Abstract
Background & Aims
Patients with diarrhea-predominant IBS (IBS-D) anecdotally report symptom improvement after initiating a very low-carbohydrate diet (VLCD). This is the first study to prospectively evaluate a VLCD in IBS-D.
Methods
Participants with moderate to severe IBS-D were provided a 2-week standard diet, then 4 weeks of a VLCD (20 grams of carbohydrates/day). A responder was defined as having adequate relief (AR) of gastrointestinal symptoms for 2 or more weeks during the VLCD. Changes in abdominal pain, stool habits, and quality of life (QOL) were also measured.
Results
Of the 17 participants enrolled, 13 completed the study and all met the responder definition, with 10 (77%) reporting AR for all 4 VLCD weeks. Stool frequency decreased (2.6 ± 0.8/day to 1.4 ± 0.6/day; p<0.001). Stool consistency improved from diarrheal to normal form (Bristol Stool Score: 5.3 ± 0.7 to 3.8 ± 1.2; p<0.001). Pain scores and QOL measures significantly improved. Outcomes were independent of weight loss.
Conclusion
A VLCD provides adequate relief, and improves abdominal pain, stool habits, and quality of life in IBS-D.
Introduction
Irritable bowel syndrome (IBS) is a heterogeneous disorder, and sub-classifications of diarrhea-predominant IBS (IBS-D) and constipation-predominant IBS (IBS-C) have been identified.1 However, the pathophysiology of IBS is not well understood. The role of diet in IBS has been investigated because patients frequently identify worsening of symptoms after meals and often cite particular foods as triggers of their IBS symptoms.2 Nevertheless, data from clinical trials is insufficient to allow for specific dietary recommendations.3-5
Previous research has suggested a role for dietary carbohydrates in worsening IBS symptoms.6, 7 Our combined clinical experience indicates that patients with IBS-D report improvement in their gastrointestinal symptoms after initiating a very low-carbohydrate diet (VLCD). However, no study has investigated the effect of a VLCD in patients with IBS-D. The purpose of this study is to prospectively assess the effect of a VLCD on gastrointestinal symptoms and quality of life among patients with IBS-D.
Methods
Subjects
A total of 17 participants who met Rome II criteria for IBS-D were enrolled in this study.8 Only individuals with moderate or severe symptoms were included, based on a score of >36 using the Functional Bowel Disorder Severity Index (FBDSI).9 Participants were required to have a body mass index of > 25kg/m2. Individuals with a history of inflammatory bowel disease, any gastrointestinal surgery, diabetes or other serious medical conditions, previous use of a VLCD, or use of narcotics or weight loss medications were ineligible. Antidepressants were allowed if the dose had been stable for at least 4 weeks prior to enrollment. Routine blood tests were performed to exclude other disorders that might produce symptoms similar to IBS-D. All subjects provided written informed consent, and the study was approved by the institutional review board of the University of North Carolina (UNC).
Study Design and Diet
All prospective participants met with a dietitian prior to study enrollment. All meals were provided for all six weeks of the study by the metabolic kitchen of the adult General Clinical Research Center (GCRC) at UNC. The energy content provided in the meals was designed to achieve weight maintenance for the entire study period. Estimates of energy expenditure were calculated using the Harris-Benedict equation with an adjustment for activity level.10 A standard diet was provided for the first two weeks of the study, approximating the diet for the average adult American, according to the NHANES study.11 Approximately 55% of calories were from carbohydrates, 30% from fat, and 15% from protein. During the final four weeks, participants consumed a VLCD in which carbohydrates were limited to 20 grams per day. For the VLCD, approximately 51% of calories were from fat, 45% from protein, and 4% from carbohydrates. This distribution is consistent with those used in previous studies evaluating very low-carbohydrate diets.12, 13 Participants received meals three times a week. They returned any uneaten portions to the GCRC so that actual energy intake could be calculated. Participants were also strongly discouraged from consuming any food other than what was provided to them by the GCRC kitchen.
Outcomes
The primary outcome was adequate relief of IBS-D symptoms during the VLCD phase.14 Participants completed a one-item questionnaire at the end of each of the four weeks of the VLCD, assessing whether they had adequate relief of their IBS symptoms for the week. A responder was defined as reporting adequate relief in at least two of the four weeks on the VLCD. Participants also completed daily diary cards for all 6 weeks of the study. They recorded the number of bowel movements for each day, stool consistency (using the Bristol Stool Scale (BSS) that ranges from 1 (hard/lumpy) to 7 (watery)), and abdominal pain.15 Daily abdominal pain scores were assessed using a 100-mm visual analog scale, with scores ranging from 0 (no pain) to 100 (severe pain). The Irritable Bowel Syndrome Quality of Life (IBS-QOL)16 and Sickness Impact Profile (SIP)17 questionnaires were completed at the end of the two-week standard diet and again at the completion of the four-week VLCD.
Statistical Methods
Paired t-tests were used to analyze changes in IBS-QOL and SIP scores. Trend regression analysis was used to assess changes in average abdominal pain rating, stool frequency, and stool consistency during the four weeks of the VLCD compared to the two weeks of the standard diet. We made an a priori decision to evaluate whether outcomes were different between individuals who lost more than 3 kg compared to those who lost less than 3 kg. Data were analyzed using SAS V.8.0 (SAS Institute Inc., Cary, NC).
Results
A total of 17 individuals were enrolled. The participants were predominantly women (n=15) and white (n=14). The mean (±SD) age in years was 46 ± 10, and the mean BMI was 32.0 ± 4.8 kg/m2. One participant dropped out during Week 1 of the study (intolerance of standard diet), and three participants dropped out during Week 3 of the study (two due to intolerance of the VLCD and one due to emotional symptoms), and 13 completed all 6 weeks. All 13 participants who completed the study were responders who reported adequate relief of IBS-D symptoms for at least 2 of the 4 weeks during the VLCD, and 10 participants (77%) reported adequate relief for all four weeks. All 13 participants reported adequate relief in the last week of the VLCD.
With regard to secondary outcomes, participants reported a significant decrease in stool frequency with the VLCD, decreasing from 2.6 ± 0.8 bowel movements/day during the standard diet to 1.4 ± 0.6 bowel movements/day during the VLCD (p<0.001). Participants reported an improvement in stool consistency (Figure 1), with the average Bristol stool score decreasing from 5.3 ± 0.7 on the standard diet to 3.8 ± 1.2 during the VLCD (p<0.001). Abdominal pain scores improved (Figure 2), with average daily abdominal pain scores decreasing from 26 ± 18 during the standard diet to 10 ± 10 during the VLCD (p=0.007).
Figure 1.
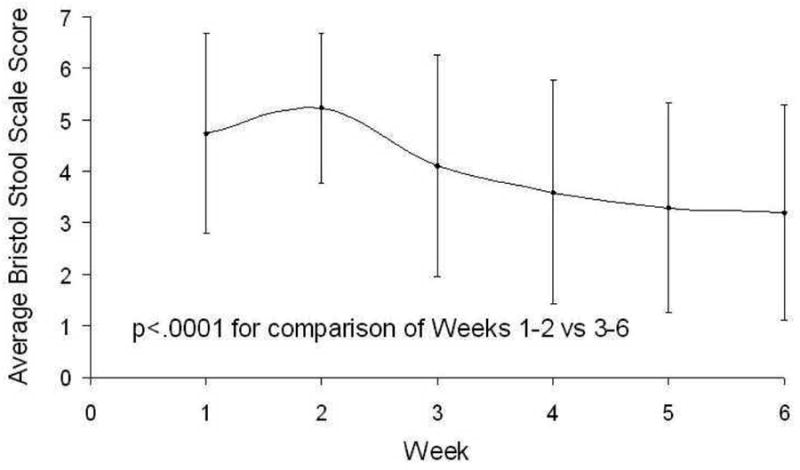
Average Daily Bristol Stool Score During the Standard Diet (Weeks 1-2) and the Very Low-Carbohydrate Diet (Weeks 3-6).
Figure 2.
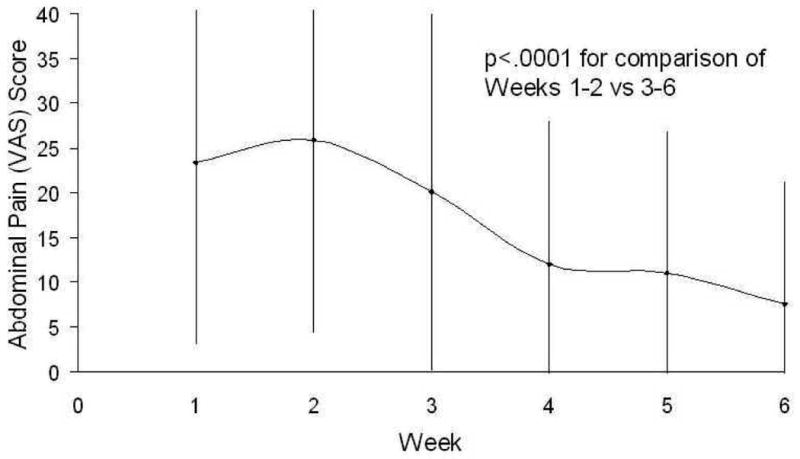
Average Daily Abdominal Pain Score During the Standard Diet (Weeks 1-2) and the Very Low-Carbohydrate Diet (Weeks 3-6).
Quality of life was significantly improved with the VLCD when compared to the participant's standard diet. IBS-QOL scores improved from 71 ± 22 on the standard diet to 81 ± 13 (p=0.02) on the VLCD. SIP scores improved from 5.5 ± 6.4 on the standard diet to 2.3 ± 3.6 on the VLCD (p=0.001). Finally, participants did lose an average of 3.1 ± 1.7 kg during the study (p<0.0001), but improvement for all outcomes were similar for those who lost less than 3 kg (n=6) compared to those who lost more than 3 kg (n=7).
Discussion
The purpose of this prospective trial was to assess the effect of a VLCD in IBS-D. The results provide preliminary evidence that a VLCD provides adequate relief of IBS-D symptoms, decreases abdominal pain, improves stool frequency and consistency, and improves quality of life. All 13 participants who completed the 6-week study reported adequate relief of their IBS-D symptoms for at least two of the four weeks. More impressively, 10 of these 13 participants reported adequate relief for all four weeks.
This is the first study to assess the effect of a low-carbohydrate diet in individuals with IBS-D, though previous research has investigated the role of carbohydrates in IBS.6, 7 One study of 239 individuals with either IBS or non-specific functional bowel complaints showed an improvement in symptoms after elimination of some combination of sorbitol, lactose, or fructose for one month.6 Additionally, King et al found that individuals with IBS have abnormal colonic fermentation of carbohydrates.7 They found that an exclusion diet that reduced the load of potential offending carbohydrates improved IBS symptoms. However, several studies have shown similarly high rates of abnormal colonic fermentation of carbohydrates in healthy volunteers.18, 19 Also, not all studies have shown benefit when the potential offending carbohydrate is removed from the diet.20
This study has a few limitations. Our study represents the experience of 17 individuals, with 13 participants completing all 6 weeks. The results must be confirmed in larger numbers. Three participants who dropped out did so primarily because of difficulty following a restrictive diet. There is no a priori reason why the drop out group would be different from those who completed the study with regard to treatment benefit. The other main limitation is the lack of a standard control group. Although participants served as their own control for several outcomes, a placebo effect may explain the positive findings, particularly for subjective outcomes such as abdominal pain. However, the use of daily diary cards helps to obviate subjective interpretation or recall bias by providing a systematic and objective measure of daily bowel habits. Finally, most of the participants were women who were overweight or obese, so it is uncertain if these responses would be seen in men or normal-weight individuals.
Despite these limitations, this study found objective evidence that overweight and obese individuals initiating a VLCD had a profound clinical response in their IBS-D symptoms. This finding requires further investigation to identify mechanisms by which a VLCD affects the symptoms of IBS-D. This will elucidate additional dietary and pharmacologic methods for managing patients with IBS-D.
Acknowledgments
This research was supported by a research grant from the Atkins Foundation, the UNC Gastrointestinal Biopsychosocial Research Center NIH R24 DK067674 and in part by a grant from the National Institutes of Health T32 DK 07634. Facility support for this study has been provided by the UNC General Clinical Research Center.
Footnotes
No conflicts of interest exist for the authors of this manuscript. The sponsors were not involved in the data collection, data analysis, or data interpretation in preparing this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional Bowel Disorders. In: Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE, editors. Rome III: The Functional Gastrointestinal Disorders. 3rd. McLean, VA: Degnon Associates, Inc.; 2006. pp. 487–555. [Google Scholar]
- 2.Simren M, Mansson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, Bjornsson ES. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–15. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 3.Burden S. Dietary treatment of irritable bowel syndrome: current evidence and guidelines for future practice. J Hum Nutr Diet. 2001;14:231–41. doi: 10.1046/j.1365-277x.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 4.Dapoigny M, Stockbrugger RW, Azpiroz F, Collins S, Coremans G, Muller-Lissner S, Oberndorff A, Pace F, Smout A, Vatn M, Whorwell P. Role of alimentation in irritable bowel syndrome. Digestion. 2003;67:225–33. doi: 10.1159/000072061. [DOI] [PubMed] [Google Scholar]
- 5.Floch MH. Use of diet and probiotic therapy in the irritable bowel syndrome: analysis of the literature. J Clin Gastroenterol. 2005;39:S243–6. doi: 10.1097/01.mcg.0000156104.67505.5b. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein R, Braverman D, Stankiewicz H. Carbohydrate malabsorption and the effect of dietary restriction on symptoms of irritable bowel syndrome and functional bowel complaints. Isr Med Assoc J. 2000;2:583–7. [PubMed] [Google Scholar]
- 7.King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187–9. doi: 10.1016/s0140-6736(98)02146-1. [DOI] [PubMed] [Google Scholar]
- 8.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Mueller-Lissner SA. Functional Bowel Disorders and D Functional Abdominal Pain. In: Drossman DA, Talley NJ, Thompson WG, Whitehead WE, Corazziari E, editors. Rome II: Functional Gastrointestinal Disorders: Diagnosis, Pathophysiology, and Treatment. 2nd. McLean, VA: Degnon Associates, Inc.; 2000. pp. 351–432. [Google Scholar]
- 9.Drossman DA, Li Z, Toner BB, Diamant NE, Creed FH, Thompson D, Read NW, Babbs C, Barreiro M, Bank L, et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986–95. doi: 10.1007/BF02064187. [DOI] [PubMed] [Google Scholar]
- 10.Pellett PL. Food energy requirements in humans. Am J Clin Nutr. 1990;51:711–22. doi: 10.1093/ajcn/51.5.711. [DOI] [PubMed] [Google Scholar]
- 11.Trends in intake of energy and macronutrients--United States, 1971-2000. MMWR Morb Mortal Wkly Rep. 2004;53:80–2. [PubMed] [Google Scholar]
- 12.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–90. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 13.Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769–77. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- 14.Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, Talley NJ, Van Zanten SJOV. Design of Treatment Trials for Functional Gastrointestinal Disorders. In: Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE, editors. Rome III: The Functional Gastrointestinal Disorders. 3rd. McLean, VA: Degnon Associates, Inc.; 2006. pp. 779–834. [Google Scholar]
- 15.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 16.Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, Jia H, Bangdiwala SI. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999–1007. doi: 10.1111/j.1572-0241.2000.01941.x. [DOI] [PubMed] [Google Scholar]
- 17.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care. 1981;19:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Lea R, Whorwell PJ. The role of food intolerance in irritable bowel syndrome. Gastroenterol Clin North Am. 2005;34:247–55. doi: 10.1016/j.gtc.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Banares F, Esteve-Pardo M, de Leon R, Humbert P, Cabre E, Llovet JM, Gassull MA. Sugar malabsorption in functional bowel disease: clinical implications. Am J Gastroenterol. 1993;88:2044–50. [PubMed] [Google Scholar]
- 20.Tolliver BA, Jackson MS, Jackson KL, Barnett ED, Chastang JF, DiPalma JA. Does lactose maldigestion really play a role in the irritable bowel? J Clin Gastroenterol. 1996;23:15–7. doi: 10.1097/00004836-199607000-00005. [DOI] [PubMed] [Google Scholar]


