood Fractions, BD Biosciences/Clontech, Palo Alto, CA). Allele specific primer sequences for NM_199249 and NM_199250 were: NM_199249 forward primer, 5′-GAGAAATGCTGGGAGACAGG-3′, NM_199249 reverse primer, 5′-GGACAGAGGCCAAGCTAACA-3′; NM_199250 forward primer, 5′-AGAAATGCTGGGGTGCAG-3′, NM_199250 reverse primer, 5′-AGTAACAGGCAGCCTCCTCTG-3′. NM_199249 and NM_199250 expression was evaluated for each sample in triplicate and normalized to GAPDH expression measured in triplicate wells run in the same tray with the following primers: GAPDH forward 5′-GAAGGTGAAGGTCGGAGTC-3′ and GAPDH reverse 5′-GAAGATGGTGATGGGATTTC-3′. Reactions were run in 25 μL volumes with RT2 SYBR Green/ROX qPCR Master Mix (SuperArray Bioscience Corporation, Frederick, MD), 400 nM forward and reverse primers, and 100 ng cDNA template for cell culture-derived samples or 2 ng of commercially prepared cDNA. Amplifications were performed on an Applied Biosystems 7900HT thermocycler. Cycling conditions were: 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 15 seconds and priming/extension at 60°C for 60 seconds. The threshold cycle (CT) was calculated using 7900HT version 2.2.2 SDS software (Applied Biosystems Incorporated, Foster City, CA) based on the default settings. CT values for triplicate reactions were averaged and relative NM_199249 or NM_199250 expression determined with the comparative CT method, using average CT values for NM_199249 or NM_199250 and GAPDH.
PCR-Restriction Fragment Length Polymorphism (RFLP) Assay to Determine Genotype at rs3745526
Genomic DNA was isolated from tumor cells with a QIAamp DNA Mini kit (QIAGEN) according to the manufacturer’s protocol. The forward primer 5′-GCACTGGGCTCATTAACCTT-3′ and reverse primer 5′-CGAGATAGCCAGCTCAGTGTC-3′ were used to amplify a 410 bp interval of the C19orf48 genomic sequence that includes rs3745526. Thermocycler parameters were 95°C denaturation for 5 minutes followed by 35 cycles of 95°C for 30 seconds, 62°C for 30 seconds and 72°C for 30 seconds and final extension at 72°C for 7 minutes. HinfI digestion of the PCR product amplified from the allele of C19orf48 with genotype T at rs3745526 produces two fragments of 291 and 119 bp, while the product amplified from the allele with genotype A at rs3745526 is insensitive to HinfI. Digested PCR products were analyzed by agarose gel electrophoresis and the genotype at rs3745526 was inferred from the banding pattern.
Results
Identification of C19orf48 by cDNA Expression Cloning as the Gene Encoding a Novel RCC-Associated Minor H Antigen
The 2B3 CTL clone was found to recognize the HLA-A*0201-expressing melanoma tumor cell line LB373-MEL (data not shown) from which a cDNA library had been previously generated (19). For library screening, plasmid DNA from 576 cDNA pools with ~100 cDNA clones each was tested for the ability to stimulate TNF-α and IFN-γ release from 2B3 CTL after transient transfection into COS-7 cells along with a cDNA encoding HLA-A*0201. Two positive pools were then subcloned and screened in a similar manner. One subclone, termed 95-4-F4, stimulated HLA-A*0201-dependent cytokine release from 2B3 CTL (data not shown).
Sequencing of the insert in 95-4-F4 identified a 1.4 kb sequence with >99% identity to a transcript of C19orf48 (RefSeq accession number NM_199250), a gene of unknown function encoded on chromosome 19q13. This chromosomal location is in immediate proximity to the KLK(AC) marker previously identified as tightly linked with the gene encoding the antigen recognized by 2B3 CTL (15). The 95-4-F4 sequence differed from that of NM_199250 at six noncontiguous nucleotide positions, all of which corresponded to previously identified single nucleotide polymorphisms (SNPs) (Table 1). The C19orf48 alleles in patient- and donor-derived LCL as well as in antigen-positive and negative tumor cell lines were sequenced and compared (data not shown). This analysis revealed that antigen-negative cell lines were homozygous at these six nucleotide positions for the allele corresponding to NM_199250. In contrast, patient-derived EBV-LCL and the antigen-positive LB373-MEL tumor from which the 95-4-F4 cDNA was derived were heterozygous at all six SNPs. Thus, on the basis of these data it was not possible to determine whether genotype at one of the six SNPs determined recognition by 2B3 CTL.
Table 1.
Single nucleotide polymorphisms in the NM_199250 transcript of C19orf48
To identify the nucleotide interval within the 95-4-F4 cDNA that encoded the antigenic peptide recognized by 2B3 CTL, a series of 5′ and 3′ deletion constructs derived from this cDNA were tested for HLA-A*0201-dependent CTL recognition in the COS-7 transfection assay. This analysis localized the epitope-encoding region to an interval in the 5′ portion of 95-4-F4 that spanned the most 5′ SNP (T ↔ A polymorphism at nucleotide 263 of the NM_199250 sequence, dbSNP reference rs3745526; Table 1). Similar deletion constructs encoding A263 (the allele carried by antigen-negative tumor lines) could not stimulate the 2B3 clone when transfected into COS-7 cells with HLA-A*0201 (data not shown). These data indicated that of the six SNPs located in the C19orf48 cDNA, only the nucleotide T263 was necessary for recognition by 2B3 CTL.
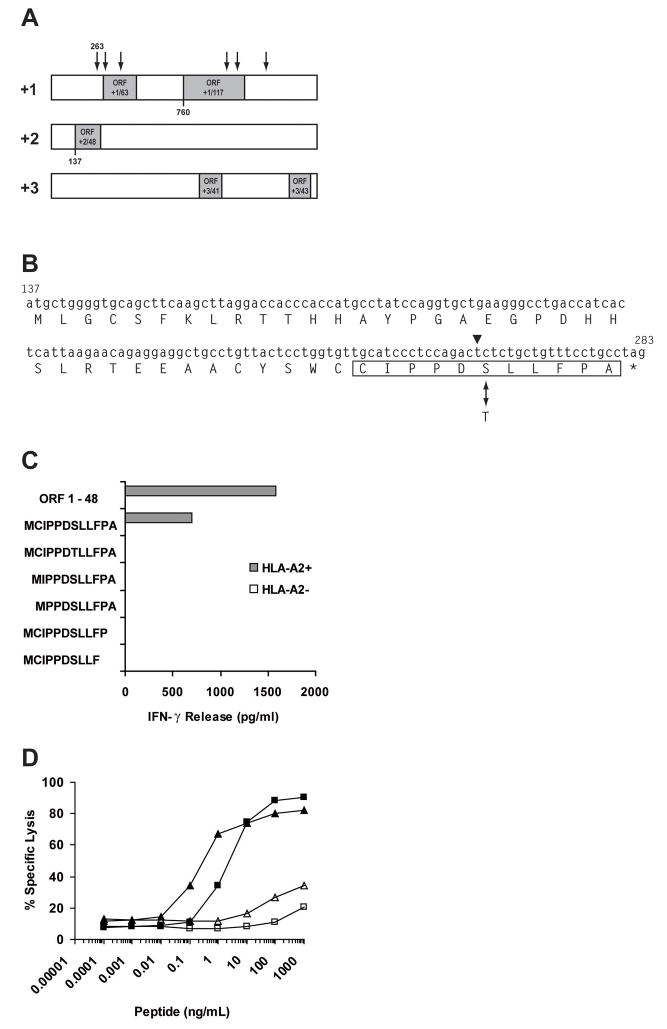
The rs3745526 polymorphism lies 5′ to the open reading frame (ORF) in frame +1 that encodes the predicted major protein product of the NM_199250 transcript of C19orf48. Analysis of reading frames +2 and +3 identified an ORF only in reading frame +2 that would encompass rs3745526 in a polypeptide sequence (Figure 1A). This +2 ORF encodes a predicted polypeptide of 48 residues in which the critical SNP creates a serine (antigenic) to threonine (nonantigenic) amino acid polymorphism (Figure 1B). A minigene construct encoding this 48-residue polypeptide with serine at the polymorphic residue stimulated IFN-γ release from 2B3 CTL in the COS-7 transfection assay (Figure 1C). Thus, the antigenic peptide encoded by C19orf48 was derived from a novel +2 ORF encoding a predicted polypeptide of 48 residues (termed ORF +2/48).
Figure 1. Identification of a CTL Epitope Within the 48-Residue Polypeptide Encoded by the +2 Reading Frame in NM_199250.
(A) Schematic representation of ORFs encoded by the sense strand of the NM_199250 transcript of C19orf48. The relative location in reading frames +1, +2, and +3 of ORFs predicted to encode polypeptides > 40 amino acids are indicated with shading. Nonsynonymous SNPs located at nucleotides 263, 311, 399, 1009, 1070, and 1235 are indicated by vertical arrows. (B) Nucleotide sequence and predicted 48-residue protein product of the ORF in the +2 reading frame starting at nucleotide 137 (ORF +2/48) and spanning the critical T↔A polymorphism at nucleotide 263 (arrowhead), which creates a serine (S) ↔threonine (T) amino acid polymorphism (double headed arrow) in the translated polypeptide. The 11mer peptide sequence recognized by the CTL clones 2B3 and 12B3 is boxed. (C) Minigenes encoding the entirety of ORF +2/48 or the shorter peptide sequences indicated were cloned into pCDNA3.1/V5-His-TOPO and transfected into COS-7 targets cells with or without co-transfection of a plasmid vector encoding HLA-A*0201. Transfections were carried out with Lipofectamine (Invitrogen) and 320 ng of plasmid DNA following the manufacturer’s protocol. 2B3 CTL were added to transfected COS-7 targets after 48 hours. Culture supernatants were harvested 18 hours after CTL addition and assayed for IFN-γ by ELISA. (D) Aliquots of 0.5 to 1.0 ×106 T2 target cells were labeled with 50–100 μCi of 51Cr overnight at 37°C and used as targets for CTL clones 2B3 (■, □) or 12B3 (▲, △) in a 4hr cytotoxicity assay as described (41). Synthetic peptides CIPPDSLLFPA (filled symbols) or CIPPDTLLFPA (open symbols) were added to T2 at the indicated concentrations before CTL addition. The E:T ratio was 5:1.
Identification of the HLA-A2-Associated CTL Epitope Encoded by ORF +2/48 in C19orf48
To identify the HLA-A2-associated peptide recognized by the 2B3 and 12B3 CTL clones, additional minigene constructs were created that corresponded to the C-terminal portion of ORF +2/48 encompassing rs3745526. Minigenes that encoded an initiating methionine followed by peptides of 13-, 12- or 11-residues comprising the C-terminal domain of ORF +2/48 and terminating in alanine were all recognized by the 2B3 CTL clone following transfection into COS-7 targets with HLA-A*0201 (Figure 1C and data not shown). Because the majority of reported HLA-A*0201-associated T cell epitopes are 9 or 10 residues in length, additional minigene constructs encoding N- or C-terminally truncated derivatives of the 11-mer sequence (CIPPDSLLFPA) were made. None of four constructs encoding derivative 10- or 9-mer peptides stimulated 2B3 CTL following transfection into COS-7 cells with HLA-A*0201 (Figure 1C). In addition, a minigene with nucleotide A263 encoding the 11mer peptide sequence CIPPDTLLFPA, corresponding to the product of the nonantigenic C19orf48 allele, was also not recognized by the 2B3 CTL clone. These data suggested that the minimal epitope recognized by the 2B3 CTL clone was the sequence CIPPDSLLFPA, the C-terminal 11 amino acids of the product of ORF +2/48 of C19orf48.
Most peptides that bind to HLA-A*0201 satisfy a sequence motif characterized by residues with aliphatic side chains at position 2 and at the C-terminus (22, 23). To determine if the peptide sequences encoded by the antigenic (CIPPDSLLFPA) or nonantigenic (CIPPDTLLFPA) alleles of C19orf48 could bind to HLA-A2, synthetic peptides corresponding to these sequences were tested for stabilization of HLA-A2 on the surface of T2 cells measured by flow cytometry with the HLA-A2-specific mAb BB7.2. The antigenic and nonantigenic peptides showed comparable stabilization of HLA-A2 expression on T2 cells, which modestly exceeded that observed with a positive control peptide, VLHDDLLEA, corresponding to the minor H antigen HA-1 (data not shown). Shorter derivatives of the undecameric peptide including nonamer (CIPPDSLLF) and octamer (CIPPDSLL) peptide sequences showed no capacity to stabilize cell surface HLA-A2 on T2 cells (data not shown). Taken together, the data from both minigene analysis as well as the binding of synthetic peptides to HLA-A2 support the requirement for an 11-mer peptide sequence with the C-terminal alanine serving as an anchor residue for peptide association with HLA-A*0201.
The peptides CIPPDSLLFPA and CIPPDTLLFPA were then tested for their ability to sensitize T2 cells or donor EBV-LCL targets for recognition by CTL clones 2B3 and 12B3 (Figure 1D and data not shown). Only the serine-containing 11-mer peptide sequence was able to efficiently sensitize target cells for lysis by C19orf48-specific clones. The peptide concentration required for half-maximal lysis of T2 cells by the 2B3 and 12B3 CTL clones was in the range of 0.1 to 1.0 ng/ml (Figure 1D).
Expression of C19orf48 in Malignant and Normal Tissues
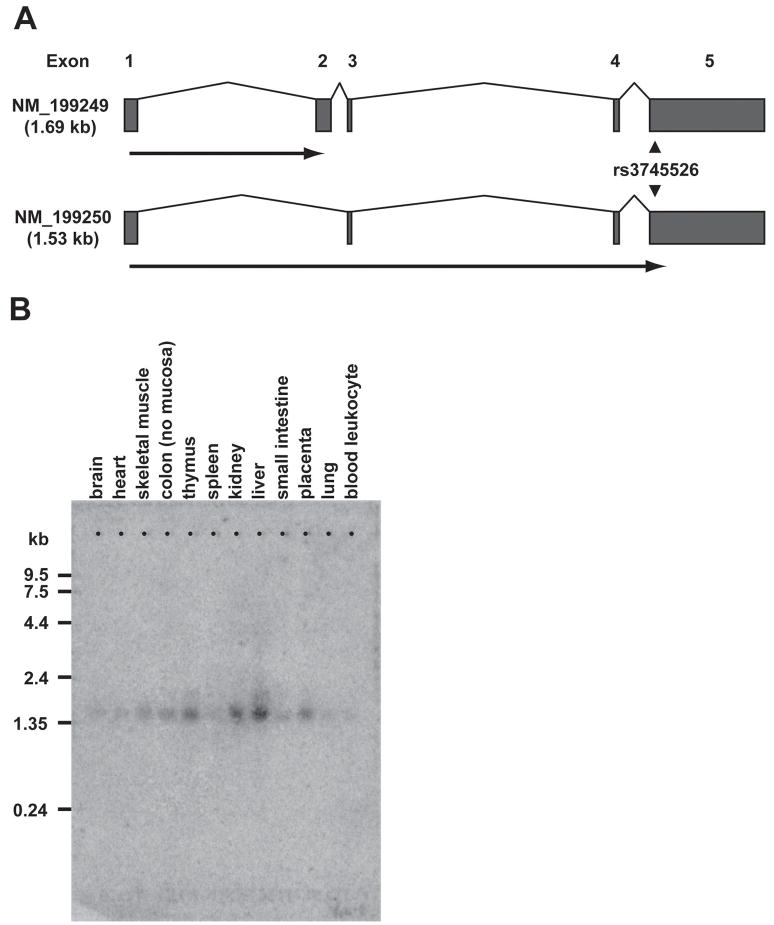
C19orf48 has two reported transcripts in the RefSeq database, which are derived from alternative splicing of 5 potential exons. These two transcripts differ by the inclusion (NM_199249) or exclusion (NM_199250) of exon 2. The novel 48-residue ORF in C19orf48 that encodes the epitope for 2B3 and 12B3 CTL is present only in the NM_199250 transcript, and spans all or part of the four exons that comprise this transcript. However, presence of the exon 2 sequence in the NM_199249 transcript introduces a stop codon 5′ of the interval that encodes the CIPPDSLLFPA CTL epitope within the +2 ORF (Figure 2A). We investigated the expression profile of C19orf48 transcripts NM_199249 and NM_199250 in both malignant and normal tissues. Northern blot analysis was performed on poly (A)+ RNA isolated from an array of normal human tissues. A probe spanning the epitope-encoding region identified a single band of approximately 1.5 kb size that was observed at variable intensity across the panel of tissues tested. A discrete second band corresponding to the 1.6 kb NM_199249 transcript was not seen (Figure 2B). Quantitative (Q)-PCR with transcript-specific primers designed to selectively amplify the NM_199249 or NM_199250 transcripts showed that the expression level of NM_199250 exceeded that of NM_199249 by 100- to 600-fold in samples of RCC tumor, normal proximal renal tubule epithelial cells, peripheral blood mononuclear cells, EBV- LCL, and dermal fibroblasts (data not shown), confirming that NM_199250 is the major transcript of C19orf48.
Figure 2. Two Alternative Transcripts from C19orf48.
(A) Schematic diagram of the C19orf48 genomic locus showing the intron/exon structure of the two RefSeq transcripts NM_199249 and NM_199250. The heavy horizontal arrows indicate the open reading frames (in frame +2) defined by the ATG at nucleotides 137–139 in both NM_199249 and NM_199250. The location of the nonsynonymous T↔A SNP at nucleotide 263 is noted (arrowheads). (B) An oligonucleotide spanning ORF +2/48 of the NM_199250 transcript of C19orf48 was amplified by PCR and purified (QIAGEN). This oligonucleotide was 32P-labeled using a Random Primed DNA Labeling Kit (Roche Diagnostics Corporation, Indianapolis, IN) and used to probe a Multiple Tissue Northern Blot nylon membrane (BD Biosciences/Clontech) loaded with poly (A)+ RNA isolated from 12 different human tissues. Hybridization was performed as described (41) at 68°C for one hour. Imaging was obtained by 7-day exposure on a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Q-PCR analysis of NM_199250 expression with transcript-specific primers was performed for 21 RCC tumor cell lines. The RCC tumor line A-498, which expresses cell surface HLA-A2 and is lysed in cytotoxicity assays by both the 2B3 and 12B3 CTL clones, was chosen as a reference standard for comparison of NM_199250 transcript expression across the panel of RCC tumor lines. All 21 RCC tumor lines expressed detectable NM_199250 transcript at levels that ranged from 0.11 to 3.35-fold of the expression level in A-498 (Figure 3A). The genotype at rs3745526 of eight of these 21 RCC tumor lines that natively expressed HLA-A*0201 was determined by PCR-RFLP. Four of the eight RCC lines were found to be heterozygous (3/8) or homozygous (1/8) for the antigenic T263 allele of NM_199250. The eight HLA-A2+ RCC lines were then tested as targets for the CTL clones 2B3 and 12B3 in cytotoxicity assays. All 4 of the HLA-A*0201-expressing RCC lines that carried the T263 allele of NM_199250 were efficiently lysed by the 2B3 and 12B3 CTL clones, suggesting that expression levels for the NM_199250 transcript of C19orf48 in RCC tumor lines are generally sufficient for effective processing and presentation of this antigen to CTL (Figure 3B).
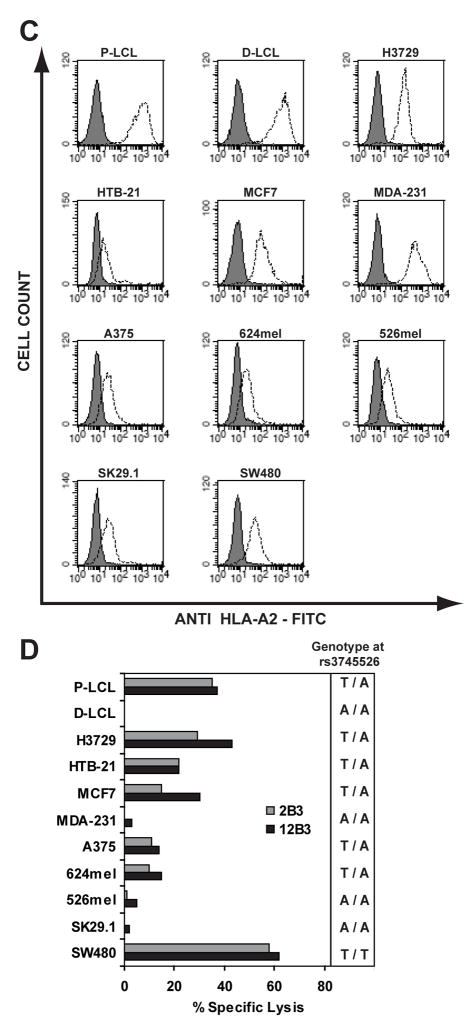
Figure 3. NM_199250 Transcript Expression and CTL Recognition of Tumor Cell Lines.
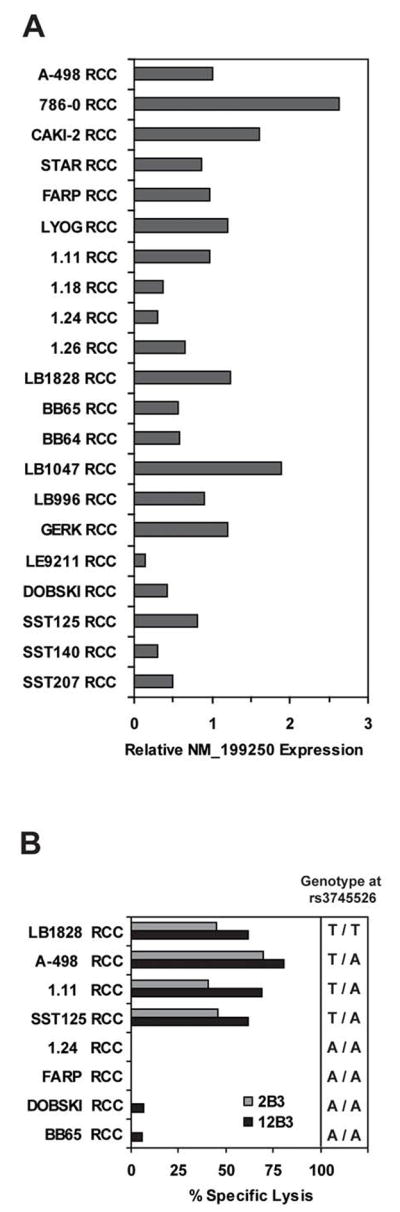
(A) Quantitative PCR analysis of NM_199250 expression in a panel of 21 RCC tumor cell lines is indicated. First strand cDNA was amplified by PCR with NM_199250 allele- as well as GAPDH-specific primers, and the expression level of NM_199250 was normalized according to the expression level of GAPDH. The expression level of NM_199250 in the A-498 RCC tumor cell line was arbitrarily defined as 1. (B) Eight RCC tumor cell lines that natively express HLA-A*0201 were labeled with 50 μCi of 51Cr overnight at 37°C and tested as targets for CTL clones 2B3 and 12B3 in a 4 hour 51Cr -release assay. The E:T was 10:1. The genotype of each line at the rs3745526 SNP as determined by PCR-RFLP is indicated. (C) Patient (P)- or donor (D)-derived EBV-LCL lines from Patient #2 (15) and nine tumor cell lines of various histologies, including ovarian carcinoma (H3729), breast carcinoma (HTB-21, MCF7, and MDA-231), melanoma (A375, 624mel, 526mel, and SK29.1), and colon carcinoma (SW480) were analyzed for cell surface HLA-A2 expression by flow cytometry. The FITC-conjugated BB7.2 mAb reactive with HLA- A*0201 (42) was used at 25 μg/ml and compared to an isotype matched FITC-conjugated control (BD Biosciences, San Diego, CA). Data were collected on a Calibur analytical flow cytometer and analyzed with CellQuest™ software (BD Biosciences). (D) The same cell lines as in panel 3C were labeled with 51Cr and tested for CTL recognition as described in panel 3B.
Q-PCR analysis also revealed the expression of NM_199250 transcript in tumor cell lines derived from AML (12/12), ALL (4/4), CLL (4/4), melanoma (5/5), ovarian carcinoma (3/3), breast carcinoma (3/3), prostate carcinoma (2/2) pancreatic adenocarcinoma (1) and colon carcinoma (1). The expression pattern for NM_199250 was further explored in silico by using the nucleotide sequence for the epitope-encoding 48-residue ORF to probe the translated human subset of the EST database (dbEST). Four hundred thirty-one exact matches for either allele of this sequence were retrieved. The tissue sources for these expressed sequences were derived predominantly (72%) from tumors or immortalized cell lines encompassing a wide variety of histologies (data not shown). Subsets of these sequences were also derived from embryonic stem cells or their derivatives (9%), embryonic/fetal tissues (5%), or from normal adult tissues (11%).
To determine if the C19orf48-encoded CTL epitope was functionally expressed by tumors other than RCC, a panel of nine tumor cell lines that natively expressed HLA-A*0201 was collected and cell surface expression of HLA-A2 was evaluated by flow cytometry (Figure 3C). The genotype at rs3745526 in each line was also determined by PCR-RFLP. Six of the nine tumor lines were found to carry the antigenic T263 allele of NM_199250, including two breast, two melanoma, one ovarian, and one colon tumor cell line. These lines were then used as targets in cytotoxicity assays with 2B3 and 12B3 CTL, both of which demonstrated significant lysis of each HLA-A2-expressing target cell line that also carried the antigenic allele of C19orf48 (Figure 3D). Taken together, the expression data for NM_199250 and CTL recognition of tumor target cell lines representing multiple different histologies provide compelling evidence that C19orf48 encodes a broadly shared tumor-associated minor H antigen.
To investigate the expression pattern of C19orf48 within normal tissues, cDNAs derived from an array of normal human tissues were analyzed by Q-PCR for expression of the NM_199250 transcript. Expression of NM_199250 was detected in both resting and activated leukocyte subsets, including T- and B-lymphocytes as well as CD14+ monocytes (data not shown). Expression of NM_199250 was also detected in a large subset of normal human tissues, including organs that can be targets of graft-versus-host reactions, such as small and large intestine, liver, and lung (Figure 4A). Dermal fibroblasts, representing nontransformed target cells, obtained from the two patients from whom the 2B3 and 12B3 CTL clones were isolated (patient #2 – UPN 18473 and patient #6 – UPN 18502) (15) were tested as targets for these two C19orf48-reactive clones. Fibroblast target cells were lysed at low levels compared to patient LCL targets (Figure 4B and 4C). Lysis of fibroblast targets was not augmented by pretreatment with IFN-γ.
Figure 4. Expression of the NM_199250 Transcript in Normal Tissues.
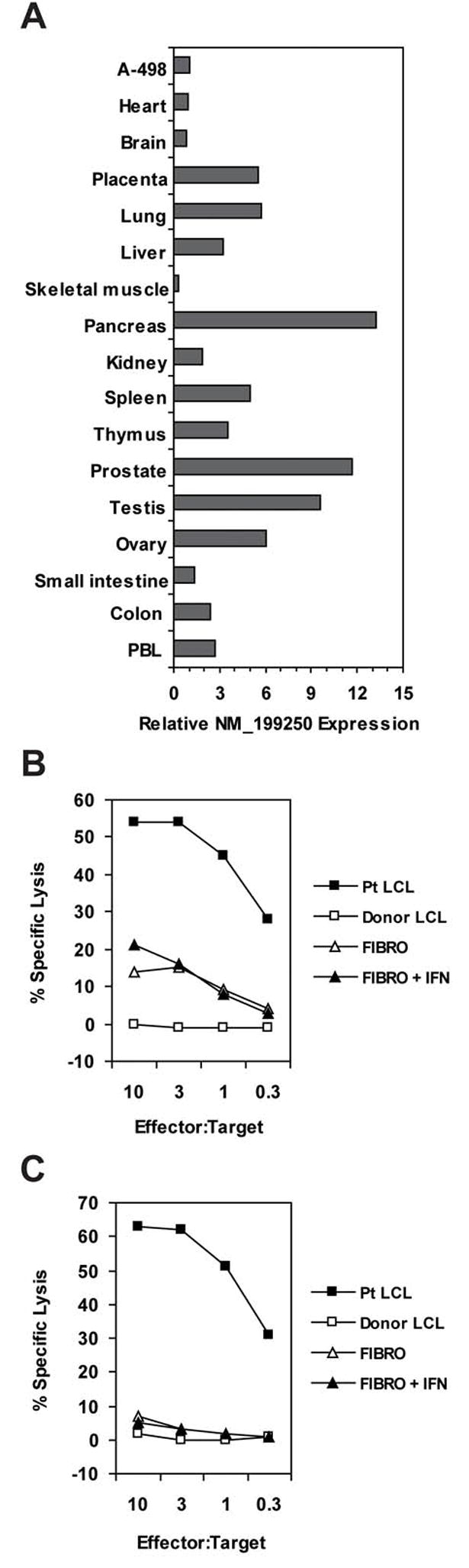
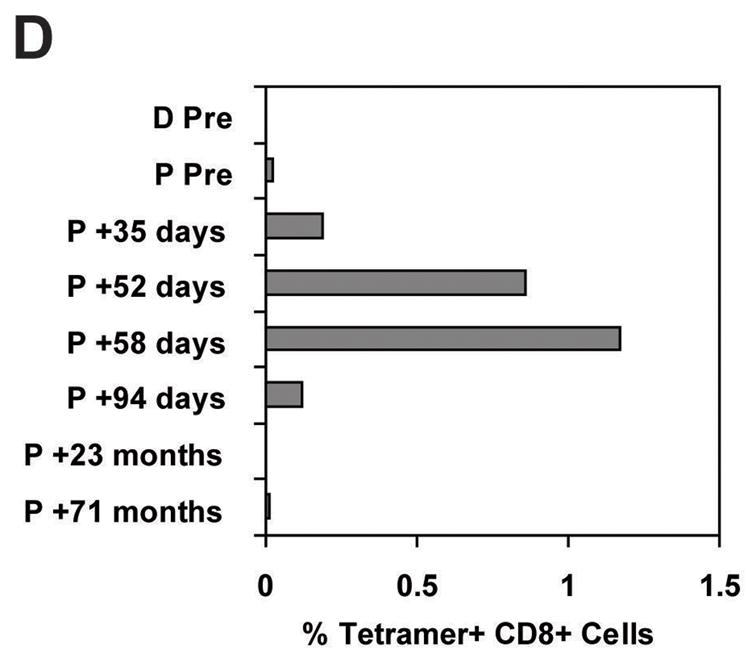
(A) CDNA isolated from the indicated human tissues (human Multiple Tissue cDNA panels I and II, BD Biosciences/Clontech) were analyzed by quantitative PCR for relative expression of the NM_199250 transcript as described for Figure 3A. (B) Patient #2 (15) or donor LCL target cells were chromium-labeled as described for panel 1D. Fibroblast cells from Patient #2 were labeled with 50–100 μCi of 51Cr for 1 to 2 hours at 37°C. One aliquot of fibroblast cells was pretreated with IFN-γ at 200 units/ml for 48 hours. Target cell lines were plated with variable numbers of the 2B3 CTL clone indicated for a 4-hour cytotoxicity assay. (C) Target cell lines were derived from Patient #6 (15) or their donor and the effector cell was CTL clone 12B3. Assay conditions were the same as described for panel 4B. (D) A PE-labeled MHC-tetramer (used at 10μg/ml) composed of HLA-A2 and the CIPPDSLLFPA peptide was used to immunostain cryopreserved PBMC previously obtained from Patient (“P”) #6 (15) pretransplant, and at the indicated time points posttransplant, or from the donor (“D”) for Patient #6. Cells were co-labeled with a FITC-conjugated CD8 antibody (BD Biosciences) and gated for exclusion of propidium iodide as a viability marker. Ten thousand CD8+ events were collected on a Calibur analytical flow cytometer and analyzed with CellQuest™ software (BD Biosciences). Tetramer staining was positive on 98.3% of cells from a culture of CTL clone 12B3 isolated from Patient #6.
Previous observations by our group and others have suggested that minor H antigens broadly expressed in normal tissues may contribute to GVHD following allogeneic HCT (24, 25). To compare the time course of the CTL response against C19orf48 in patients #2 and #6 (15) with their clinical GVHD status, cryopreserved PBMC samples from the two patients were screened by flow cytometry with a tetramer composed of HLA-A2 and CIPPDSLLFPA peptide. CD8+/tetramer+ CTL were detected in the peripheral blood of Patient #2 at low frequency (range, 0.06 to 0.17% of total CD8+ T cells) on posttransplant days 196, 385, 420 and 720, and T cell lines generated by stimulating PBMC from the first three time points with pretransplant patient PBMC or EBV-LCL showed 7 to 50 fold enhancement of CD8+/tetramer+ cells (data not shown). CIPPDSLLFPA-specific CTL clones were also isolated at each time point studied. Patient #2 ultimately achieved near-complete regression of bulky metastatic disease following three infusions of donor lymphocytes and treatment with IFN-α in the complete absence of acute and chronic GVHD, but died at 31 months posttransplant from probable sepsis (Supplementary Figures 1 and 2).
PBMC obtained from patient #6 on posttransplant days 35, 52, 58, and 94 also contained detectable CD8+/tetramer+ cells (Figure 4D). This patient was treated for acute and chronic GVHD involving the skin, gut, mouth, lacrimal glands, and lungs with corticosteroids and thalidomide starting at posttransplant day 92. Tetramer analysis of PBMC obtained at 23 months and 70 months posttransplant demonstrated no detectable CD8+/tetramer+ cells above background levels (Figure 4D). Patient #6 had stable disease to 23 months posttransplant. Subsequent disease progression was managed with IFN-α followed by temsirolimus, and the patient was alive at last follow-up 6.2 years posttransplant.
Two additional HLA-A2+ HCT patients (1 RCC, 1 ALL) who were discordant with their HLA-matched donors at rs3745526 in the direction of a donor anti-host response targeting the antigenic allele of C19orf48 were assessed for CIPPSLLFPA-specific T cell responses. Posttransplant PBMC from these two patients were screened by flow cytometry for CIPPDSLLFPA/HLA-A2 tetramer binding directly ex vivo and after in vitro stimulation with irradiated CIPPDSLLFPA-loaded T2 cells. This analysis demonstrated 21% CD8+/tetramer+ T cells following two cycles of in vitro stimulation of PBMC collected at posttransplant day 27 from the patient with ALL (data not shown). No CD8+/tetramer+ T cells were observed in PBMC from the other (RCC) patient analyzed directly ex vivo or after in vitro stimulation.
Processing of C19orf48 for HLA-A2 Presentation by a TAP-Independent Pathway
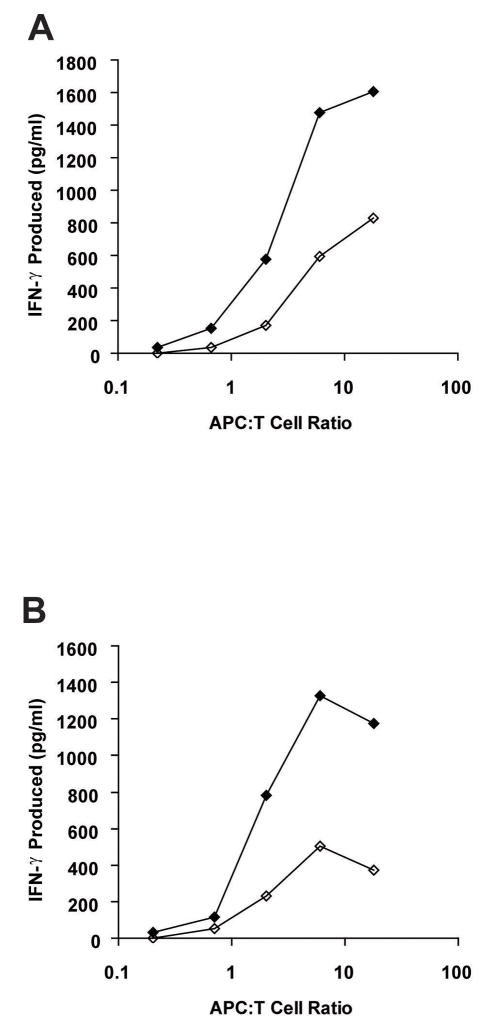
In the absence of exogenous CIPPDSLLFPA peptide, 2B3 and 12B3 CTL showed reproducible recognition of T2 target cells in cytotoxicity assays at low levels (specific lysis of 10–20%, compared with antigen-negative EBV-LCL targets, for which specific lysis values were routinely < 5%; Figure 1E, 4B, 4C, and data not shown). PCR-RFLP genotyping of genomic DNA from T2 for the rs3745526 polymorphism indicated that T2 are heterozygous for the antigenic T263 allele of C19orf48, and suggested that CTL recognition of T2 cells reflected endogenous processing and presentation of the C19orf48-encoded epitope despite the absence of the TAP-1, TAP-2, LMP-2 and LMP-7 genes in these cells (26, 27). CTL recognition of T2 targets was compared to recognition of T2 cells stably transfected with the TAP1 and TAP2 genes (17). The T2/TAP targets were a more potent stimulus for IFN-γ release from 2B3 CTL than T2 (Figure 5A). Q-PCR analysis of NM_199250 expression in T2 or T2/TAP demonstrated similar expression levels (1 to 2-fold) compared to that in the A-498 cell line (data not shown). T2 cells genetically modified via retroviral transduction to overexpress the epitope-encoding ORF +2/48 of NM_199250 also stimulated more IFN-γ release from 2B3 CTL than untransduced T2 cells (Figure 5B). These observations collectively suggest that the processing and presentation of the C19orf48-encoded CTL epitope may occur via both TAP-dependent and TAP-independent pathways.
Figure 5. Recognition of T2 and genetically modified T2 by 2B3 CTL.
(A) The 2B3 CTL clone was cultured overnight with T2 target cells (◇) or T2 cells stably transfected with TAP1/2 (◆) at the indicated antigen presenting cell (APC):T cell ratios. Supernatants were harvested and assayed for IFN-γ by ELISA. (B) T2 cells were transduced with a retrovirus encoding the epitope-encoding ORF +2/48 of NM_199250 coupled to an HA epitope tag followed by an IRES-GFP, and GFP-expressing cells were purified by flow cytometry. The 2B3 CTL clone was then cultured overnight with T2 cells (◇) or sorted GFP-positive T2 cells transduced with the ORF +2/48-encoding retrovirus (◆), and the supernatants were harvested and assayed for IFN-γ by ELISA. The data in (A) and (B) are representative of those obtained in four independent experiments.
Discussion
In this report, we have identified C19orf48 as the gene encoding a novel minor H antigen presented by HLA-A*0201 and recognized by CTL clones isolated from two patients with metastatic RCC who were treated with nonmyeloablative HLA-identical allogeneic HCT. The C19orf48 gene, first identified by the Mammalian Gene Collection Program (28), was subsequently cloned from a cisplatin-resistant human lung adenocarcinoma cell line (29). Sequence analysis of C19orf48 transcripts predicted a 117-residue translation product encoded by the largest ORF that is located in both the NM_199250 and NM_199249 transcripts. However, our studies of T cell recognition of C19orf48 provide direct evidence for a translation product derived from a novel ORF in the +2 frame of NM_199250 that encodes a predicted polypeptide of only 48 residues. Although differential splicing of 5 exons result in the alternative C19orf48-derived transcripts NM_199249 or NM_199250 that are discordant for the ORF encoding the antigenic 48-residue protein, our analyses of transcript expression by both northern blot and Q-PCR demonstrate that the NM_199250 transcript encoding the antigenic 48 residue polypeptide is the dominant transcript, with expression levels that are 100- to 600-fold greater than the expression levels of NM_199249. Taken together, our findings represent the first experimental evidence for a translation product derived from C19orf48. Discovery of the C19orf48-encoded minor H antigen within an unrecognized ORF adds to a growing body of data demonstrating that minor H peptides can be derived from surprisingly short protein species encoded by unconventional or occult ORFs (4, 30, 31). The cellular function of the C19orf48-encoded 48-residue polypeptide is currently unknown. The C19orf48 genomic locus lies between the androgen-regulated testicular acid phosphatase (ACPT) gene and the androgen-regulated kallikrein gene cluster on chromosome 19q (32). Preliminary analysis in our lab suggests that transcription of C19orf48 is likewise androgen-regulated (NF and EHW, unpublished observations), which may explain the high level of NM_199250 expression seen in prostate tissue.
CTL recognition of minigene constructs and epitope reconstitution assays with synthetic peptides pulsed onto T2 target cells identified the 11-mer peptide sequence CIPPDSLLFPA as the minimal sequence recognized by CTL in association with HLA-A*0201, suggesting that this sequence represents the naturally-processed epitope. The peptide CIPPDTLLFPA encoded by the nonantigenic allele of NM_199250 was able to stabilize HLA-A2 on the surface of T2 to a similar degree as the antigenic peptide. However, when pulsed onto target cells, CIPPDTLLFPA was not efficiently recognized by CTL, suggesting that discrimination between the two peptides occurred at the level of the TCR. It is unknown if the CIPPDTLLFPA peptide is presented at the cell surface in association with HLA-A2, and it remains formally possible that CIPPDTLLFPA may also be immunogenic in HCT donor/recipient pairs with appropriately discordant genotypes at rs3745526. It is notable that extensive biochemical analyses of HLA-A2 associated peptide ligands isolated from RCC tumor cells (33) including the A-498 tumor cell line (34) failed to identify peptides encoded by C19orf48. Thus, our data support the observation that T cell-directed identification of tumor-associated antigens remains a valuable tool for the tumor immunologist.
The frequencies of the antigenic and non-antigenic alleles of NM_199250 (0.354 and 0.646, respectively), and of the corresponding antigen-positive T/A and T/T and antigen-negative A/A genotypes (0.458, 0.125, and 0.417, respectively), observed in a group of 24 adults drawn from the CEPH European reference population (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=rs3745526) predicts a higher probability of discordant C19orf48 antigen expression in HLA-A2+ HCT donor/recipient pairs than that observed for several other autosomally-encoded HLA-A2-restricted minor H antigens, including HA-1, HA-2, and HA-8 (35). Studies of posttransplant CTL response in four HLA-A2+ HCT recipients (3 RCC, 1 ALL) discordant for the antigenic allele of NM_199250 revealed evidence for CIPPDSLLFPA-specific CTL in three of the four patients (2 RCC, 1 ALL). Thus, these data suggest that further study of the contribution of C19orf48-specific CTL responses to posttransplant GVT and GVHD effects is warranted. The potent in vitro effector function demonstrated by the 2B3 and 12B3 CTL clones against all HLA-A2+ allogeneic RCC lines that expressed the antigenic allele of NM_199250, combined with the ubiquitous expression of NM_199250 demonstrated in 21 independent RCC tumor samples, support the hypothesis that the CTL response against C19orf48 could have contributed to antitumor effects in these two RCC patients. Posttransplant tumor regression has also been observed in patients with metastatic melanoma, breast, ovarian, or colorectal carcinomas after nonmyeloablative MHC-matched allogeneic HCT (36–38). The HLA-A2- and rs3745526 genotype-dependent recognition of melanoma, breast, ovarian, and colorectal tumor targets by the 2B3 and 12B3 CTL clones suggests that CTL responses against the C19orf48-encoded minor H antigen could also contribute to antitumor activity in HLA-A2+ patients with these tumor histologies who undergo allogeneic HCT from MHC-matched but minor H antigen-mismatched donors.
Further study will be required to clarify the potential qualitative and/or quantitative contribution of C19orf48-specific CTL responses to GVHD. Patient #2, in whose peripheral blood CIPPDSLLFPA-specific CTL were detected at time points ranging from posttransplant days +196 to +720, experienced a RCC-directed GVT effect in the complete absence of acute and chronic GVHD. Our data demonstrate that NM_199250 transcript levels as measured by Q-PCR do not correlate closely with recognition by 2B3 and 12B3 CTL of the NM_199250-encoded antigenic peptide, and may therefore represent a poor surrogate marker to predict effector activity against normal tissues. The large 3′ UTR of NM_199250 contains several sequences that are predicted to be targets for defined microRNAs (JC and EHW, unpublished observations), suggesting a plausible mechanism for modulating the efficiency of translation of the epitope-encoding ORF +2/48 from NM_199250 without altering the transcript level. Ongoing studies in our laboratory are addressing this issue.
Target cell recognition by C19orf48-specific CTL clones was observed to extend to the antigen processing mutant T2, a cell line with a genetic deletion spanning the HLA-linked genes TAP-1, TAP-2, LMP-2 and LMP-7 (26, 27). T2 was found to be heterozygous for the antigenic allele of C19orf48 and expresses the NM_199250 transcript at levels comparable to target cell lines recognized by the 2B3 and 12B3 CTL clones. In the absence of exogenously added peptide, CTL specific for the C19orf48-encoded epitope still recognized the T2 target line measured by assays for cytotoxicity or IFN-γ release. These data suggest a TAP-independent processing pathway for the generation of the C19orf48-encoded epitope. Sequence and hydrophobicity analysis of the product of the +2/48 ORF suggests that the presentation of CIPPDSLLFPA in T2 cells does not likely occur via the route that enables presentation of signal sequence-derived peptides. Further study of the processing of the antigenic C19orf48-encoded peptide in T2 cells represents an area of ongoing investigation in our laboratories. Low constitutive levels of MHC class I processing-associated molecules including TAP-1 and TAP-2 in RCC and other tumor types has been proposed as a potential mechanism of tumor escape from immune surveillance (39, 40). The 2B3 and 12B3 CTL clones show reproducible recognition of tumor lines with very low cell surface HLA-A2 expression (HTB-21, A375, 624mel), demonstrating that low MHC class I expression does not preclude target recognition by these CTL. TAP-independent processing and presentation of the CIPPDSLLFPA antigenic peptide by RCC or other tumor cells may enhance its ability to both stimulate and serve as a target for CTL responses that contribute to GVT activity after MHC-matched allogeneic HCT.
Supplementary Material
Acknowledgments
This research was supported in part by National Institutes of Health grants CA121912 (SST), CA106512 (EHW), CA78902, an American Cancer Society Postdoctoral Fellowship (SST), and a Lilly Clinical Investigator Award from the Damon Runyon Cancer Research Foundation (EHW).
References
- 1.Baron F, Sandmaier BM. Current status of hematopoietic stem cell transplantation after nonmyeloablative conditioning. Curr Opin Hematol. 2005;12:435–43. doi: 10.1097/01.moh.0000177830.63033.9d. [DOI] [PubMed] [Google Scholar]
- 2.Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer. 2004;4:371–80. doi: 10.1038/nrc1365. [DOI] [PubMed] [Google Scholar]
- 3.den Haan JM, Meadows LM, Wang W, et al. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science. 1998;279:1054–7. doi: 10.1126/science.279.5353.1054. [DOI] [PubMed] [Google Scholar]
- 4.Dolstra H, Fredrix H, Maas F, et al. A human minor histocompatibility antigen specific for B cell acute lymphoblastic leukemia. J Exp Med. 1999;189:301–8. doi: 10.1084/jem.189.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brickner AG, Warren EH, Caldwell JA, et al. The immunogenicity of a new human minor histocompatibility antigen results from differential antigen processing. J Exp Med. 2001;193:195–206. doi: 10.1084/jem.193.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce RA, Field ED, Mutis T, et al. The HA-2 minor histocompatibility antigen is derived from a diallelic gene encoding a novel human class I myosin protein. J Immunol. 2001;167:3223–30. doi: 10.4049/jimmunol.167.6.3223. [DOI] [PubMed] [Google Scholar]
- 7.Mommaas B, Kamp J, Drijfhout JW, et al. Identification of a novel HLA-B60-restricted T cell epitope of the minor histocompatibility antigen HA-1 locus. J Immunol. 2002;169:3131–6. doi: 10.4049/jimmunol.169.6.3131. [DOI] [PubMed] [Google Scholar]
- 8.Akatsuka Y, Nishida T, Kondo E, et al. Identification of a polymorphic gene, BCL2A1, encoding two novel hematopoietic lineage-specific minor histocompatibility antigens. J Exp Med. 2003;197:1489–500. doi: 10.1084/jem.20021925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spierings E, Brickner AG, Caldwell JA, et al. The minor histocompatibility antigen HA-3 arises from differential proteasome-mediated cleavage of the lymphoid blast crisis (Lbc) oncoprotein. Blood. 2003;102:621–9. doi: 10.1182/blood-2003-01-0260. [DOI] [PubMed] [Google Scholar]
- 10.Torikai H, Akatsuka Y, Miyazaki M, et al. The human cathepsin H gene encodes two novel minor histocompatibility antigen epitopes restricted by HLA-A*3101 and -A*3303. Br J Haematol. 2006;134:406–16. doi: 10.1111/j.1365-2141.2006.06205.x. [DOI] [PubMed] [Google Scholar]
- 11.Warren EH, Vigneron NJ, Gavin MA, et al. An antigen produced by splicing of noncontiguous peptides in the reverse order. Science. 2006;313:1444–7. doi: 10.1126/science.1130660. [DOI] [PubMed] [Google Scholar]
- 12.Frazer KA, Ballinger DG, Cox DR, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–61. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullally A, Ritz J. Beyond HLA: the significance of genomic variation for allogeneic hematopoietic stem cell transplantation. Blood. 2007;109:1355–62. doi: 10.1182/blood-2006-06-030858. [DOI] [PubMed] [Google Scholar]
- 14.Yang JC, Childs R. Immunotherapy for renal cell cancer. J Clin Oncol. 2006;24:5576–83. doi: 10.1200/JCO.2006.08.3774. [DOI] [PubMed] [Google Scholar]
- 15.Tykodi SS, Warren EH, Thompson JA, et al. Allogeneic hematopoietic cell transplantation for metastatic renal cell carcinoma after nonmyeloablative conditioning: toxicity, clinical response, and immunological response to minor histocompatibility antigens. Clin Cancer Res. 2004;10:7799–811. doi: 10.1158/1078-0432.CCR-04-0072. [DOI] [PubMed] [Google Scholar]
- 16.Vigneron N, Ooms A, Morel S, Ma W, Degiovanni G, Van den Eynde BJ. A peptide derived from melanocytic protein gp100 and presented by HLA-B35 is recognized by autologous cytolytic T lymphocytes on melanoma cells. Tissue Antigens. 2005;65:156–62. doi: 10.1111/j.1399-0039.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 17.Karttunen JT, Lehner PJ, Gupta SS, Hewitt EW, Cresswell P. Distinct functions and cooperative interaction of the subunits of the transporter associated with antigen processing (TAP) Proc Natl Acad Sci U S A. 2001;98:7431–6. doi: 10.1073/pnas.121180198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren EH, Greenberg PD, Riddell SR. Cytotoxic T-lymphocyte-defined human minor histocompatibility antigens with a restricted tissue distribution. Blood. 1998;91:2197–207. [PubMed] [Google Scholar]
- 19.Serrano A, Lethe B, Delroisse JM, et al. Quantitative evaluation of the expression of MAGE genes in tumors by limiting dilution of cDNA libraries. Int J Cancer. 1999;83:664–9. doi: 10.1002/(sici)1097-0215(19991126)83:5<664::aid-ijc16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Seed B, Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987;84:3365–9. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 22.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351:290–6. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 23.Ruppert J, Sidney J, Celis E, Kubo RT, Grey HM, Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993;74:929–37. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- 24.Dickinson AM, Wang XN, Sviland L, et al. In situ dissection of the graft-versus-host activities of cytotoxic T cells specific for minor histocompatibility antigens. Nat Med. 2002;8:410–4. doi: 10.1038/nm0402-410. [DOI] [PubMed] [Google Scholar]
- 25.Akatsuka Y, Warren EH, Gooley TA, et al. Disparity for a newly identified minor histocompatibility antigen, HA-8, correlates with acute graft-versus-host disease after haematopoietic stem cell transplantation from an HLA-identical sibling. Br J Haematol. 2003;123:671–5. doi: 10.1046/j.1365-2141.2003.04676.x. [DOI] [PubMed] [Google Scholar]
- 26.Momburg F, Ortiz-Navarrete V, Neefjes J, et al. Proteasome subunits encoded by the major histocompatibility complex are not essential for antigen presentation. Nature. 1992;360:174–7. doi: 10.1038/360174a0. [DOI] [PubMed] [Google Scholar]
- 27.Spies T, Bresnahan M, Bahram S, et al. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990;348:744–7. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- 28.Strausberg RL, Feingold EA, Grouse LH, et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A. 2002;99:16899–903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou XD, Liu LZ, Qian GS, Huang GJ, Chen J. [Cloning and sequence analysis of a new, full-length cDNA fragment of drug resistance-related gene in human lung adenocarcinoma] Ai Zheng. 2002;21:341–5. [PubMed] [Google Scholar]
- 30.Brickner AG, Evans AM, Mito JK, et al. The PANE1 gene encodes a novel human minor histocompatibility antigen that is selectively expressed in B-lymphoid cells and B-CLL. Blood. 2006;107:3779–86. doi: 10.1182/blood-2005-08-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torikai H, Akatsuka Y, Miyazaki M, et al. A novel HLA-A*3303-restricted minor histocompatibility antigen encoded by an unconventional open reading frame of human TMSB4Y gene. J Immunol. 2004;173:7046–54. doi: 10.4049/jimmunol.173.11.7046. [DOI] [PubMed] [Google Scholar]
- 32.Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev. 2001;22:184–204. doi: 10.1210/edrv.22.2.0424. [DOI] [PubMed] [Google Scholar]
- 33.Kruger T, Schoor O, Lemmel C, et al. Lessons to be learned from primary renal cell carcinomas: novel tumor antigens and HLA ligands for immunotherapy. Cancer Immunol Immunother. 2005;54:826–36. doi: 10.1007/s00262-004-0650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flad T, Spengler B, Kalbacher H, et al. Direct identification of major histocompatibility complex class I-bound tumor-associated peptide antigens of a renal carcinoma cell line by a novel mass spectrometric method. Cancer Res. 1998;58:5803–11. [PubMed] [Google Scholar]
- 35.Spierings E, Hendriks M, Absi L, et al. Phenotype frequencies of autosomal minor histocompatibility antigens display significant differences among populations. PLoS Genet. 2007;3:e103. doi: 10.1371/journal.pgen.0030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hentschke P, Barkholt L, Uzunel M, et al. Low-intensity conditioning and hematopoietic stem cell transplantation in patients with renal and colon carcinoma. Bone Marrow Transplant. 2003;31:253–61. doi: 10.1038/sj.bmt.1703811. [DOI] [PubMed] [Google Scholar]
- 37.Ueno NT, Cheng YC, Rondon G, et al. Rapid induction of complete donor chimerism by the use of a reduced-intensity conditioning regimen composed of fludarabine and melphalan in allogeneic stem cell transplantation for metastatic solid tumors. Blood. 2003;102:3829–36. doi: 10.1182/blood-2003-04-1022. [DOI] [PubMed] [Google Scholar]
- 38.Blaise D, Bay JO, Faucher C, et al. Reduced-intensity preparative regimen and allogeneic stem cell transplantation for advanced solid tumors. Blood. 2004;103:435–41. doi: 10.1182/blood-2003-07-2236. [DOI] [PubMed] [Google Scholar]
- 39.Johnsen AK, Templeton DJ, Sy M, Harding CV. Deficiency of transporter for antigen presentation (TAP) in tumor cells allows evasion of immune surveillance and increases tumorigenesis. J Immunol. 1999;163:4224–31. [PubMed] [Google Scholar]
- 40.Seliger B, Maeurer MJ, Ferrone S. TAP off--tumors on. Immunol Today. 1997;18:292–9. doi: 10.1016/s0167-5699(97)01052-9. [DOI] [PubMed] [Google Scholar]
- 41.Warren EH, Gavin MA, Simpson E, et al. The human UTY gene encodes a novel HLA-B8-restricted H-Y antigen. J Immunol. 2000;164:2807–14. doi: 10.4049/jimmunol.164.5.2807. [DOI] [PubMed] [Google Scholar]
- 42.Parham P, Brodsky FM. Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981;3:277–99. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.