Abstract
Amphiphilic block copolymer self-assembly provides a versatile means to prepare nanoscale micelles in solution. The utilization of these structures as targeted drug delivery vehicles has motivated efforts to prepare bioactive ligand-functionalized polymer micelles. The impact of ligand conjugation on micelle morphology was examined through use of well-characterized poly(ethylene oxide)-b-poly(butadiene) (OB) block copolymers functionalized to varying extents with a biologically relevant RGD-containing peptide sequence. Micelle morphology and dilute solution behavior of RGD-functionalized OB (RGD-OB) copolymers were examined using cryogenic transmission electron microscopy (cryo-TEM) and dynamic mechanical analysis. The direct dispersion of RGD-OB copolymers into deionized water yielded a variety of structures; the observed morphologies deviated from the canonical series predicted by the overall change in amphiphile composition due to peptide conjugation. RGD functionalized spherical micelles, cylindrical micelle networks and annular multilayer vesicles were prepared. The morphological behavior was attributed to interactions between peptide moieties conjugated to the termini of coronal chains and has implications in the design of targeting micelles for drug delivery applications.
Introduction
Aqueous self-assembly of amphiphilic block copolymers has emerged as a powerful tool for the preparation of nanoscale aggregates of various shapes. The utilization of these self-assembled structures as platforms for a host of applications is facilitated by the chemical and structural diversity found in modern block copolymers.1,2 Control over micelle characteristics and morphology is achieved through the manipulation of monomers, chain architecture, composition, molecular weight and the method employed to disperse amphiphiles into the desired media.3 Spherical, cylindrical, bilayered, and more intricate morphologies have been prepared from a variety of chemically distinct polymeric amphiphiles.4
Extremely small critical micelle concentrations and the inherent core-shell geometry of micellar aggregates formed from block copolymers5 has spurred entrance of these materials into the biomedical arena as delivery vehicles for therapeutic applications.6-8 When sequestered within hydrophobic micellar cores, a number of drugs have shown markedly improved pharmacokinetic profiles, namely prolonged plasma circulation9,10 and decreased systemic toxicity.11,12 Additionally, the ability of micellar delivery vehicles to avoid rapid clearance by the reticuloendothelial system facilitates the passive targeting of certain tissues through the enhanced permeation and retention effect.12-14
Spherical micelles are the most common morphology used in drug delivery,6 due in part to the ready dispersion and self-assembly of polymeric amphiphiles containing a majority hydrophilic component and the monodispersity of the aggregates formed. However, controlling aggregate morphology (e.g., through the manipulation of block copolymer composition) provides a means to examine the effect of vehicle shape on drug delivery efficacy, or to explore other applications utilizing specific micelle geometries. For example, cylindrical micelles have been examined as drug delivery vehicles and prolonged in vivo circulation relative to spherical micelles has been demonstrated.15 Vesicles or polymersomes have also shown prolonged in vivo circulation times16 and provide the added capacity for simultaneous conveyance of hydrophobic and hydrophilic agents, contained within the vesicle wall and lumen, respectively.17
Efforts to increase the efficacy of micellar drug delivery protocols has motivated research in the formulation of stimuli-responsive (i.e., pH, temperature, etc.) micelles to facilitate site-specific release and ligand functionalization to promote targeting and cellular internalization.18-20 The active targeting of cells or tissues by micellar delivery vehicles is made possible through the conjugation of specific ligands to the hydrophilic termini of the constituent block copolymers. For example, the over-expression of receptors or antigens on the surfaces of certain cells may facilitate the targeted treatment of specific diseases through uptake of micelles functionalized with a complementary ligand.19-21 However, as potential ligands vary extensively in molecular weight, hydrophobicity and ionic nature, the effect of ligand conjugation on block copolymer self-assembly and aggregate morphology must be considered.
The balance of free energies associated with core chain stretching, corona chain repulsion, and core/corona interfacial tension dictates the micellar morphology of nonionic block copolymers.5 Typically, the extreme hydrophobicity of block copolymer surfactants,22 the glassy nature of common hydrophobic blocks, and the lack of free chain exchange between micelles in pure water23 necessitates the use of organic cosolvents to aid dispersion.24 The utilization of low glass transition temperature (Tg), amorphous hydrophobic blocks can mitigate the need for plasticizers or increased temperature to speed chain dynamics, allowing for the direct dissolution of polymeric amphiphiles into water.25,26 Though polymer chain exchange between micelles is still arrested, the mobility of polymer chains constituting the micellar core allows for the local equilibration of individual aggregates.27
The self-assembled structures of block copolymers are more stable than those formed from small molecule surfactants,28 yet morphologies are still susceptible to minor changes in overall composition25,29,30 and solvent quality.31,32 This is especially true for the cylindrical micelle phase, where perturbations in amphiphile molecular weight,29 composition25 or polydispersity27 can lead to micelle structures with higher or lower interfacial curvature or to the coexistence of multiple morphologies. Consequently, the conjugation of ligands to the hydrophilic termini of cylindrical micelle forming copolymers is expected to impact solution morphology if the hydrophilic / hydrophobic balance of the amphiphile is significantly altered. Electrostatic interactions between ligands may also have a strong effect on the aggregate structure. Additionally, ionic character imparted to amphiphiles through ligand conjugation may restrict uniform dispersion while adding new means for manipulating aggregate morphology through the addition of external ionic species.33-35
In this work we have utilized an arginine-glycine-aspartic acid (RGD) containing peptide sequence as a model ligand to explore the impact of peptide conjugation on short-range block copolymer self-assembly and longer range dispersant aggregation in globally dilute aqueous dispersions. The RGD-peptide sequence, one of the shortest known cell adhesion motifs, facilitates cellular interaction through integrin receptors common to a number of cell types.36,37 Over-expression of the αvβ3 integrin by vascular cells during tumor angiogenesis38 has been exploited for cancer-targeted drug delivery of RGD-bearing vehicles.39-41 Additionally, cell-adhesive substrates for modeling cellular interaction and matrices for tissue engineering have been produced through functionalization of surfaces with RGD-peptides.42,43 The high water solubility, relatively low molecular weight, and variety of chemistries available for conjugation has motivated the incorporation of RGD-containing peptides into a range of biomaterials. The utilization of peptide-functionalized micellar structures in targeted drug delivery and tissue engineering applications has prompted our efforts in the preparation of RGD-functionalized polymer micelles with controlled morphologies.
Herein we report the synthesis, characterization and aqueous self-assembly of a series of RGD-functionalized poly(ethylene oxide)-b-poly(butadiene) (OB) copolymers. Hydroxyl-terminated OB was initially reacted with divinyl sulfone to yield OB copolymers with vinyl sulfone terminated poly(ethylene oxide) blocks (VS-OB). RGD-OB copolymer conjugates were then prepared through the Michael-type addition of a thiol (-SH) containing RGD peptide sequence to VS-OB. A modification of the Sakaguchi reaction described by Van Pilsum et al.44 for the determination of arginine in biological fluids was employed to quantify the extent of peptide conjugation to VS-OB copolymers. The series of OB block copolymers studied spanned the composition (weight fraction of PEO) range 0.64 ≥ wPEO ≥ 0.34, where spherical, cylindrical, and bilayered micelles (polymersomes) have been observed. Characterization of these solutions using cryogenic transmission microscopy (cryo-TEM) and solution rheometry revealed the self-assembly of RGD-functionalized amphiphiles failed to yield the expected micelle morphologies, and aggregate geometries exhibited anomalies attributed to attractive interactions between peptide groups. This study provides insight into the impact of peptide-functionalization on polymeric amphiphile self-assembly and the challenges involved in controlling the morphology of ligand-functionalized aggregates.
Experimental Section
Materials
Poly(ethylene oxide)-poly(butadiene) block copolymers were synthesized by sequential anionic polymerization45 and contain a common poly(1,2-butadiene) (PB) block with a number average molecular weight of 2500 g mol−1 and poly(ethylene oxide) (PEO) block number average molecular weights that range from 2300 to 4500 g mol−1. The molecular characteristics and dilute solution morphologies of this series of OB diblocks have been reported previously.27,46 The peptide gylcine-argenine-glycine-aspartic acid-serine-cysteine (GRGDSC, 96.3% pure, 593 g mol−1) was purchased from GenScript (Piscataway, NJ) and stored at 0 °C. Dichloromethane (CH2Cl2, HPLC grade, Aldrich) was dried by passing through molecular sieve-based columns (MBraun, Stratham, NH). All other chemicals were used as received.
Measurements
1H NMR spectra were acquired on a Varian VI-500 spectrometer at room temperature. All polymer samples were dissolved in CDCl3 (Cambridge) at approximately 1 w/v %. Polydispersity indices were determined by size exclusion chromatography (SEC) using a Hewlett-Packard series 1100 liquid chromatography system equipped with a Hewlett-Packard 1047A RI detector and three PLgel 5 μm MIXED-C columns (Polymer Laboratories). Chloroform was used as the mobile phase (35 °C, 1 mL/min) and the SEC was calibrated with polystyrene standards (Polymer Laboratories). Infrared analysis was performed on thin films of polymer cast onto NaCl plates from chloroform. FT-IR spectra were collected using a Nicolet Magna-IR Spectrometer 550. UV-Vis spectra were recorded on a Spectronic Genesys-5 spectrometer using 4 mL acryl cuvettes (Sarstedt 67.738) with disposable polyethylene caps.
Synthesis of Vinyl Sulfone Poly(ethylene oxide)-b-poly(butadiene) (VS-OB)
The OB starting material was initially dried under vacuum at 40 °C for 72 h. The diblock was then dissolved in CH2Cl2 (∼ 3.5 mM), and sequentially under argon a 10:1 molar excess of sodium hydride:hydroxyl-OB and a 100:1 molar excess of divinyl sulfone:hydroxyl-OB was added to the polymer solution. The reaction flask was then sealed and stirred at 37 °C for 3.5 days. Excess sodium hydride in solution was neutralized by the drop-wise addition of concentrated acetic acid, the solution was filtered, and CH2Cl2 was removed under reduced pressure at room temperature. The crude product was then redissolved in tetrahydrofuran (THF, HPLC grade) and transferred to a hydrated dialysis membrane (MWCO 1000 Da, Spectrum Laboratories, Inc.). Free divinyl sulfone was then removed from VS-OB by dialysis against three 2 L volumes of THF/H2O (10 vol % DI H2O); the dialysate was changed every 24 h. The final product was recovered by drying under reduced pressure and stored at 4 °C. 1H NMR (CDCl3): δ 6.8 (dd, -SO2CH=), 6.4 (d, -SO2CH=CH2), 6.1 (d, -SO2CH=CH2), 5.4 (m, CH2-CH=CH-CH2- and CH2=CH-CH-), 4.9 (m, CH2=CH-CH-), 3.9 (t, -SO2-CH2-CH2-O-), 3.65 (b, -O-CH2-CH2-O-), 3.3 (t, -SO2-CH2-CH2-O-), 2.1 (b, CH2-CH=CH-CH2- and CH2=CH-CH-), 1.2 (b, CH2=CH-CH-CH2-), 0.9 (m, -CH3 resonances from initiator). The polydispersities of OB diblocks measured using SEC were nearly identical before and after vinyl sulfone functionalization (PDI < 1.10).
Synthesis of RGD-poly(ethylene oxide)-b-poly(butadiene) (RGD-OB)
VS-OB was initially dissolved in THF (∼ 25 w/v %) and buffer (pH 8.0, 0.45 M triethanolamine solution containing 5mM EDTA) was added drop-wise to reach a final concentration of ∼ 50 mg/mL polymer (reaction solution was 20 vol % THF in buffer). The polymer dispersion was shaken until visually homogeneous and degassed under reduced pressure. A specific amount of GRGDSC (0.25−1.5:1 GRGDSC:VS-OB mole ratio) was dissolved in 0.2 mL of degassed buffer solution and the peptide solution was then added to the polymer solution under argon, the reaction flask sealed, and the mixture was stirred at 25 °C for approximately 2 days. The polymer solution was then transferred to a dialysis membrane (MWCO 3500 Da, Spectrum Laboratories, Inc.) and free peptide removed through dialysis at 25 °C against one 2L volume of 10 vol % THF in DI H2O and two 2L volumes of DI H2O; the dialysate was changed every 24 h. This aqueous dispersion was then transferred to a round bottom flask, the solution was frozen in liquid nitrogen, and water removed from the product under vacuum at 0 °C. The lyophilized product was stored at 4 °C.
Quantitative Determination of GRGDSC Conjugation
A colorimetric assay (i.e., modified Sakaguchi reaction) for the determination of arginine (Arg, R) in biological fluids44,47 was used to quantify GRGDSC conjugation to VS-OB copolymers. Initially, 100 μL aliquots of an OB stock solution prepared in THF were added to glass vials. Pure H2O was then added to the polymer solution, followed by varying amounts of a peptide stock solution (prepared in HPLC H2O) to achieve a total volume of 1 mL. The vials were then sealed, shaken vigorously and the calibration samples cooled in an ice bath. RGD-OB samples were prepared by adding 100−60 μL of an RGD-OB stock solution prepared in THF to glass vials, followed by the addition of pure THF to bring the volume in individual vials to 100 μL. 900 μL of HPLC H2O was then added to each polymer solution, the vials sealed, shaken vigorously, and the unknown samples cooled in an ice bath. Reagent solutions used in the Van Pilsum et al. method of arginine quantification were prepared daily and the procedure for sample preparation was performed as reported.44 Briefly, 3.5 mL of a 10% NaOH solution containing 20 mg/mL thymine was added to 3.5 mL of absolute ethanol containing 0.04% 1-naphthol. 0.5 mL of this basic, 1-naphthol solution was then added to each of the calibration and unknown samples, the solutions were mixed thoroughly and the vials were returned to the ice bath. 0.2 mL of a 1% sodium hypochlorite solution was then added to a single vial, and after shaking vigorously for exactly 1 min, 0.2 mL of a 2% sodium thiosulfate solution was added. The vial was shaken briefly, returned to the ice bath, and this process repeated for each sample vial. Calibration and unknown samples were then transferred to acryl cuvettes and kept on ice until UV-Vis spectra were recorded. Care was taken to remove all condensation from the outer surfaces of the cuvette just prior to analysis. Absorbance measurements were taken over a range of wavelengths (380−650nm) and the colored complex obtained from reaction with arginine was observed as a broad absorbance centered near 515 nm. The UV-Vis absorbance spectra for six calibration samples containing the same concentration of polymer and varying amounts of GRGDSC (0−0.125 μmol) were recorded and used to prepare calibration curves. Relative to the calibration samples, unknown (RGD-OB) samples exhibited overall higher apparent absorbance across the wavelength range examined due to increased solution turbidity. Subsequently, a linear baseline correction was utilized for both calibration and RGD-OB samples. The amount of arginine present in RGD-OB solutions was determined from calibration curves constructed from normalized peak absorbance values at 515 nm and integrated peak areas. A minimum of three absorbance spectra were recorded for each RGD-OB polymer and the calculated values for mole fraction of peptide conjugation were averaged.
Sample Preparation
Aqueous dispersions of OB and RGD-OB were prepared at a concentration of 1 wt% by the direct addition of bulk copolymer into HPLC water. OB samples were initially dissolved in CH2Cl2, the solutions filtered through a 0.2 μm PTFE syringe filter, and thin polymer films formed by solvent evaporation and drying under reduced pressure at room temperature. Water was then added to the dried films and the solutions were stirred at 25 °C for at least 7 days prior to analysis. Aqueous dispersions of RGD-OB were prepared by two methods; a similar thin-film hydration protocol and by the direct addition of water to a known mass of the white powder recovered upon lyophilization of RGD-OB products (see above). Thin films of RGD-OB polymer were prepared in the same manner as OB samples, excluding filtration of the RGD-OB/CH2Cl2 dispersion prior to solvent evaporation. After the addition of water to either polymer films or bulk lyophilized polymer, RGD-OB samples were sealed and gently stirred at 25 °C for at least 7 days prior to analysis. Though characterized by differing degrees of turbidity, the OB and RGD-OB solutions appeared homogeneous to the eye.
Cryo-TEM
Samples for cryo-TEM analysis were prepared using a controlled environment vitrification system (CEVS),48 the isolated chamber of which was humidified to near saturation to prevent evaporative water loss from the polymer solutions. A drop of the micellar dispersion at 25 °C was placed on a lacey carbon supported TEM grid, which was held by tweezers suspended within the chamber of the CEVS. Excess solution was removed through blotting with a piece of filter paper and the remaining solution, held as films spanning the holes in the lacey carbon film, was allowed to relax for approximately 10 s prior to the grid being plunged into a reservoir of liquid ethane just above its freezing point (−183 °C). Vitrified specimens were stored in liquid nitrogen prior to transferring and mounting on a cryogenic sample holder (Gatan 626) which was held near −180 °C. Imaging was performed on a JEOL 1210 TEM operated at 120 kV, with nominal underfocus (3−10 μm) used to obtain adequate phase-contrast. Images were recorded on a Gatan 724 multiscan CCD and processed with DigitalMicrograph version 3.3.1.
Shear Rheometry
The solution behavior of dilute aqueous dispersions was examined with steady shear experiments performed on a strain-controlled Rheometrics Fluid Spectrometer (RFS-II, Rheometrics Inc., Piscataway, NJ). The RFS-II was equipped with a force rebalance transducer, the most sensitive setting of which can measure a torque range of 0.002 to 10 g cm to within 1% accuracy. The fixture employed was a standard Couette cell (34 mm cup diameter, 33 mm bob diameter, and 33 mm bob length) with a recessed bottom. Approximately 10 mL of sample was initially loaded into the cup, and the bob was slowly lowered until the solution filled the concentric gap formed by the length of the inner cylinder. Measurements were taken at 23 ± 0.5 °C and the use of a moist sponge-lined cover minimized water loss from the sample. The dependence of viscosity on shear rate was examined through steady rate sweep experiments, with shear rates varying from 0.02−1000 s−1. Prior to actual measurements, a pre-shear delay of 120 s at low shear rates (< 10 s−1) and 10 s at higher shear rates was utilized. Reported data was recorded in both the clockwise and counterclockwise direction and averaged.
Results and Discussion
Synthesis of RGD-OB
RGD-OB block copolymers were prepared through the functionalization of OB with divinyl sulfone, and the subsequent Michael-type addition of peptide thiol groups to VS-OB (Scheme 1). The vinyl sulfone functional group has been shown to be stable toward hydrolysis49 and selective for nucleophilic thiol addition versus biological amines,50 allowing for the efficient single addition of unprotected, cysteine-containing peptides to OB diblocks. Table 1 lists key characteristics of the OB block copolymers used in the peptide conjugation reactions and includes the solution morphologies observed by cryo-TEM analysis for the hydroxyl terminated OB samples. Initially, VS-OB was prepared through the addition of divinyl sulfone to the hydroxyl terminus of the PEO block.51 The quantitative functionalization of hydroxyl terminated OB with vinyl sulfone was determined by 1H NMR spectroscopy through the comparison of relative peak intensities characteristic of the vinyl group adjacent to the sulfone (6.8, 6.4, 6.1 ppm) to those of the PEO (3.65 ppm) and PB (5.4, 4.9 ppm) blocks (Figure S1). The absence of a coupling peak in the SEC traces of VS-OB products also confirmed the single addition of OB chains to divinyl sulfone (Figure S2).
Scheme 1.
Synthetic Route for the Preparation of RGD-OB
Table 1.
Molecular Characteristics of OB Diblock Copolymers
| Sample IDa | Mn (kg mol−1)b | wPEOc | Morphologyd |
|---|---|---|---|
| O95-B46 | 6.7 | 0.63 | S |
| O49-B46 | 4.7 | 0.46 | C |
| O44-B46 | 4.4 | 0.43 | C, B |
| O39-B46 | 4.2 | 0.40 | C, B |
| O32-B46 | 4.0 | 0.36 | B, C |
| O28-B46 | 3.8 | 0.33 | B |
Subscripts denote degree of polymerization of the O and B blocks.
Number average molecular weight of OB diblock copolymers as determined by 1H NMR spectroscopy.
Weight fraction of the O block as determined by 1H NMR spectroscopy.
Micelle structures formed in water identified by cryo-TEM analysis: B = bilayers, C = cylinders, S = spheres. Predominant coexisting structure is listed first.
VS-OB block copolymers were dissolved in THF prior to the addition of triethanolamine buffer in order to speed dissolution. Upon the addition of buffer, the VS-OB dispersions were shaken until optically homogeneous. Reaction solutions were quite viscous, and dispersions prepared with VS-OB diblocks having a low PEO weight fraction (wPEO<0.63) were turbid, indicative of polymer micellization. The addition of EDTA to the triethanolamine buffer and the degassing of reaction solutions minimized spurious oxidation of peptide thiol groups. Selective addition of thiol groups to vinyl sulfone at pH 8 yield polymer-peptide adducts via a stable covalent linkage (Scheme 1). The removal of free or unconjugated peptide was achieved through extensive dialysis of the block copolymer solution against DI water.
A comparison of FT-IR spectra before and after GRGDSC conjugation to VS-OB showed the presence of resonances characteristic of the peptide (Figure S3). Direct determination of the extent of peptide conjugation to VS-OB by 1H NMR spectroscopy proved problematic due to an inability to find a suitable solvent in which to completely dissolve RGD-OB polymers. However, the slightly cloudy solutions formed upon dissolution of RGD-OB in chloroform (likely due to aggregation of the peptide termini, see below) did allow for a determination of the remaining vinyl sulfone functionality present after the peptide conjugation reaction. Given the demonstrated stability of vinyl sulfone groups to hydrolysis in the buffered medium over the reaction period,49 the extent of peptide conjugation should be equal to one minus the fraction of the remaining vinyl sulfone functionality.
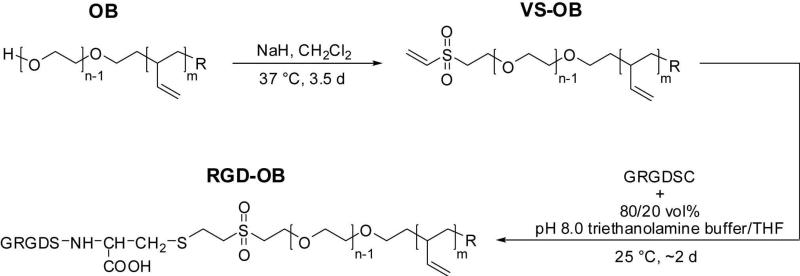
A modification of the Sakaguchi reaction described by Van Pilsum et al.44 for the determination of arginine in biological fluids was used for the direct quantification of GRGDSC conjugation to the VS-OB copolymers. Calibration curves prepared from UV-Vis spectra for each RGD-OB polymer were constructed from six samples containing a constant amount of the unconjugated OB and varying amounts of free GRGDSC (Figure 1a). As evident from the spectrum containing no peptide, the presence of OB diblock in solution does not significantly interfere with absorbance measurements over the experimentally relevant wavelength range (400−650 nm); however, a slight decrease in measured absorbance for the arginine-complex peak centered at 515nm was observed at increasing polymer concentration (OB) and when vinyl sulfone functionalized polymer (VS-OB) was used to prepare the calibration samples. Accordingly, to avoid overestimation of peptide conjugation, calibration samples were prepared with OB at concentrations that fell within the concentration range of RGD-OB used to prepare unknown samples. Unknown (RGD-OB) samples appeared more turbid than the respective calibration samples, and the spectra were characterized by increased absorbance across the measured wavelength range (Figure 1b). Quantification of peptide conjugation to VS-OB required a normalization of measured absorbance values. This was achieved through use of a linear baseline correction which facilitated the construction of calibration curves based on normalized absorbance at 515 nm (Figure S4a) and integrated peak area (Figure S4b).
Figure 1.
Representative UV-Vis spectra obtained for quantifying peptide conjugation to VS-OB. (a) Calibration samples: 0.000 μmol GRGDSC (X squares), 0.025 μmol GRGDSC (inverted triangles), 0.050 μmol GRGDSC (triangles), 0.075 μmol GRGDSC (diamonds), 0.100 μmol GRGDSC (squares), 0.125 μmol GRGDSC (circles). Calibration samples contained 360 mg of O44-B46 (b) RGD-OB samples with a maximum amount of GRGDSC = 0.075 μmol (diamonds), 0.100 μmol (squares), 0.125 μmol (circles).
Table 2 lists the molecular characteristics of RGD functionalized OB diblock copolymers prepared in this study. Overall, the mole fraction of peptide conjugated to VS-OB as determined from the calibration curves based on normalized absorbance and integrated area were in good agreement. When RGD-OB products were analyzed with 1H NMR the residual vinyl sulfone functionality correlated well with expected values, assuming the stability of the vinyl sulfone group in solution and selective nucleophilic thiol addition. The extent of peptide conjugation was controlled through manipulation of the mole ratio of GRGDSC to VS-OB in the reaction mixture. Conjugation efficiencies decreased for VS-OB samples that formed opaque solutions (wPEO < 0.63) when dispersed in the reaction medium. This may be due to the increased effect of steric constraints when attaching peptides to less curved micellar surfaces or to the inaccessibility of some polymer chains for reaction (i.e., those which comprise the inner bilayer surface of vesicles).
Table 2.
Molecular Characteristics of RGD-OB Diblock Copolymer Conjugates
| Sample IDa | RGD:VSb | XRGD-OB (Area)c | XRGD-OB (Abs)d | 1–XVS-OBe | 〈whydrophilic〉f |
|---|---|---|---|---|---|
| RGD1.0-O95-B46 | 1.1:1 | 0.96 ± 0.01 | 1.00 ± 0.01 | 0.86 | 0.66 |
| RGD0.8-O49-B46 | 1:1 | 0.76 ± 0.04 | 0.81 ± 0.07 | 0.77 | 0.52 |
| RGD0.2-O49-B46 | 0.25:1 | 0.20 ± 0.01 | 0.23 ± 0.01 | 0.28 | 0.48 |
| RGD0.8-O44-B46 | 1.1:1 | 0.80 ± 0.05 | 0.84 ± 0.04 | 0.78 | 0.50 |
| RGD0.4-O44-B46 | 0.50:1 | 0.37 ± 0.06 | 0.39 ± 0.05 | 0.37 | 0.47 |
| RGD0.6-O39-B46 | 1.1:1 | 0.61 ± 0.01 | 0.66 ± 0.01 | 0.58 | 0.47 |
| RGD0.6-O32-B46 | 1.1:1 | 0.58 ± 0.02 | 0.63 ± 0.02 | 0.58 | 0.43 |
| RGD0.6-O28-B46 | 1.1:1 | 0.55 ± 0.02 | 0.60 ± 0.01 | 0.66 | 0.40 |
RGD subscript denotes mole fraction of peptide conjugated chains, O and B subscripts denote degree of polymerization of the respective blocks.
Mole ratio of GRGDSC to VS-OB used in the coupling reactions.
Mole fraction peptide conjugation from Sakaguchi reaction- integrated peak area.
Mole fraction peptide conjugation from Sakaguchi reaction- Abs at 515 nm.
One minus mole fraction remaining vinyl sulfone functionality as determined by 1H NMR spectroscopy.
Average weight fraction of peptide plus O chains in the RGD-OB product.
Aqueous Self-Assembly Behavior of RGD-OB
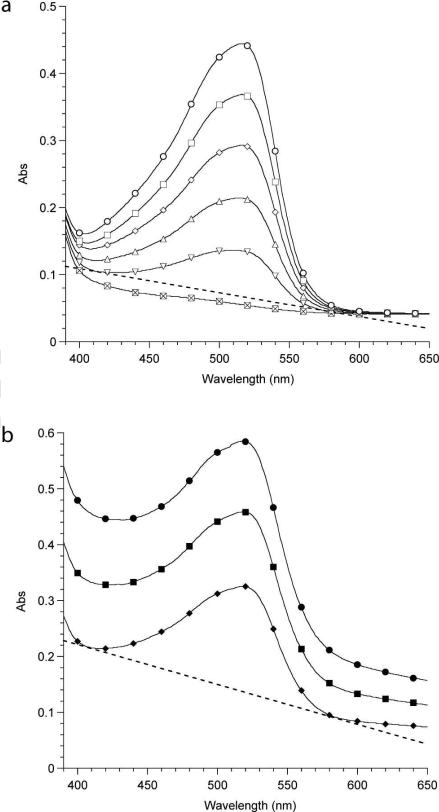
Cryo-TEM was employed as the primary method for the determination of micelle morphologies, as it allows for direct visualization of the aggregate structures formed in water. Dispersions of hydroxyl and vinyl sulfone terminated OB diblock copolymers prepared via the thin-film hydration technique showed no noticeable difference in solution morphologies. Similar attempts were made to prepare micellar solutions of RGD-OB copolymers by thin-film hydration. Initially, the peptide-functionalized polymers were dispersed in CH2Cl2, and thin films formed on the sides of vials by removal of the solvent through evaporation and subsequent drying in vacuo overnight at 25 °C. Hydration of RGD-OB films yielded aqueous dispersions that, though characterized by differing degrees of turbidity, appeared optically homogenous. Dark aggregates (10−100 nm in diameter) were commonly observed by cryo-TEM as bulbous defects in cylindrical and bilayered micelles or as detached micelle-like aggregates (Figure 2). We propose these dark aggregates are the result of peptide aggregation induced by the dissolution of RGD-OB copolymers into CH2Cl2 given the ionic nature of the GRGDSC moeities. Upon the hydration of polymer thin-films, attractive interactions between RGD moieties may facilitate the clustering peptide groups in the corona of the structures. Coupled with the mobility of the amorphous hydrophobic component, a flatting and eventual inversion of the PB/water interface would lead to the formation of a vesicle or a stable, solubilizing bilayer that surrounds aggregated peptide-functionalized copolymer chains. Such kinetically trapped structures are consistent with the dark (i.e., electron dense) spherical or disc-like features observed by cryo-TEM. These aggregates are rimmed by a poly(butadiene) bilayer (7.0 ± 0.9 nm). The sulfur atoms present on the peptide (i.e., cysteine) and the termini of hydrophilic chains (i.e., sulfone) that comprise the inner coronal layer likely gives rise to the enhanced contrast between the aggregate core and the hydrocarbon bilayer (i.e., mass-thickness contrast).52
Figure 2.
Cryo-TEM micrographs of 1 wt% aqueous dispersions highlighting peptide aggregate structures. Solutions were formed by thin-film hydration of (a) RGD0.6-O28-B46 and (b), (c) RGD1.0-O49-B46. Scale bars indicate 100 nm. (d) Schematic representation of peptide aggregate structures. The bulbous protrusions evident in panel (a) are believed to contain aggregated RGD, which is responsible for the core contrast.
In efforts to eliminate the aggregation of peptide groups within these types of structures, aqueous dispersions of RGD-OB copolymers were subsequently prepared by the direct hydration of lyophilized polymers recovered from peptide conjugation reactions (see Experimental Section). Formation of the dark aggregates represented in Figure 2 was minimized when solutions were prepared by the direct hydration of lyophilized powders, as evidenced by their near complete disappearance from cryo-TEM micrographs. An examination of the aggregate structures formed upon direct dissolution of RGD-OB copolymers follows. Here we emphasize that, as micelle morphology is influenced by the bulk state of the polymer prior to hydration, the nonergodic27 and nonequilibrium nature of these micelles must be recognized.
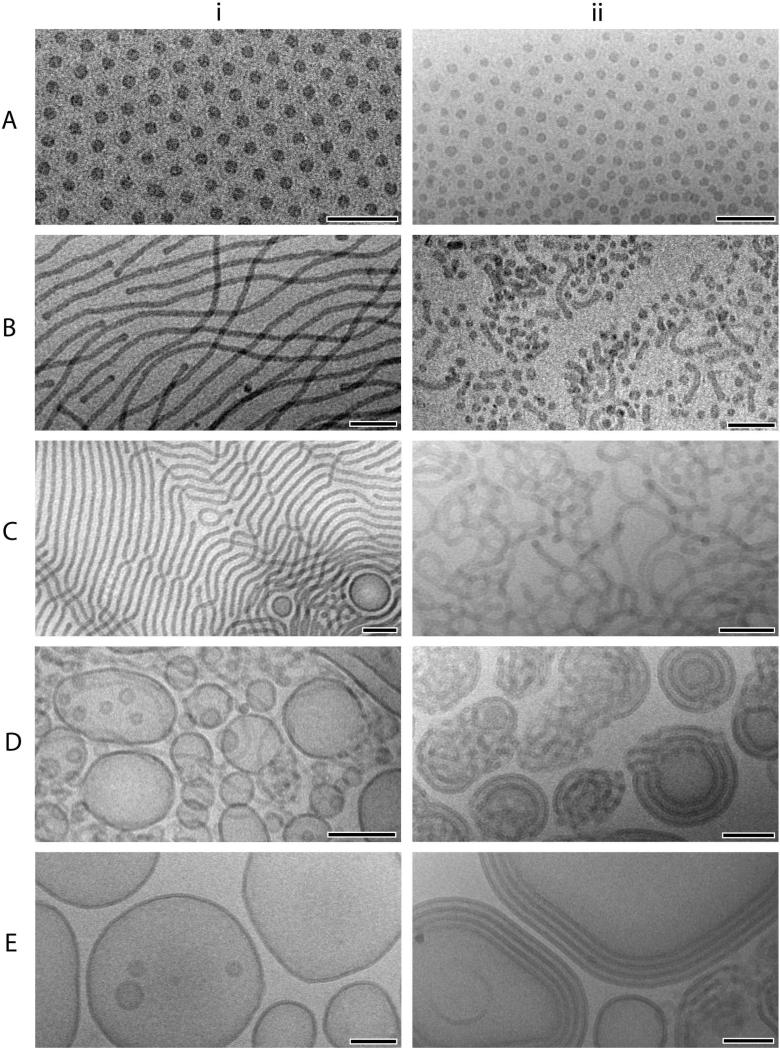
Cryo-TEM micrographs representative of solution morphologies for OB (i) and RGD-OB (ii) block copolymers are shown in Figure 3, and the dimensions of observed aggregate structures are listed in Table 3. The spherical micelles obtained from O95-B46 before and after peptide conjugation are shown in Figure 3A(i) and 3A(ii), respectively. Though the self-assembled structures of RGD1.0-O95-B46 retain the spherical morphology, we speculate that attractive interactions between the zwitterionic peptide groups result in micelle clustering that is apparent in cryo-TEM images and the increased cloudy appearance of aqueous dispersions. Oligopeptides containing the RGD-sequence can assume a β-turn in solution through intramolecular hydrogen bonding.53,54 Presenting a high concentration of ligands in the micellar corona provides the potential for intermolecular hydrogen binding between proximal peptide groups. Additionally the charged state of arginine (+) and aspartic acid (−) at pH 7 may give rise to complementary attractive electrostatic interactions.55 These types of peptide interactions may facilitate the aggregation of spherical micelles upon hydration of the bulk copolymer.
Figure 3.
Cryo-TEM micrographs obtained from 1 wt % aqueous dispersions of A: i) O95-B46, ii) RGD1.0-O95-B46, B: i) O49-B46, ii) RGD0.8-O49-B46, C: i) O44-B46, ii) RGD0.8-O44-B46, D: i) O32-B46, ii) RGD0.6-O32-B46, E: i) O28-B46, ii) RGD0.6-O28-B46. Scale bars indicate 100 nm.
Table 3.
Dimensions of OB Micellar Structures
| Sample ID | Morphologya | d (nm)b | Sample ID | Morphologya | d (nm)b |
|---|---|---|---|---|---|
| O95-B46 | S | 18.4 ± 1.8 | RGD1.0-O95-B46 | S | 16.9 ± 2.9 |
| O49-B46 | C | 14.1 ± 0.9 | RGD0.8-O49-B46 | S | 17.7 ± 3.4 |
| C | 14.9 ± 1.2 | ||||
| RGD0.2-O49-B46 | S | 16.5 ± 3.3 | |||
| C | 14.7 ± 0.9 | ||||
| O44-B46 | C | 15.0 ± 1.1 | RGD0.8-O44-B46 | C | 14.5 ± 0.7 |
| S | 17.2 ± 3.4 | ||||
| RGD0.4-O44-B46 | S | 17.9 ± 3.5 | |||
| C | 14.9 ± 1.1 | ||||
| O39-B46 | C | 14.8 ± 1.0 | RGD0.6-O39-B46 | C | 15.4 ± 1.0 |
| B | 8.8 ± 0.7 | B | 9.5 ± 0.5 | ||
| O32-B46 | C | 14.2 ± 1.0 | RGD0.6-O32-B46 | C | 14.2 ± 0.8 |
| B | 9.0 ± 0.5 | B | 9.1 ± 0.6 | ||
| O28-B46 | B | 9.1 ± 0.4 | RGD0.6-O28-B46 | B | 9.4 ± 0.5 |
Micelle structures formed in water identified with cryo-TEM: B = bilayers, C = cylinders, S = spheres. Predominant coexisting structure is listed first.
Core diameter of spherical and cylindrical micelles, thickness of the vesicle bilayer.
Figure 3B shows the aggregate structures observed before (i) and after (ii) peptide conjugation to a cylinder forming OB diblock. Peptide addition to 80 mol % of O49-B46 chains shifts the average hydrophilic weight fraction (〈whydrophilic〉) of amphiphiles from 0.46 to 0.52, and the effective increase in corona volume drives a morphological transition from cylindrical (Dcyl= 14.1 ± 0.9 nm) to spherical (Dsph= 17.7 ± 3.4 nm) micelles. The presence of short rods (Dcyl=14.9 ± 1.2 nm) observed in aqueous dispersions of RGD0.8-O49-B46 may be due to incomplete peptide functionalization or to the corresponding increase in overall coronal chain polydispersity. However, the inability of amphiphilic polymer dispersions to reach equilibrium through single chain exchange between micelles23 and the inherent ability of polymeric amphiphiles to accommodate slight distortions of core and coronal chain dimensions can provide for the coexistence of structures with differing interfacial curvatures. Examples can be found in a number of amphiphilic diblock copolymer systems,25,30 including OB diblocks of similar composition (wPEO 0.50−0.54).26,27
At a poly(ethylene oxide) weight fraction of 0.43, OB aqueous dispersions are characterized by the presence of long (> 5 μm) worm-like micelles with a small population of vesicles (Figure 3C(i)). Upon peptide addition to 80 mol % of O44-B46 chains, short cylinders (≤ 1 μm), highly branched cylindrical network structures, and spherical micelles populate the solution (Figure 3C(ii)). Based on the overall amphiphile composition, RGD0.8-O44-B46 is expected to self-assemble into cylindrical and/or spherical micelles. Analysis of cryo-TEM images reveals that although a cylindrical micelle structure is retained, the morphology is dominated by extensive branch and loop defects. The RGD0.8-O44-B46 network structure closely resembles that formed in aqueous dispersions of higher molecular weight OB diblocks (O110-B170).29 However, unlike the phase separated Y-junction based network reported by Jain et al.,29 which coalesce into a macroscopic network with time, dispersions of RGD0.8-O44-B46 remain homogeneous when left undisturbed for several weeks.
The branch points and loops evident in the cylindrical micelles found in Figure 3C(ii) could be the result of peptide-peptide interactions; the presence of these features does not depend solely on the bulk state of the copolymer prior to hydration.56 A low bending modulus and the ease of copolymer redistribution within a micelle provides a small energy barrier to oppose cylinder bending for aggregates containing fluid-like (i.e., low Tg) hydrophobic cores. Peptide-peptide interactions active upon corona hydration could strongly influence aggregate morphology by promoting the intermicellar attraction of cylindrical micelles and possibly the collapse of nascent aggregates onto the bulk polymer surface. Coupled with the nonergodicity common to highly amphiphilic block copolymers, it is unlikely that an intramicellar redistribution of RGD0.8-O44-B46 chains would yield a long cylindrical micelle once the aggregate contained loops, thus resulting in the formation of network fragments.
A slight decrease in the hydrophilic weight fraction of OB (wPEO = 0.40) leads to self-assembly into vesicular and cylindrical aggregates (Figure 3D(i)). Peptide conjugation to 60 mol % of O32-B46 chains shifts the whydrophilic to 0.43, a composition at which unfunctionalized OB diblock solution morphology is dominated by cylindrical micelles (e.g., the solution morphology of O44-B46 (wPEO = 0.43) is shown in Figure 3C(i)). The self-assembly of RGD0.6-O32-B46 results in the formation of bilayer structures with multiple lamella and dense or collapsed cylindrical micelle networks as observed in Figure 3D(ii). Combinations of these structural elements emerge as multilayer vesicles perforated to various extents by highly branched network fragments.
Unilamellar vesicles emerge as the predominant species in solution when the hydrophilic content in the OB diblock series falls below wPEO ∼ 0.33 (Figure 3E(i)).29 After peptide conjugation to 60 mol % O28-B46 polymer chains, cryo-TEM image analysis reveals retention of the bilayered aggregate structure (Figure 3E(ii)), though a small amount of the collapsed cylindrical network observed in RGD0.6-O32-B46 solutions (Figure 3D(ii)) is also present. Interestingly, the formation of vesicle structures with multiple, precisely correlated, wall layers is prominent in RGD0.6-O28-B46 solutions. This morphology has not been seen in unmodified OB diblock copolymers.
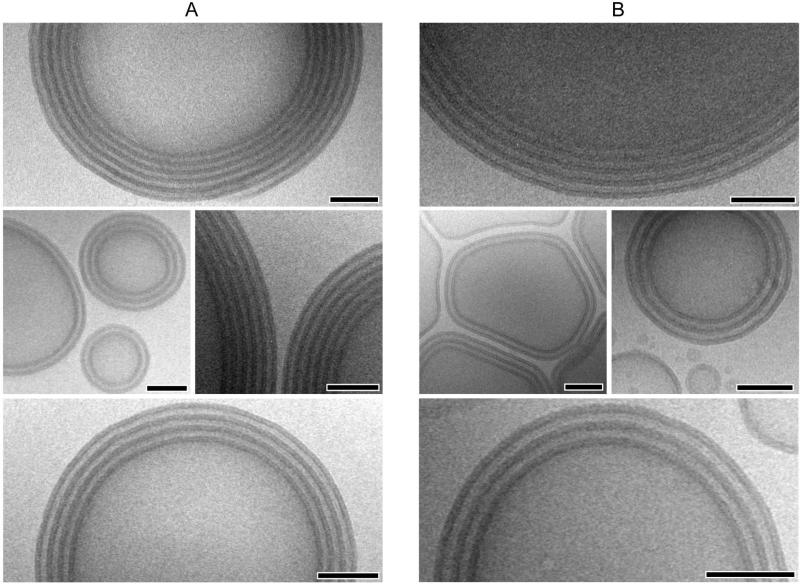
The sporadic examples of multiple lamellar vesicles observed in O28-B46 solutions are typically single bilayer vesicles located at random within the enclosed volume of larger unilamellar vesicles (e.g., notice the small vesicles present inside another larger vesicle in Figure 3E(i). The multilayer vesicles formed from RGD0.6-O28-B46 have a distinctly annular appearance, with individual vesicle bilayers (ranging from 2 to 7 in number) appearing as concentric rings with repeat spacing of 9.3 ± 1.1 nm (Figure 4). We assume that the alternating dark and light domains are PB and hydrated PEO, respectively. Assuming a degree of stretching for the corona chains that is at least as large as that determined for cylindrical micelle forming OB diblocks,57 the minimum expected brush length for a tethered poly(ethylene oxide) chain with 28 repeat units is expected to be about 8 nm. This reveals that PEO chains in the alternating coronal layers of the multilayer vesicles are in a compressed or highly interdigitated state as compared to the well-hydrated chains found in the corona of OB spherical and cylindrical micelles. Multilayer vesicle formation was found to have no dependence on the bulk state of the polymer prior to dispersion, as evident in the annular structures formed from hydration of RGD0.6-O28-B46 lyophilized powders (Figure 4A) and thin polymer films (Figure 4B). The organization and regular spacing of bilayers within the multilamellar vesicles formed from RGD0.6-O28-B46 lends further credence to the influence of attractive peptide-peptide interactions in the corona on self-assembly and aggregate morphology.
Figure 4.
Cryo-TEM micrographs of multi-lamellar vesicles obtained from 1 wt % aqueous dispersion of RGD0.6-O28-B46. A: Solution prepared by hydration of a bulk, lyophilized powder, B: Solution prepared by hydration of polymer thin-film. Scale bars indicate 100 nm.
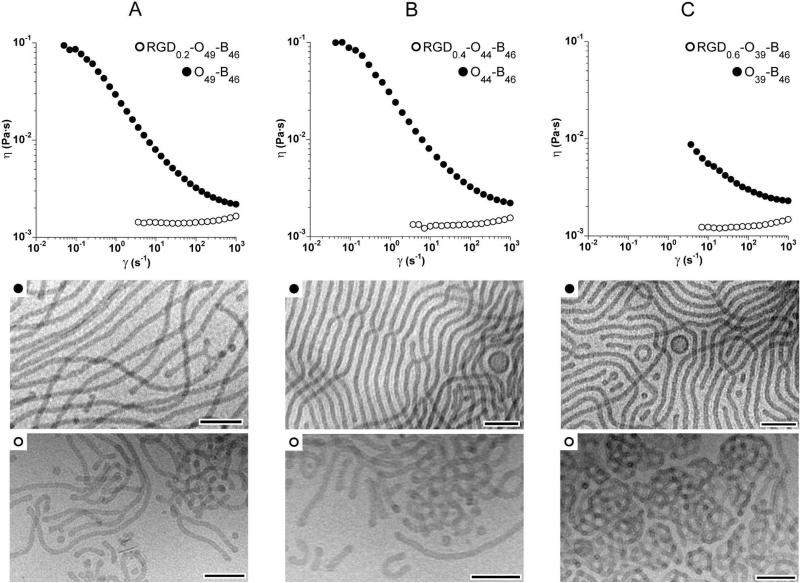
In attempts to prepare extended wormlike micelles from RGD-OB copolymers we prepared series of block copolymer conjugates having similar overall composition but with varying extents of peptide functionality. Long wormlike micelles were the predominant structure formed upon self-assembly of each parent OB (wPEO = 0.46, 0.43, 0.40) as evident from cryo-TEM micrographs and the shear thinning behavior observed in 1 wt % aqueous dispersions (the filled circle data shown in Figure 5 A–C. Manipulating the relative amount of peptide conjugated to OB diblocks allowed for the targeting of RGD-OB polymers with overall compositions (〈whydrophilic〉 ∼ 0.47) conducive to wormlike micelle formation (Table 1). However, in the three cases examined, self-assembly of the peptide functionalized diblock failed to yield a majority population of extended cylindrical micelles. This is evident from cryo-TEM images and the dramatic change in solution behavior exhibited by OB dispersions upon peptide conjugation (Figure 5 A–C). All of the RGD-OB dispersions were only slightly more viscous than water over the range of experimentally accessible shear rates. Additionally, shear rate dependent viscosity exhibited by OB diblocks was not observed in dispersions of RGD-OB. The observed Newtonian behavior in the RGD-conjugated samples indicates an absence of long, entangled wormlike micelles in these solutions. A comparison cryo-TEM images from RGD0.8-O49-B46 (Figure 3B(ii)) and RGD0.2-O49-B46 (Figure 5 A) samples reveals a qualitative increase in overall contour length of cylindrical micelles formed from the same OB when extent of peptide conjugation is decreased. However, the incorporation of just 20 mol% peptide functionality into O49-B46 impeded self-assembly into extended cylindrical aggregates.
Figure 5.
Cryo-TEM micrographs and shear rate dependent viscosity of 1 wt % aqueous dispersions: A: (filled circles) O49-B46, (open circles) RGD0.2-O49-B46, B: (filled circles) O44-B46, (open circles) RGD0.4-O44-B46, C: (filled circles) O39-B46, (open circles) RGD0.6-O39-B46. Scale bars indicate 100 nm.
Increasing the level of peptide conjugated to cylinder forming OB diblocks, while keeping the overall hydrophilic content constant (〈whydrophilic〉 = 0.47), led to the increased formation of cylindrical micelle network fragments as evident when comparing solutions of RGD0.4-O44-B46 (Figure 5 B) and RGD0.6-O39-B46 (Figure 5 C). Similar observations can be made when comparing solutions of O44-B46 with 40 mol % (Figure 5 B) and 80 mol % (Figure 3 C(ii)) peptide conjugation. Though looped cylindrical network fragments were the dominant aggregate species observed for RGD0.6-O39-B46 (Figure 5 C), samples also contained a minor population of long wormlike micelles in coexistence with more exotic cylinder-bilayer hybrid structures. Examples include wormlike micelles emanating from bilayer ribbons and unilamellar vesicles that are ringed repeatedly by cylindrical micelle windings (Figure 6). However, like all other RGD-OB samples prepared, the extent of long wormlike micelle formation was not appreciable enough for solutions to exhibit the shear rate dependent viscosity indicative of entangled wormlike micelles.
Figure 6.
Cryo-TEM micrographs of cylinder-bilayer hybrid structures observed in solutions of RGD0.6-O39-B46. Black arrows highlight a bilayer sheet in coexistence with cylindrical micelles. Scale bars indicate 100 nm.
Summary
This report details the conjugation of an RGD-containing peptide to a series of OB diblock copolymer amphiphiles and examines the effect of peptide addition on micelle morphology. Measurements of peptide functionality on OB diblocks, made via a modified procedure for the quantification of arginine, correlated well with 1H NMR calculations of residual vinyl sulfone groups. Attempts to form dispersions of RGD-OB via a thin-film hydration method generally resulted in micelle-like structures with cores of aggregated peptide functionalized polymer chains. The direct dispersion of RGD-OB polymers into deionized water yielded a variety of micelle structures that showed a tendency to aggregate in solution. The aggregate morphologies of RGD-OB polymers were affected beyond the extent predicted by the overall change in amphiphile composition due to peptide conjugation, and we attribute these abnormalities in solution behavior to attractive interactions between peptide moieties conjugated to the termini of coronal chains.
Aggregate morphology was retained upon peptide conjugation to an OB diblock copolymer that was observed to form spherical micelles. Additionally, peptide conjugation to a vesicle forming OB diblock resulted in preservation of the bilayer structure, though precisely correlated multilayer vesicles were prevalent. Various amounts of peptide were conjugated to a number of cylinder forming OB diblock copolymers, covering the range of compositions at which cylindrical micelles are expected to form. Though cylindrical aggregates were observed via cryo-TEM, attempts to form extended cylindrical micelles from peptide functionalized OB diblocks were unsuccessful, as evidenced by the Newtonian behavior of aqueous dispersions. Additionally, a fragmented cylindrical network phase emerged upon peptide conjugation to certain OB diblocks that had only previously been observed in solutions of OB amphiphiles with higher molecular weight cores.
Supplementary Material
Acknowledgments
The authors thank Dr. Sumeet Jain for providing the OB diblock copolymers used in this study. We thank Professor Robert Tranquillo for helpful discussions. Parts of this work were carried out in the University of Minnesota I.T. Characterization Facility, which receives partial support from NSF through the NNIN program. This work was supported by the NIH (1R21EB00989-01).
Footnotes
Supporting Information Available: Supporting NMR, SEC, IR, and UV-Vis data for the VS and peptide functionalized OB block polymers. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Zana R, Kaler EW, editors. Giant Micelles: Properties and Applications. Vol. 140. CRC Press; Boca Raton: 2007. [Google Scholar]
- 2.Torchilin VP, editor. Nanoparticulates as Drug Carriers. Imperial College Press; London: 2006. [Google Scholar]
- 3.Gohy J-F. Advances in Polymer Science. 2005;190:66–136. [Google Scholar]
- 4.Hamley IW. Block Copolymers in Solution: Fundamentals and Applications. Wiley; Hoboken, NJ: 2005. [Google Scholar]
- 5.Halperin A, Tirrell M, Lodge TP. Advances in Polymer Science. 1992;100:31–71. [Google Scholar]
- 6.Torchilin VP. Journal of Controlled Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 7.Kwon GS, Kataoka K. Advanced Drug Delivery Reviews. 1995;16:295–309. [Google Scholar]
- 8.Kakizawa Y, Kataoka K. Advanced Drug Delivery Reviews. 2002;54:203–222. doi: 10.1016/s0169-409x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- 9.Danson S, Ferry D, Alakhov V, Margison J, Kerr D, Jowle D, Brampton M, Halbert G, Ranson M. British Journal of Cancer. 2004;90:2085–2091. doi: 10.1038/sj.bjc.6601856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura Y, Hamaguchi T, Ura T, Muro K, Yamada Y, Shimada Y, Shirao K, Okusaka T, Ueno H, Ikeda M, Watanabe N. British Journal of Cancer. 2004;91:1775–1781. doi: 10.1038/sj.bjc.6602204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchino H, Matsumura Y, Negishi T, Koizumi F, Hayashi T, Honda T, Nishiyama N, Kataoka K, Naito S, Kakizoe T. British Journal of Cancer. 2005;93:678–687. doi: 10.1038/sj.bjc.6602772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, Nakatomi I, Yokoyama M, Kataoka K, Kakizoe T. British Journal of Cancer. 2005;92:1240–1246. doi: 10.1038/sj.bjc.6602479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Journal of Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 14.Hamaguchi T, Kato M, Yasui H, Morizane C, Ikeda M, Ueno H, Muro K, Yamada Y, Okusaka T, Shirao K, Shimada Y, Nakahama H, Matsumura Y. British Journal of Cancer. 2007;97:170–176. doi: 10.1038/sj.bjc.6603855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Nature Nanotechnology. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. Journal of Controlled Release. 2003;90:323–334. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 17.Discher DE, Ortiz V, Srinivas G, Klein ML, Kim Y, Christian D, Cai S, Photos P, Ahmed F. Progress in Polymer Science. 2007;32:838–857. doi: 10.1016/j.progpolymsci.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nature Nanotechnology. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 19.Sutton D, Nasongkla N, Blanco E, Gao J. Pharmaceutical Research. 2007;24:1029–1046. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 20.Mahmud A, Xiong X-B, Aliabadi HM, Lavasanifar A. Journal of Drug Targeting. 2007;15:553–584. doi: 10.1080/10611860701538586. [DOI] [PubMed] [Google Scholar]
- 21.Krag DN, Shukla GS, Shen G-P, Pero S, Ashikaga T, Fuller S, Weaver DL, Burdette-Radoux S, Thomas C. Cancer Res. 2006;66:7724–7733. doi: 10.1158/0008-5472.CAN-05-4441. [DOI] [PubMed] [Google Scholar]
- 22.Booth C, Attwood D. Macromolecular Rapid Communications. 2000;21:501–527. [Google Scholar]
- 23.Won Y-Y, Davis HT, Bates FS. Macromolecules. 2003;36:953–955. [Google Scholar]
- 24.Munk P. In: Solvents and Self-Organization of Polymers. Webber SE, Munk P, Tuzar Z, editors. Vol. 327. Kluwer Academic Publishers; Dordrecht: 1996. [Google Scholar]
- 25.Zupancich JA, Bates FS, Hillmyer MA. Macromolecules. 2006;39:4286–4288. [Google Scholar]
- 26.Won Y-Y, Brannan AK, Davis HT, Bates FS. J. Phys. Chem. B. 2002;106:3354–3364. [Google Scholar]
- 27.Jain S, Bates FS. Macromolecules. 2004;37:1511–1523. [Google Scholar]
- 28.Discher BM, Won Y-Y, Ege DS, Lee JC-M, Bates FS, Discher DE, Hammer DA. Science. 1999;284:1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 29.Jain S, Bates FS. Science. 2003;300:460–464. doi: 10.1126/science.1082193. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Eisenberg A. J. Am. Chem. Soc. 1996;118:3168–3181. [Google Scholar]
- 31.Zhang L, Yu K, Eisenberg A. Science. 1996;272:1777–1779. doi: 10.1126/science.272.5269.1777. [DOI] [PubMed] [Google Scholar]
- 32.Shen H, Eisenberg A. J. Phys. Chem. B. 1999;103:9473–9487. [Google Scholar]
- 33.Pochan DJ, Chen Z, Cui H, Hales K, Qi K, Wooley KL. Science. 2004;306:94–97. doi: 10.1126/science.1102866. [DOI] [PubMed] [Google Scholar]
- 34.Cui H, Chen Z, Zhong S, Wooley KL, Pochan DJ. Science. 2007;317:647–650. doi: 10.1126/science.1141768. [DOI] [PubMed] [Google Scholar]
- 35.Cornelissen J. J. n. L. n. M., Fischer M, Sommerdijk N. A. n. J. n. M., Nolte R. J. n. M. Science. 1998;280:1427–1430. doi: 10.1126/science.280.5368.1427. [DOI] [PubMed] [Google Scholar]
- 36.Ruoslahti E. Annual Review of Cell and Developmental Biology. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 37.Eble J, Kuhn K, editors. Integrin-Ligand Interaction. R.G. Landes; Austin: 1997. [Google Scholar]
- 38.Brooks PC, Clark RAF, Cheresh DA. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 39.Burke PA, DeNardo SJ, Miers LA, Lamborn KR, Matzku S, DeNardo GL. Cancer Res. 2002;62:4263–4272. [PubMed] [Google Scholar]
- 40.Bibby DC, Talmadge JE, Dalal MK, Kurz SG, Chytil KM, Barry SE, Shand DG, Steiert M. International Journal of Pharmaceutics. 2005;293:281–290. doi: 10.1016/j.ijpharm.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Schiffelers RM, Koning GA, ten Hagen TLM, Fens MHAM, Schraa AJ, Janssen APCA, Kok RJ, Molema G, Storm G. Journal of Controlled Release. 2003;91:115–122. doi: 10.1016/s0168-3659(03)00240-2. [DOI] [PubMed] [Google Scholar]
- 42.Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 43.Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 44.Van Pilsum JF, Martin RP, Kito E, Hess J. J. Biol. Chem. 1956;222:225–236. [PubMed] [Google Scholar]
- 45.Hillmyer MA, Bates FS. Macromolecules. 1996;29:6994–7002. [Google Scholar]
- 46.All OB polymers used in this work were prepared by Dr. Sumeet Jain. All reported molecular characteristics were determined independently from measurements done on purified OB copolymers and products.
- 47.Parniak M, Lange G, Viswanatha T. Journal of Biochemical and Biophysical Methods. 1983;7:267–276. doi: 10.1016/0165-022x(83)90051-9. [DOI] [PubMed] [Google Scholar]
- 48.Bellare JR, Davis HT, Scriven LE, Talmon Y. Journal of Electron Microscopy Technique. 1988;10:87–111. doi: 10.1002/jemt.1060100111. [DOI] [PubMed] [Google Scholar]
- 49.Morpurgo M, Veronese FM, Kachensky D, Harris JM. Bioconjugate Chem. 1996;7:363–368. doi: 10.1021/bc9600224. [DOI] [PubMed] [Google Scholar]
- 50.Friedman M, Cavins JF, Wall JS. Journal of the American Chemical Society. 1965;87:3672–3682. [Google Scholar]
- 51.Lutolf MP, Hubbell JA. Biomacromolecules. 2003;4:713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 52.Misell DL. Image Analysis, Enhancement and Interpretation. Elsevier/North-Holland Biomedical Press; Amsterdam: 1978. [Google Scholar]
- 53.Johnson WC, Pagano TG, Basson CT, Madri JA, Gooley P, Armitage IM. Biochemistry. 1993;32:268–273. doi: 10.1021/bi00052a034. [DOI] [PubMed] [Google Scholar]
- 54.Park HS, Kim C, Kang YK. Biopolymers. 2002;63:298–313. doi: 10.1002/bip.10067. [DOI] [PubMed] [Google Scholar]
- 55.Mart RJ, Osborne RD, Stevens MM, Ulijn RV. Soft Matter. 2006;2:822–835. doi: 10.1039/b607706d. [DOI] [PubMed] [Google Scholar]
- 56.Zupancich JA. Amphiphilic Block Copolymers for Biomedical Applications; Chemical Engineering and Materials Science. University of Minnesota; Minneapolis: 2008. Defected cylinderical micelles were formed from RGD-OB copolymers upon hydration of thin-films or lyophilized powders. [Google Scholar]
- 57.Won Y-Y, Davis HT, Bates FS, Agamalian M, Wignall GD. J. Phys. Chem. B. 2000;104:7134–7143. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.