Abstract
Pioneer axons from the cingulate cortex initiate corpus callosum (CC) development, yet nothing is known about the molecules that regulate their guidance. We demonstrate that neuropilin 1 (Npn1) plays an integral role in the development of the CC. Npn1 is localized to axons of cingulate neurons as they cross the midline, and multiple class 3 semaphorins (Semas) are expressed around the developing CC, implicating these guidance molecules in the regulation of Npn1-expressing axons emanating from the cingulate cortex. Furthermore, axons from the cingulate cortex display guidance errors in Npn1Sema- mice, a knockin mouse line in which Npn1 is unable to bind Semas. Analysis of mice deficient in the transcription factor Emx2 demonstrated that the cingulate cortex of these mice was significantly reduced in comparison to wild-type controls at E17 and that the CC was absent in rostral sections. Expression of Npn1 was absent in rostral sections of Emx2 mutants, suggesting that Npn1-expressing cingulate pioneers are required for CC formation. These data highlight a central role for Npn1 in the development of projections from the cingulate cortex and further illustrate the importance of these pioneer axons in the formation of the CC.
Keywords: axon guidance, cingulate cortex, Emx2, neuropilin 1, Sema
Introduction
The corpus callosum (CC) comprises the largest fiber tract within the cortex and consists of axons that join the 2 cerebral hemispheres, enabling interhemispheric communication and subsequent coordination of activity (Yorke and Caviness 1975). In rodents, axons crossing the CC originate predominantly from neurons located in layers II, III, and V of the cerebral cortex. Development of the CC begins embryonically and continues postnatally; for instance, in mice, CC development begins at approximately embryonic day 15 (E15) and continues to postnatal day 14 (P14) (Plachez and Richards 2005). Many molecular determinants regulating the guidance of neocortical axons across the CC have recently been identified (Lindwall et al. 2007). These include axon guidance molecules such as Slit2 (Shu and Richards 2001; Bagri et al. 2002) and Netrin1 (Serafini et al. 1996) and axon guidance receptors including Robo (Andrews et al. 2006), Deleted in Colorectal Cancer (DCC; Fazeli et al. 1997) and Ryk (Keeble et al. 2006). Furthermore, midline glial populations, including the glial wedge and indusium griseum glia, are also critical for CC development. Defects in midline glial development, such as that observed in mice lacking Nfia or Fgfr1, correlate with deficits in CC formation (Shu et al. 2003; Smith et al. 2006).
Pioneer neurons are the first to project axons along pathways in the nervous system that later form axon tracts. The first pioneer axons to cross the midline, and so initiate CC development, derive from the most medial aspect of the cortex, the cingulate cortex. These pioneering axons cross the midline at around E15 in mice and E17 in rats (Koester and O'Leary 1994; Rash and Richards 2001). Such pioneers are critical for axon tract development. For instance, the other main efferent axonal projection from the mammalian cortex, the corticothalamic pathway, is pioneered by subplate neurons that project into the internal capsule (McConnell et al. 1989; De Carlos and O'Leary 1992). Ablation of subplate pioneer neurons results in aberrant corticothalamic (McConnell et al. 1994) and thalamocortical pathway formation (Ghosh et al. 1990; Ghosh and Shatz 1993). Two distinct groups of pioneer neurons, Cajal–Retzius cells and GABAergic neurons, have also been demonstrated to regulate the development of entorhinal and commissural connections to the hippocampus (Super et al. 1998), further emphasizing the importance of pioneering neurons in cortical development. However, although Pax6 and R-cadherin have been implicated in regulating the guidance of longitudinal axons pioneering the tract of the postoptic commissure (Andrews and Mastick 2003) and Slit–Robo signaling has recently been shown to regulate the guidance of axons that pioneer longitudinal tracts between the brain and spinal cord (Farmer et al. 2008), little is known about the molecules that regulate the guidance of neocortical pioneers, including those of the CC.
Here, we demonstrate that neuropilin 1 (Npn1) is expressed on the axons of cingulate pioneers, as they traverse the CC, and plays an important role in the development of this axon tract. Npn1 is a high-affinity receptor for the class 3 semaphorins (Semas) (He and Tessier-Lavigne 1997; Takahashi et al. 1999) and regulates axon guidance in a variety of contexts, including hippocampal formation (Pozas et al. 2001; Gu et al. 2003), dorsal root ganglion development (He and Tessier-Lavigne 1997), and the innervation of the inner ear and spinal cord by sensory afferents (Gu et al. 2003). Using immunohistochemisty and in situ hybridization, we demonstrate that cingulate pioneer neurons express Npn1, L1, and members of the plexin A subfamily. Multiple class 3 Semas are also expressed at the cortical midline from E15 to E17, and using an in vitro coculture paradigm, we show that Sema3C acts as an attractant for cingulate cortex neurites. Furthermore, we use mice lacking the transcription factor Emx2, which have a reduced cingulate cortex (Pellegrini et al. 1996), to demonstrate a strong correlation between the presence of Npn1-expressing pioneers and the presence of the CC. These data provide a description of the molecular mechanisms driving guidance of cortical pioneers during development and demonstrate that Npn1 plays a crucial role in the formation of the CC.
Materials and Methods
Mouse Strains
All the animals were bred on-site at The University of Queensland with approval from The University of Queensland Animal Ethics and Animal Welfare Unit. Animals used in this study were wild-type C57Bl/6J, Npn1Sema- (Gu et al. 2003), Emx2-deficient (Pellegrini et al. 1996), and Sema3A-deficient (Behar et al. 1996) mice. Timed-pregnant females were obtained by placing male and female mice together overnight. The following day was designated as embryonic day 0 (E0) if the female had a vaginal plug. Heterozygous mice were bred to obtain wild-type, heterozygous, and homozygous progeny. Embryos were genotyped by polymerase chain reaction.
Immunohistochemistry
On the required gestational day, embryos were transcardially perfused with 0.9% saline, followed by 4% paraformaldehyde (PFA), and then postfixed in 4% PFA for 24 h before being stored in phosphate buffered saline at 4 °C until sectioning. Brains were removed, blocked in 3% noble agar (DIFCO, Sparks, MS), and then sectioned coronally at 45 μm on a vibroslicer (Leica, Nussloch, Germany). Sections were processed free floating for immunohistochemistry using the chromogen 3,3′-diaminobenzidine as described previously (Campbell et al. 2008; Plachez et al. 2008). Primary antibodies used for immunohistochemistry were anti-Npn1 (a gift from Prof. David Ginty, Johns Hopkins University; 1/75 000), anti-L1 (Chemicon International, Bedford, MA; 1/5000), and anti-DCC (a gift from Assoc. Prof. Helen Cooper, Queensland Brain Institute, The University of Queensland; 1/30 000). Secondary antibodies used were biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA; 1/1000) and biotinylated donkey anti-rat IgG (Jackson ImmunoResearch, West Grove, PA; 1/500).
In Situ Hybridization
For in situ hybridization, embryos were transcardially perfused with PFA, blocked, and sectioned at 45 μm on a vibroslicer as described above. Sections were then mounted onto Superfrost slides (Menzel-Glaser, Brunswick, Germany) and allowed to dry. In situ hybridization was performed as described previously (Piper et al. 2000) with minor modifications. All hybridizations were carried out at 68 °C using the color substrate BM Purple (Roche, Mannheim, Germany). Expression patterns were assessed using digoxygenin-labeled antisense riboprobes specific for Npn1, Sema3A, Sema3B, Sema3C, Sema3F, plexin A1, plexin A2, or plexin A3.
Image Acquisition and Analysis
Once mounted and coverslipped, sections were imaged using an upright microscope (Zeiss Z1, Zeiss, Goettingen, Germany) attached to a digital camera (Zeiss AxioCam HRc), and images were captured using AxioVision software (Zeiss). When comparing wild-type to knockout tissue, sections from matching positions along the rostrocaudal axis were selected. For all immunohistochemistry and in situ hybridization experiments, sections from n = 3 different brains of each genotype were analyzed.
Sema3A and Sema3C Transfections in HEK293T Cells
HEK293T cells were transfected either with a full-length mammalian expression vector containing Sema3A or a Sema3C (in which the first furin cleavage site was mutated; both constructs were a gift from Dr Andreas Püschel, Genetik, Westfälische Wilhelms-Universität Münster, Münster, Germany) (Adams et al. 1997) or mock transfected as a control with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) using standard protocols. For both Sema3C- and mock-transfected controls, cells were cotransfected with a plasmid encoding red fluorescent protein (RFP). Following transfection, cells were cultured for 24 h and then checked for transfection efficiency via expression of the RFP marker. Cells were then trypsinized, centrifuged, and resuspended in 100 μl of 1× Opti-MEM (Invitrogen), 0.09% v/v NaHCO3 (Biowhittaker, Charles City, IA), 1% v/v Penicillin–Streptomycin–Fungizone (Invitrogen), and 58% v/v type 1 rat-tail collagen (BD Biosciences, Franklin Lakes, NJ). To make cell blocks, 20 μl of cells were added to 120 μl of warm 2% low melting point agarose (Lonza, Basel, Switzerland). Once set, the agar was cut into cubes of approximately 500 μm.
Collagen Gel Assay for Neurite Guidance and Outgrowth
The neocortex or cingulate cortex were dissected from E15.5 or E16 embryos and cut into 350 μm explants using a McIlwain Tissue Chopper (The Mickle Laboratory Engineering Co., Guildford, United Kingdom). For hemisected slices, E16 embryonic brains were cut into 300 μm slices using a vibroslicer. Brain slices were then hemisected for the culture assay. Four-well plates were prepared by layering 250 μl of 0.2% collagen mix (in 1× Opti-MEM, Invitrogen; 0.09% v/v NaHCO3, Biowhittaker; 1% v/v Penicillin–Streptomycin–Fungizone, Invitrogen; and 58% v/v type 1 rat-tail collagen, BD Biosciences) in each well, which was then allowed to set. On top of this bottom layer of collagen, 3 explants surrounding a mock-transfected control, Sema3A, or Sema3C-transfected cell block, were then quickly embedded in a further 50 μl of 0.2% collagen mix. Explants were placed approximately 500 μm from the cell block. After positioning the explants, the top collagen layer was allowed to set before incubating for 48 h in a 5% CO2 humidified incubator at 37 °C. Explants were then fixed overnight in 10% formaldehyde, before being rinsed and processed for immunohistochemistry. For the Sema3A culture assay, explants were injected with DiI (Invitrogen; in a 10% solution of dimethylformamide) to measure neurite outgrowth. Cultures were then kept at 37 °C in the dark to allow dye transport and then counterstained with Sytox green (Invitrogen) to visualize the cell blocks and explants. Cultures were imaged using a confocal microscope (Fluoview FV5000, Olympus, NY). For the Sema3C culture assay, the primary antibody used was antineuronal-specific βIII tubulin (TuJ-1 clone, R&D Systems, Minneapolis, MN; 1/1000). The secondary antibody was goat anti-mouse Alexa Fluor 488 (Invitrogen; 1/1000). Following staining for neurites, explants were imaged at 5× magnification. Approximately 10 to 15 optical sections were imaged through each explant with an upright Axio-Imager Z1 (Zeiss) microscope fitted with ApoTome (Zeiss) and an AxioCam HRm camera (Zeiss) at 20 μm across the explant to obtain all neurites in focus. Optical sections were then flattened into a multiple image projection. To quantify neurite outgrowth, each explant image was processed using an algorithm and implementation modified from Weaver et al. (2003). This consisted of pre- and postprocessing in MATLAB (The Mathworks Inc., Natick, MA) and C code, which automatically identified explants and used a ridge-tracing algorithm to locate neurite pixels. The total number of neurite pixels per explant was quantified, as was the total number of neurite pixels on both the side of the explant proximal to, and that distal to, the cell block. These results were then used to calculate the guidance ratio (GR) and the outgrowth for each explant (Rosoff et al. 2004). The GR was defined as: GR = (proximal neurite pixels − distal neurite pixels)/(total neurite pixels). Outgrowth was defined as: outgrowth = (proximal neurite pixels + distal neurite pixels)/total explant pixels. Explants displaying low growth were eliminated by removing all explants with an outgrowth value less than one standard deviation below the mean for that experiment. Statistical analyses were performed using a 2-tailed unpaired t-test. Error bars indicate standard error of the mean (SEM). Data represent pooled results from 3 independent experiments.
Hematoxylin Staining and Statistical Analysis
In all, 45 μm coronal sections of E17 wild-type C57Bl/6J or Emx2 −/− brains were mounted and stained with Mayer's hematoxylin as described previously (Barry et al. 2008). Sections were imaged as above, and the dorsoventral width of the brain and of the cingulate cortex was measured. Data from sections encompassing rostral, middle, and caudal CC were pooled from wild-type and mutant embryos. Statistical analyses were performed using a 2-tailed unpaired t-test. Error bars represent the SEM. Data represent pooled results from 4 wild-type and 4 Emx2 −/− brains.
DiI and DiA Labeling
For carbocyanine tract tracing, brains were fixed in 4% PFA and small injections of DiI and DiA (Invitrogen; each in a 10% solution of dimethylformamide) were made into the cingulate cortex (DiI) or hippocampus (DiA) using pulled glass pipettes attached to a Picospritzer. Brains were stored in the dark at 37 °C in 4% PFA for at least 4 weeks to allow for dye transport. They were then sectioned coronally at 45 μm using a vibroslicer and imaged using a confocal microscope (Fluoview FV5000, Olympus). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). (n = 3 brains analyzed for each genotype.)
Results
Cingulate Pioneering Axons Express Npn1
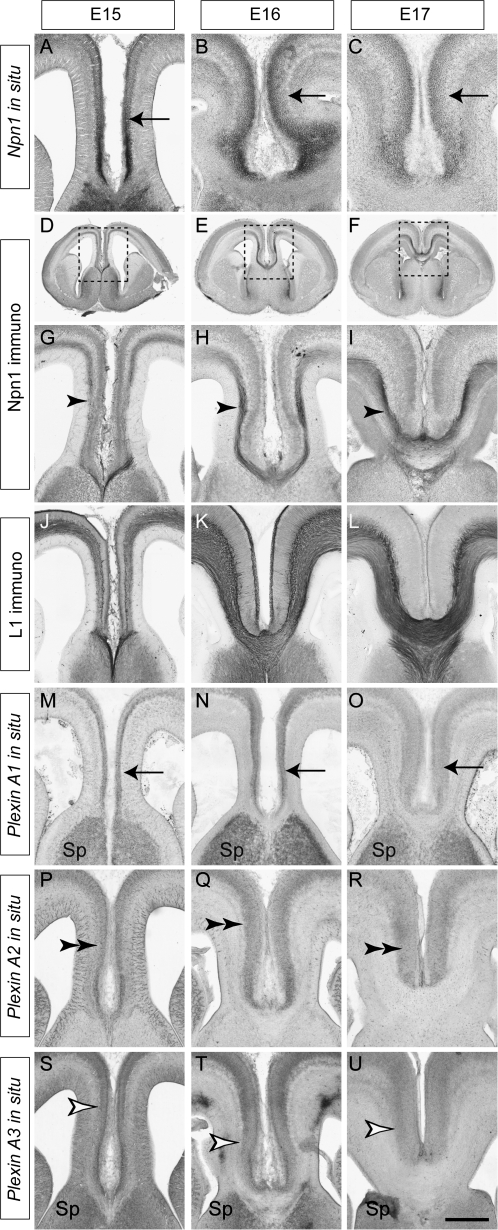
Mouse models have proven extremely useful in studying the mechanisms underlying the development of the CC (Lindwall et al. 2007; Piper et al. 2007). However, surprisingly little is known about the molecules involved in the guidance of pioneer axons from the cingulate cortex. We recently reported expression of Npn1 on the axons of cingulate pioneers during human embryonic development (Ren et al. 2006). To determine if such expression was evolutionarily conserved, we analyzed the expression of Npn1 during the period in which cingulate axons first cross the CC in mouse (E15–E17). In situ hybridization showed the localization of Npn1 mRNA to the cingulate cortex at E15–E17 (Fig. 1A–C), and immunohistochemical analysis further demonstrated that Npn1 protein is localized to axons of cingulate cortex neurons as they pioneer the CC (Fig. 1D–I). L1, a component of the Npn1 receptor complex (Castellani et al. 2000; Castellani 2002), is also expressed on cingulate axons as well as on other callosal and corticofugal axons (Fig. 1J–L). Axons of the cingulate cortex occupy the dorsal region of the tract, pioneering a path for the later arriving neocortical axons that make up the bulk of the CC (compare Fig. 1I with Fig. 1L). Thin optical sections have revealed that ventrally crossing neocortical axons likely fasciculate with the cingulate pioneers as they cross the midline (Rash and Richards 2001). These data suggest that Npn1 may regulate the guidance of cingulate pioneer axons.
Figure 1.
Npn1 and plexin expression in the cingulate cortex. Coronal sections of wild-type brains. Npn1 mRNA is expressed by cells in the cingulate cortex at E15, E16, and E17 (arrows, A–C). Npn1 protein is expressed on the axons of cingulate pioneer neurons (arrowheads) at E15 (D, G), E16 (E, H), and E17 (F, I). Panels G, H, and I are higher magnification views of the boxed regions in D, E, and F, respectively. L1, a coreceptor for Sema signaling, is expressed by all axons crossing the CC at E15 (J), E16 (K), and E17 (L). Plexin A1 mRNA is expressed in the cingulate cortex (arrows) and septum at E15, E16, and E17 (M–O), whereas plexin A2 mRNA expression is restricted to the cingulate cortex (double arrowheads, P–R). Plexin A3 mRNA is expressed in the cingulate cortex (open arrowheads) and the septum between E15 and E17 (S–U). Sp, septum. Scale bar: 600 μm (D), 550 μm (E), 500 μm (F), 250 μm (A, G, J, M, P, and S), 235 μm (B, H, K, N, Q, and T), and 200 μm (C, I, L, O, R, and U).
Expression of Plexins in the Cingulate Cortex
The plexin family of transmembrane receptors are involved in Sema signaling via either direct interactions with Semas or in conjunction with neuropilins in multimeric receptor complexes (Tamagnone et al. 1999). Multiple plexins are expressed in the developing cortex (Perala et al. 2005), and expression of plexins A1, A2, and A3 has been reported in the cortical plate of the developing neocortex (Murakami et al. 2001). As these plexins interact with Npn1 to transduce secreted class 3 Sema signaling (Tamagnone et al. 1999), we investigated their expression in the cingulate cortex by in situ hybridization. Plexin A1 and plexin A3 were both expressed in the cingulate cortex and in the septum from E15 to E17 (Fig. 1M–O, S–U), whereas the expression of plexin A2 was restricted to the cingulate cortex (Fig. 1P–R). These expression patterns implicate members of the plexin A subfamily in cingulate axon guidance.
Class 3 Sema Expression at the Cortical Midline
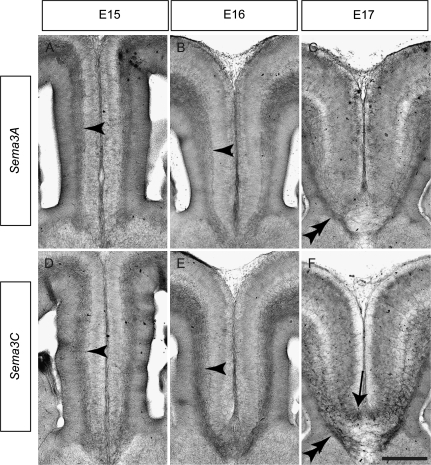
The cortical midline is a source of axon guidance cues such as Slit2 (Shu and Richards 2001). As Npn1–plexin receptor complexes transduce class 3 Sema signaling (Takahashi et al. 1999; Tamagnone et al. 1999), we next used in situ hybridization to determine if ligands from this family were expressed at the cortical midline. Sema3A is expressed in the neocortex and is a known repellent for neocortical axons in vitro (Bagnard et al. 1998; Castellani et al. 2000). At E15, Sema3A mRNA was expressed in the intermediate zone (IZ) of the cingulate cortex, where it could act to regulate the guidance of cingulate axons (Fig. 2A). A similar expression pattern was observed at E16 (Fig. 2B), but by E17, expression had become restricted to a band below the CC (Fig. 2C), corresponding to the subcallosal sling (Shu et al. 2003). Expression of Sema3B and Sema3F was also observed in the IZ at E15 and E16 and in the subcallosal sling at E17, albeit at lower levels than Sema3A (data not shown). Interestingly, Sema3C, a known attractant for neocortical axons in vitro (Bagnard et al. 1998), was also highly expressed at the cortical midline between E15 and E17. Expression was observed in the IZ at E15 (Fig. 2D), and this had intensified by E16 (Fig. 2E). At E17, Sema3C mRNA expression was clearly evident in the subcallosal sling as well as the indusium griseum (Fig. 2F), a population of glia located just dorsal to the CC that is required for callosal formation (Smith et al. 2006).
Figure 2.
Expression of class 3 Semas in the cingulate cortex. Coronal sections of wild-type brains demonstrating expression of Sema3A (A–C) and Sema3C (D–F) mRNA at the cortical midline. Sema3A is expressed in the IZ (arrowheads) at E15 (A) and E16 (B) and is expressed in the subcallosal sling (double arrowhead) at E17 (C). Sema3C is highly expressed in the IZ (arrowheads) at E15 (D) and E16 (E) and is expressed in both the subcallosal sling (double arrowhead) and the indusium griseum glia (arrow) at E17 (F). Scale bar: 250 μm (A and D), 235 μm (B and E), and 200 μm (C and F).
Sema3A and Sema3C Guide Cingulate Cortex Axons In Vitro
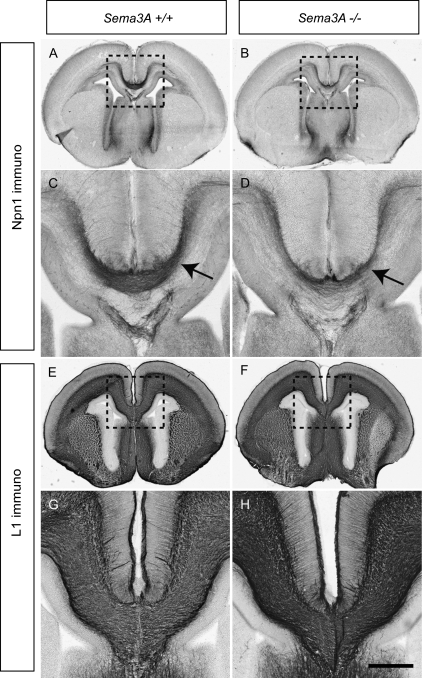
The expression of Sema3A in the IZ and the subcallosal sling from E15 to E17 indicated that this ligand was ideally placed to guide cingulate axons. In vitro coculture experiments showed that Sema3A secreted by cell blocks repelled axons from explants taken from both the neocortex (Bagnard et al. 1998) and the cingulate cortex at E16 (Supplementary Fig. 1). Furthermore, the ability of Sema3A to repel callosal axons was supported by experiments in which coronal sections of the rostral cortex were hemisected at the midline and cultured next to Sema3A-expressing cell blocks. The axons emanating from the hemisected slices, which correspond to callosal axons, were also repelled by Sema3A (Supplementary Fig. 1). We further investigated a potential role for Sema3A in guiding cingulate pioneering axons by analyzing Sema3A-deficient mice. However, these mice did not possess any gross defects in CC formation, with no apparent deficits in cingulate pioneer axon guidance (Npn1 expression, Fig. 3A–D) or callosal axon guidance (L1 expression, Fig. 3E–H) at E17 or in the adult (data not shown). This is perhaps not surprising as other major central nervous system axon tracts are relatively normal in Sema3A knockout mice (Catalano et al. 1998). Major axonal defects seen in the axonal projections of primary sensory neurons (Ulupinar et al. 1999) are also corrected later in development (White and Behar 2000), suggesting that a level of redundancy and compensation may ameliorate any deficiencies in this guidance cue.
Figure 3.
The CC forms normally in Sema3A-deficient mice. Coronal sections of wild-type and Sema3A-deficient brains at E17. Npn1 is expressed on the axons of cingulate pioneers in both wild-type (A; arrow in C) and Sema3A mutants (B; arrow in D), although a possible reduction in Npn1-expressing axons in the mutant is evident. The CC in Sema3A-deficient mice appears grossly normal in comparison to wild-type controls as assessed by L1 expression (E–H). Panels C, D, G, and H are higher magnification views of the boxed regions A, B, E, and F, respectively. Scale bar: 500 μm (A, B, E, and F) and 200 μm (C, D, G, and H).
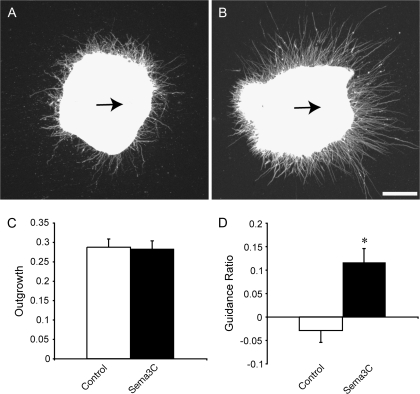
The expression of Sema3C dorsally and ventrally to the developing CC makes it another ideal candidate to attract and guide Npn1-expressing cingulate pioneer axons as they first traverse the CC. A Sema3C knockout has been generated recently, and these mice display aortic arch interruption and persistent truncus arteriosus (Feiner et al. 2001). However, the development of axon tracts in these mice, including the CC, has not been investigated. To determine if Sema3C can guide axons from the cingulate cortex, we performed in vitro coculture experiments. Explants from E15.5 cingulate cortex were cultured for 48 h beside cell blocks expressing either Sema3C or a mock-transfected control (Fig. 4A,B). Neurite outgrowth from explants cultured next to Sema3C-expressing cell blocks was not significantly different from controls (Fig. 4C). However, explants grown next to Sema3C-expressing cell blocks displayed a significantly higher GR in comparison to those grown next to mock-transfected control cell blocks (P < 0.0005, t-test; Fig. 4D), indicating that Sema3C attracts cingulate cortex neurites in vitro. Together, these results suggest that cingulate pioneering axons are guided by both repellent and attractant Sema activities at the midline.
Figure 4.
Sema3C attracts cingulate cortex neurites in vitro. Cingulate cortex explants grown next to mock-transfected cell blocks (A) or Sema3C-expressing cell blocks (B) for 48 h. Explants have been stained with antineuronal-specific βIII tubulin to visualize neurites. There is no significant difference in neurite outgrowth from explants from either group (C). However, the GR (defined as [(proximal neurite pixels − distal neurite pixels)/(total neurite pixels)) was significantly greater in explants grown next to Sema3C-expressing cell blocks in comparison to controls (*P < 0.0005), indicating that Sema3C acts as an attractant for neurites from cingulate cortex explants. The arrows in A and B indicate the direction of the cell block relative to the explant. Scale bar: 200 μm.
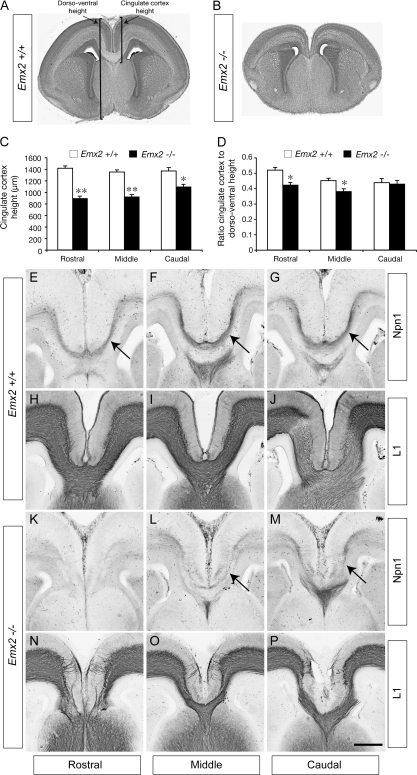
Emx2-Deficient Mice Have a Reduced Cingulate Cortex
The homeobox gene Emx2 is expressed in the presumptive cerebral cortex in a high caudomedial to low rostrolateral gradient, where it functions in cortical arealization (Bishop et al. 2000). As well as exhibiting deficiencies in cortical patterning, Emx2-deficient mice display morphological abnormalities in medial allocortical structures such as the dentate gyrus (Pellegrini et al. 1996). Pellegrini et al. (1996) also reported that the medial limbic cortex, which includes the cingulate cortex, was shortened in these mutants, but a quantification of this defect and, crucially, how it relates to cingulate pioneers and subsequent CC development was not addressed. We investigated this by first quantifying the size of the cingulate cortex in Emx2 mutants. Coronal sections of E17 wild-type or Emx2-deficient brains were mounted and stained with hematoxylin (Fig. 5A,B). Sections were then imaged and the dorsoventral width of the cingulate cortex at rostral, middle, and caudal levels measured in wild-type and mutant samples. The dorsoventral width of the cingulate cortex was significantly reduced in comparison to the wild-type control at rostral (P < 0.0001, t-test), middle (P < 0.0001, t-test), and caudal levels (P < 0.005, t-test; Fig. 5C). Importantly, because the brains of these mutants are also comparatively smaller (Pellegrini et al. 1996), we also analyzed the width of the cingulate cortex as a ratio of the total dorsoventral width of the brain to determine if there was a disproportionate decrease in cingulate size in Emx2-deficient mice. These analyses revealed that the ratio of cingulate cortex width to dorsoventral width was significantly reduced in Emx2 mutants compared with controls at both the rostral and middle levels (P < 0.005, t-test) but not at the caudal level (Fig. 5D). This implies that there is indeed a significant decrease in the size of the cingulate cortex in mice lacking Emx2. As this region is the source of Npn1-expressing neurons that pioneer the CC, we next investigated expression of this receptor in Emx2 mutants.
Figure 5.
Expression of Npn1 correlates with the presence of the CC in Emx2 mutants. Coronal sections of E17 wild-type (A) and Emx2 knockout (B) brains stained with hematoxylin. (C) The dorsoventral size of the cingulate cortex in Emx2 mutants at rostral, middle, and caudal levels is significantly reduced in comparison to wild-type controls. (D) At rostral and middle levels, the ratio of the cingulate cortex dorsoventral size to the total dorsoventral size is significantly reduced in comparison to the control (**P < 0.0001, *P < 0.005). (E–P) Coronal sections of E17 wild-type (E–J) or Emx2 mutant (K–P) brains. Npn1 expression is observed on cingulate pioneer axons at rostral, middle, and caudal levels of the wild type (arrows in E–G), and L1-expressing axons are seen crossing the CC (H–J). In the mutant, no Npn1 expression is observed in rostral sections (K), and no axons can be seen crossing the CC using L1 staining (N). At more caudal levels in the mutant, Npn1 expression becomes detectable (arrows in L, M) and L1-expressing axons can be seen crossing the midline through the CC (O, P). Scale bar: 500 μm (A, B) and 200 μm (E–P).
CC Formation Correlates with the Presence of Npn1-Expressing Pioneering Axons
Analysis of sections stained with hematoxylin demonstrated that in Emx2-deficient mice, no callosal axons were observed crossing the midline at rostral levels (Fig. 5A,B), but at more caudal levels, the CC was evident, though much reduced. Immunohistochemical analysis of wild-type brains at E17 demonstrated that Npn1 was expressed by cingulate pioneers at rostral, middle, and caudal levels of the CC (Fig. 5E–G) and that L1-expressing callosal axons form the entire CC at these levels (Fig. 5H–J). In contrast, at rostral levels in Emx2-deficient mice, Npn1 expression was absent (Fig. 5K) and L1-expressing callosal axons did not cross the midline (Fig. 5N). Importantly, at middle and caudal levels, Npn1 expression became apparent, though at reduced levels (Fig. 5L,M), and the CC was present, as demonstrated by L1 expression (Fig. 5O,P). These data indicate that the presence of Npn1-expressing cingulate pioneers and the presence of the CC are correlated, suggesting that Npn1 expression on cingulate pioneers may be critical for the formation of this axon tract.
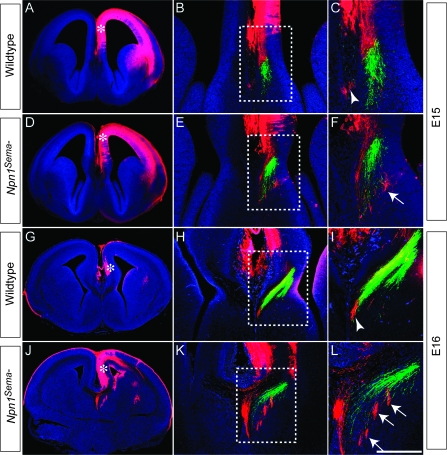
Cingulate Pioneering Axons Are Misguided in Npn1Sema- Mutants
Mice lacking Npn1 die at ∼E13.5 (Kawasaki et al. 1999), and as such, the role of Npn1 in the formation of the CC cannot be ascertained by the study of these animals. To examine the role of Npn1-Sema signaling in callosal axon pathfinding, we used a knockin mouse line, in which the Sema-binding domain of Npn1 was mutated such that Npn1 could no longer bind Semas (termed Npn1Sema- knockin mice) but retained its other functions, including the ability to bind vascular endothelial growth factor (VEGF; Gu et al. 2003). These mice are perinatal lethal and display defects in CC development, including Probst bundle formation (Gu et al. 2003). However, this study did not specifically address whether axons from the cingulate cortex were misguided in these mice. To address this, we performed carbocyanine tract tracing on Npn1Sema- mice at E15 and E16, when cingulate pioneers are approaching the midline to initiate CC formation. In both wild-type and mutant mice at these ages, we injected DiI into the cingulate cortex and DiA into the hippocampus. At E15 in the wild type, axons from the cingulate cortex (red) could be seen near the cortical midline, medial, and dorsal to hippocampal axons (green, Fig. 6A–C). In Npn1Sema- mice at E15, however, axons emanating from the cingulate cortex were misguided, appearing more lateral and ventral than hippocampal axons (Fig. 6D–F). This phenotype was even more apparent at E16, with cingulate pioneers in the mutant forming ectopic bundles medial to hippocampal axons and appearing to extend toward the septum (Fig. 6G–L). Furthermore, in rostral sections of E17 Npn1Sema- mice, we observed aberrant extension of axons into the septum (Supplementary Fig. 2) using another marker for cingulate pioneer axons, DCC (Shu et al. 2000). These data demonstrate that pioneering axons from the cingulate cortex are misguided in Npn1Sema- mutants, suggesting that the callosal defects reported in these mice (Gu et al. 2003) are likely to stem from this defect. This further implicates Npn1 as a key player in CC development.
Figure 6.
Cingulate axons are misguided in Npn1Sema- mutants. Carbocyanine tract tracing in wild-type (A–C, G–I) and Npn1Sema- knockin mice (D–F, J–L) at E15 (A–F) and E16 (G–L). Axons from the cingulate cortex were labeled with DiI in the cingulate cortex (red), whereas hippocampal axons were labeled with DiA in the dentate gyrus (green). Low-magnification images in A, D, G, and J show the injection site for DiI (asterisks). (B, C) In the wild type at E15, cingulate cortex axons are found close to the site of the presumptive CC (arrowhead in C). (E, F) In the mutant at E15, cingulate axons are misguided, being found laterally to the hippocampal axons (arrow in F). (H, I) At E16 in the wild type, cingulate axons are located just medially to the hippocampal axons (arrowhead in I). (K, L) In Npn1Sema- mutants at E16 however, cingulate axons are observed lateral to hippocampal axons, projecting into the septum (arrows in L). Panels C, F, I, and L are higher magnification views of the boxed regions B, E, H, and K, respectively. Scale bar: 600 μm (A and D), 550 μm (G and J), 250 μm (H and K), 200 μm (B and E), 75 μm (I and L), and 50 μm (C and F).
Discussion
The formation of mature axon tracts is preceded by the extension of axons from pioneer neurons, whose processes initiate tract formation by projecting along specific trajectories. For instance, the 2 major efferent projections from the cortex, the corticofugal pathway, and the CC are pioneered by axons originating in the subplate (McConnell et al. 1989) and the cingulate cortex (Koester and O'Leary 1994), respectively. We have demonstrated here that Npn1 plays a pivotal role in guiding pioneer axons from the cingulate cortex, providing an insight into the molecular mechanisms regulating how cortical pioneering axons are guided as they initiate tract formation. Three pieces of evidence, in particular, emphasize the importance of Npn1 for the guidance of cingulate pioneer axons. First, the spatiotemporal expression of Npn1 and its coreceptors in cingulate neurons and axons coupled with the expression of class 3 Semas at the cortical midline is indicative of a role in axon guidance during the period in which the first axons cross the CC (Rash and Richards 2001). Second, the presence of guidance defects in cingulate pioneer axons from Npn1Sema- knockin mice demonstrates a requirement for Npn1 function in the pathfinding of these processes. Third, the absence (rostral) or presence (caudal) of Npn1-expressing pioneers correlates with the presence of the CC in Emx2-deficient mice, a finding that is indicative of a causal link between expression of this receptor on cingulate pioneers and subsequent formation of the callosal tract.
The expression of Npn1, its coreceptors, and its ligands within the cingulate cortex and the cortical midline, during the period in which the CC is first formed, provide us with a framework in which to comprehensively investigate the molecular determinants underlying callosal formation. One immediate challenge is to determine which molecules are the primary ligands for Npn1 expressed on cingulate pioneers. Our data indicate that axons from cingulate cortex explants or from hemisected cortical slices are repelled from cell blocks transiently transfected with Sema3A. These findings are consistent with the published reports that in in vitro culture experiments, axons from cortical explants (collectively including both callosally and subcortically projecting axons) are repelled by Sema3A (Bagnard et al. 1998) and imply that the repulsive activity of Sema3A in the IZ and subcallosal sling may channel cingulate pioneers to the midline during development. Indeed, Sema3A has been demonstrated to be a repellent for axons in a variety of experimental paradigms in vitro. However, although Sema3A-deficient mice demonstrate defects in sensory axon projections in the periphery and the brainstem (Ulupinar et al. 1999), these defects are later corrected (White and Behar 2000), and moreover, no significant defects have been noted in the forebrain axonal projections of these mice (Catalano et al. 1998), including the CC (this study). We did observe that Npn1 expression was slightly reduced in the Sema3A mutants, indicating that some Npn1 positive axons may have been eliminated during development. Thus, although relevant in an in vitro context, the contribution of Sema3A in vivo to the guidance of axonal populations, including cingulate pioneers, is unclear. Our in situ hybridization analysis also indicates that Sema3C is an excellent candidate for guiding cingulate pioneer axons. Sema3C, expressed in the IZ (E15 and E16) and the indusium griseum glia and subcallosal sling (E17), is an attractant in vitro for neurites from neocortical explants (Bagnard et al. 1998) and cingulate cortex explants (this study, Fig. 4). Although a Sema3C knockout mouse has been generated (Feiner et al. 2001), a thorough analysis to determine if CC formation is aberrant in these mice has yet to be performed.
Our study demonstrates that axons from cingulate pioneers are misguided in Npn1Sema- knockin mice. This confirms the requirement for Npn1-Sema signaling in the guidance of the callosal pioneer axons. Our data suggest that both attractive (Sema3C) and repulsive (Sema3A) cues may be required for the guidance of the callosal pioneers. For example, Sema3C could be required to attract the pioneer axons to the midline, where after crossing, they could then be repelled by Sema3A. Such differential guidance mechanisms would be possible through specific coreceptor binding or different intracellular signaling pathways being activated at the appropriate developmental stage.
When interpreting the phenotype of Npn1Sema- knockin mice, it is important to note that Npn1, although being unable to bind Semas (Gu et al. 2003), is still expressed on cingulate axons and may be capable of binding to VEGF (Gu et al. 2002) or promoting cell–cell adhesion (Shimizu et al. 2000). As such, we cannot determine the full extent of Npn1 function in the Npn1Sema- knockin mice. A recent report using RNA interference-mediated knockdown of Npn1 in vivo has demonstrated that this receptor is required for radial migration of cortical neurons during development (Chen et al. 2008). A similar experimental approach may provide insights into the function of Npn1 during CC development, as would examining callosal development in a conditional knockout of Npn1.
Emx2 is a homeodomain transcription factor expressed in a graded pattern within the developing neocortex that is high caudally and medially and low rostrally and laterally (Mallamaci et al. 1998). Emx2 is postulated to regulate arealization within the neocortex in a combinatorial fashion with Pax6 (Bishop et al. 2000). This hypothesis is supported by analyses of Emx2-deficient mice, in which the rostral and lateral neocortical areas are expanded at the expense of caudal and medial areas (Bishop et al. 2000). How can we frame our findings within this hypothesis? Although Emx2 is a transcription factor, it seems unlikely that it directly regulates Npn1 expression, as Npn1 expression is only lost in rostral regions of Emx2-deficient brains. Although another transcription factor may be compensating for Emx2 function in more caudal areas of the mutant, a more likely explanation is that the expansion of rostral and lateral regions results in a more caudal localization of the area of the cingulate cortex that would normally express Npn1. Driving expression of Npn1 in vivo using electroporation directly into the rostral cingulate cortex in Emx2 mutants would provide a way to rigorously analyze the contribution of this receptor to the guidance of cingulate pioneers and, moreover, to address the importance of these pioneers to the guidance of later arriving neocortical axons. In summary, our study indicates that Npn1 plays a central role in the guidance of pioneering axons from the cingulate cortex.
Supplementary Material
Supplementary Figures 1 and 2 can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Health and Medical Research Council (NHMRC; 401616 and 456027 to L.J.R.); National Institutes of Health (NS039050 and NS049048 to R.E.); L.J.R. is a NHMRC Senior Research Fellow; M.P. holds an NHMRC Biomedical Career Development Award.
Supplementary Material
Acknowledgments
We thank Sarah Croft, Daphne Kusters, Lynnette Knowles, Chantelle Reid, Erica Little, Barri King, and John Baisden for technical assistance. We are very grateful to Dr David Ginty (John Hopkins University, Baltimore, MD) for providing the Npn1Sema- knockin mouse line, the Npn1 antibody, and Npn1 in situ probe; Dr Helen Cooper (Queensland Brain Institute, Brisbane, Australia) for providing the DCC antibody; Dr Peter Gruss (Max Planck Society, Munich, Germany) for providing the Emx2-deficient line; and Dr Andreas W. Püschel (Genetik, Westfälische Wilhelms-Universität Münster, Münster, Germany) for providing the Sema expression constructs and Sema in situ probes. We also thank Jonathan Hunt (Queensland Brain Institute, Brisbane, Australia) for help in modifying and implementing the algorithm for measuring neurite outgrowth and guidance. Conflict of Interest: None declared.
References
- Adams RH, Lohrum M, Klostermann A, Betz H, Puschel AW. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J. 1997;16:6077–6086. doi: 10.1093/emboj/16.20.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews GL, Mastick GS. R-cadherin is a Pax6-regulated, growth-promoting cue for pioneer axons. J Neurosci. 2003;23:9873–9880. doi: 10.1523/JNEUROSCI.23-30-09873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, Murakami F, Parnavelas JG, Sundaresan V, Richards LJ. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Bagnard D, Lohrum M, Uziel D, Puschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–5053. doi: 10.1242/dev.125.24.5043. [DOI] [PubMed] [Google Scholar]
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Barry G, Piper M, Lindwall C, Moldrich R, Mason S, Little E, Sarkar A, Tole S, Gronostajski RM, Richards LJ. Specific glial populations regulate hippocampal morphogenesis. J Neurosci. 2008;28:12328–12340. doi: 10.1523/JNEUROSCI.4000-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O'Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Campbell CE, Piper M, Plachez C, Yeh YT, Baizer JS, Osinski JM, Litwack ED, Richards LJ, Gronostajski RM. The transcription factor Nfix is essential for normal brain development. BMC Dev Biol. 2008;8:52. doi: 10.1186/1471-213X-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V. The function of neuropilin/L1 complex. Adv Exp Med Biol. 2002;515:91–102. doi: 10.1007/978-1-4615-0119-0_8. [DOI] [PubMed] [Google Scholar]
- Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27:237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- Catalano SM, Messersmith EK, Goodman CS, Shatz CJ, Chedotal A. Many major CNS axon projections develop normally in the absence of semaphorin III. Mol Cell Neurosci. 1998;11:173–182. doi: 10.1006/mcne.1998.0687. [DOI] [PubMed] [Google Scholar]
- Chen G, Sima J, Jin M, Wang KY, Xue XJ, Zheng W, Ding YQ, Yuan XB. Semaphorin-3A guides radial migration of cortical neurons during development. Nat Neurosci. 2008;11:36–44. doi: 10.1038/nn2018. [DOI] [PubMed] [Google Scholar]
- De Carlos JA, O'Leary DD. Growth and targeting of subplate axons and establishment of major cortical pathways. J Neurosci. 1992;12:1194–1211. doi: 10.1523/JNEUROSCI.12-04-01194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer WT, Altick AL, Nural HF, Dugan JP, Kidd T, Charron F, Mastick GS. Pioneer longitudinal axons navigate using floor plate and Slit/Robo signals. Development. 2008;135:3643–3653. doi: 10.1242/dev.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Feiner L, Webber AL, Brown CB, Lu MM, Jia L, Feinstein P, Mombaerts P, Epstein JA, Raper JA. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development. 2001;128:3061–3070. doi: 10.1242/dev.128.16.3061. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ. A role for subplate neurons in the patterning of connections from thalamus to neocortex. Development. 1993;117:1031–1047. doi: 10.1242/dev.117.3.1031. [DOI] [PubMed] [Google Scholar]
- Gu C, Limberg BJ, Whitaker GB, Perman B, Leahy DJ, Rosenbaum JS, Ginty DD, Kolodkin AL. Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165. J Biol Chem. 2002;277:18069–18076. doi: 10.1074/jbc.M201681200. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- Keeble TR, Halford MM, Seaman C, Kee N, Macheda M, Anderson RB, Stacker SA, Cooper HM. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci. 2006;26:5840–5848. doi: 10.1523/JNEUROSCI.1175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester SE, O'Leary DD. Axons of early generated neurons in cingulate cortex pioneer the corpus callosum. J Neurosci. 1994;14:6608–6620. doi: 10.1523/JNEUROSCI.14-11-06608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall C, Fothergill T, Richards LJ. Commissure formation in the mammalian forebrain. Curr Opin Neurobiol. 2007;17:3–14. doi: 10.1016/j.conb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Mallamaci A, Iannone R, Briata P, Pintonello L, Mercurio S, Boncinelli E, Corte G. EMX2 protein in the developing mouse brain and olfactory area. Mech Dev. 1998;77:165–172. doi: 10.1016/s0925-4773(98)00141-5. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245:978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. Subplate pioneers and the formation of descending connections from cerebral cortex. J Neurosci. 1994;14:1892–1907. doi: 10.1523/JNEUROSCI.14-04-01892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Suto F, Shimizu M, Shinoda T, Kameyama T, Fujisawa H. Differential expression of plexin-A subfamily members in the mouse nervous system. Dev Dyn. 2001;220:246–258. doi: 10.1002/1097-0177(20010301)220:3<246::AID-DVDY1112>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Mansouri A, Simeone A, Boncinelli E, Gruss P. Dentate gyrus formation requires Emx2. Development. 1996;122:3893–3898. doi: 10.1242/dev.122.12.3893. [DOI] [PubMed] [Google Scholar]
- Perala NM, Immonen T, Sariola H. The expression of plexins during mouse embryogenesis. Gene Expr Patterns. 2005;5:355–362. doi: 10.1016/j.modgep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Piper M, Dawson AL, Lindwall C, Barry G, Plachez C, Richards LJ. Emx and Nfi genes regulate cortical development and axon guidance in the telencephalon. Novartis Found Symp. 2007;288:230–242. [discussion 242–235, 276–281] [PubMed] [Google Scholar]
- Piper M, Georgas K, Yamada T, Little M. Expression of the vertebrate Slit gene family and their putative receptors, the Robo genes, in the developing murine kidney. Mech Dev. 2000;94:213–217. doi: 10.1016/s0925-4773(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Plachez C, Lindwall C, Sunn N, Piper M, Moldrich RX, Campbell CE, Osinski JM, Gronostajski RM, Richards LJ. Nuclear factor I gene expression in the developing forebrain. J Comp Neurol. 2008;508:385–401. doi: 10.1002/cne.21645. [DOI] [PubMed] [Google Scholar]
- Plachez C, Richards LJ. Mechanisms of axon guidance in the developing nervous system. Curr Top Dev Biol. 2005;69:267–346. doi: 10.1016/S0070-2153(05)69010-2. [DOI] [PubMed] [Google Scholar]
- Pozas E, Pascual M, Nguyen Ba-Charvet KT, Guijarro P, Sotelo C, Chedotal A, Del Rio JA, Soriano E. Age-dependent effects of secreted Semaphorins 3A, 3F, and 3E on developing hippocampal axons: in vitro effects and phenotype of Semaphorin 3A (−/−) mice. Mol Cell Neurosci. 2001;18:26–43. doi: 10.1006/mcne.2001.0999. [DOI] [PubMed] [Google Scholar]
- Rash BG, Richards LJ. A role for cingulate pioneering axons in the development of the corpus callosum. J Comp Neurol. 2001;434:147–157. doi: 10.1002/cne.1170. [DOI] [PubMed] [Google Scholar]
- Ren T, Anderson A, Shen WB, Huang H, Plachez C, Zhang J, Mori S, Kinsman SL, Richards LJ. Imaging, anatomical, and molecular analysis of callosal formation in the developing human fetal brain. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:191–204. doi: 10.1002/ar.a.20282. [DOI] [PubMed] [Google Scholar]
- Rosoff WJ, Urbach JS, Esrick MA, McAllister RG, Richards LJ, Goodhill GJ. A new chemotaxis assay shows the extreme sensitivity of axons to molecular gradients. Nat Neurosci. 2004;7:678–682. doi: 10.1038/nn1259. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Murakami Y, Suto F, Fujisawa H. Determination of cell adhesion sites of neuropilin-1. J Cell Biol. 2000;148:1283–1293. doi: 10.1083/jcb.148.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Butz KG, Plachez C, Gronostajski RM, Richards LJ. Abnormal development of forebrain midline glia and commissural projections in Nfia knock-out mice. J Neurosci. 2003;23:203–212. doi: 10.1523/JNEUROSCI.23-01-00203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Richards LJ. Cortical axon guidance by the glial wedge during the development of the corpus callosum. J Neurosci. 2001;21:2749–2758. doi: 10.1523/JNEUROSCI.21-08-02749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Valentino KM, Seaman C, Cooper HM, Richards LJ. Expression of the netrin-1 receptor, deleted in colorectal cancer (DCC), is largely confined to projecting neurons in the developing forebrain. J Comp Neurol. 2000;416:201–212. doi: 10.1002/(sici)1096-9861(20000110)416:2<201::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Smith KM, Ohkubo Y, Maragnoli ME, Rasin MR, Schwartz ML, Sestan N, Vaccarino FM. Midline radial glia translocation and corpus callosum formation require FGF signaling. Nat Neurosci. 2006;9:787–797. doi: 10.1038/nn1705. [DOI] [PubMed] [Google Scholar]
- Super H, Martinez A, Del Rio JA, Soriano E. Involvement of distinct pioneer neurons in the formation of layer-specific connections in the hippocampus. J Neurosci. 1998;18:4616–4626. doi: 10.1523/JNEUROSCI.18-12-04616.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Ulupinar E, Datwani A, Behar O, Fujisawa H, Erzurumlu R. Role of semaphorin III in the developing rodent trigeminal system. Mol Cell Neurosci. 1999;13:281–292. doi: 10.1006/mcne.1999.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CM, Pinezich JD, Lindquist WB, Vazquez ME. An algorithm for neurite outgrowth reconstruction. J Neurosci Methods. 2003;124:197–205. doi: 10.1016/s0165-0270(03)00017-7. [DOI] [PubMed] [Google Scholar]
- White FA, Behar O. The development and subsequent elimination of aberrant peripheral axon projections in Semaphorin3A null mutant mice. Dev Biol. 2000;225:79–86. doi: 10.1006/dbio.2000.9822. [DOI] [PubMed] [Google Scholar]
- Yorke CH, Jr, Caviness VS., Jr Interhemispheric neocortical connections of the corpus callosum in the normal mouse: a study based on anterograde and retrograde methods. J Comp Neurol. 1975;164:233–245. doi: 10.1002/cne.901640206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.