Abstract
The excitatory neurons of the mammalian cerebral cortex arise from asymmetric divisions of radial glial cells in the ventricular zone and symmetric division of intermediate progenitor cells (IPCs) in the subventricular zone (SVZ) of the embryonic cortex. Little is known about the microenvironment in which IPCs divide or whether a stem cell niche exists in the SVZ of the embryonic cortex. Recent evidence suggests that vasculature may provide a niche for adult stem cells but its role in development is less clear. We have investigated the vasculature in the embryonic cortex during neurogenesis and find that IPCs are spatially and temporally associated with blood vessels during cortical development. Intermediate progenitors mimic the pattern of capillaries suggesting patterns of angiogenesis and neurogenesis are coordinated during development. More importantly, we find that IPCs divide near blood vessel branch points suggesting that cerebral vasculature establishes a stem cell niche for intermediate progenitors in the SVZ. These data provide novel evidence for the presence of a neurogenic niche for intermediate progenitors in the embryonic SVZ and suggest blood vessels are important for proper patterning of neurogenesis.
Keywords: capillaries, eomesodermin, migration, Tbr2, RC2
Introduction
The development of the mammalian cerebral cortex involves a series of orchestrated events that lead to the sequential inside-out assembly of the 6-layered cortex (Smart 1973; McConnell 1995; Kriegstein et al. 2006; Rakic 2006; Cheung et al. 2007; Dehay and Kennedy 2007; Pontious et al. 2008). Two distinct progenitor cell types, radial glia and intermediate progenitors, participate in cortical neurogenesis. Radial glia, bipolar cells with radial fibers that extend to the pial surface, divide asymmetrically at the ventricular surface with vertical cleavage planes to produce a radial glial cell and either a neuron (direct neurogenesis) or an intermediate progenitor cell (IPC, indirect neurogenesis) (Misson et al. 1988; Takahashi et al. 1995; Noctor et al. 2001; Tamamaki et al. 2001; Tarabykin et al. 2001; Anthony et al. 2004; Haubensak et al. 2004; Zimmer et al. 2004). IPCs are multipolar cells, express the T-box transcription factor Tbr2, and migrate away from the ventricular surface to undergo symmetrical divisions. They generally have horizontal 45 cleavage planes and produce 2 neurons (Takahashi et al. 1995; Haubensak et al. 2004; Noctor et al. 2004, 2008; Englund et al. 2005). Although IPCs play a critical role in cortical development by expanding the neuronal population, little is known about the factors that influence IPC production, mitosis, or whether a niche for neurogenesis exists in the embryonic cortical subventricular zone (SVZ) where the majority of IPC divisions occur. Observations in other systems have shown that vasculature can provide a proliferative niche for stem cells (Palmer et al. 2000; Louissaint et al. 2002; Kiel et al. 2005; Nikolova et al. 2006; Yoshida et al. 2007). Recent work also hints at a relationship between cortical neurogenesis and angiogenesis (Palmer et al. 2000; Louissaint et al. 2002; Gerhardt et al. 2004; Shen et al. 2004; Vasudevan et al. 2008). Progenitors are often associated with blood vessels in the dentate gyrus of the adult hippocampus as well as during neurogenesis in zebra finches (Palmer et al. 2000; Louissaint et al. 2002). Blood vessels also play a role in adult SVZ progenitors and neuroblasts in the adult SVZ are found to migrate on blood vessels toward the olfactory bulb in rodents (Bovetti et al. 2007; Riquelme et al. 2008). Moreover, media conditioned by endothelial cells stimulates proliferation and neurogenesis of mouse embryonic cortical stem cells, suggesting that a secreted factor from endothelial cells promotes neurogenesis (Shen et al. 2004). We therefore investigated the development of cortical vasculature and its potential role as a neurogenic niche for IPCs.
Recent work suggests that endothelial cells destined to make up the venous circulation appear on the pia prior to neurogenesis, whereas endothelial cells of the arterial vasculature migrate tangentially from the ventral telencephalon to the SVZ of the dorsal cortex shortly thereafter (Hiruma et al. 2002; Vasudevan et al. 2008). The arterial endothelial cells construct the capillary beds that will supply the cortex (Vasudevan et al. 2008). Angiogenesis occurs through the coordinated activity of 2 distinct endothelial cell types: tip cells and stalk cells (Gerhardt et al. 2003). Endothelial tip cells are specialized nonmitotic migratory cells with many highly dynamic filopodia (Gerhardt et al. 2003). Tip cells are only present during angiogenesis and are responsible for vascular patterning. Endothelial stalk cells divide in the wake of the tip cells to generate the tubular structure of blood vessels (Gerhardt et al. 2003). Endothelial tip cells have been observed to extend filopodia to the ventricular surface, where radial glia divide, and to interact with the fibers of radial glia in the hindbrain, suggesting communication between progenitors of the vasculature and central nervous system (Gerhardt et al. 2004). For example, endothelial cells in the brain share a similar molecular profile with neighboring neural stem cells (i.e., ventral endothelial cells express Dlx1/5 and Nkx2.1, whereas dorsal endothelial cells express Pax6), strongly suggesting a relationship between the mechanism of patterning during angiogenesis and neurogenesis in the brain (Vasudevan et al. 2008).
We examined the initial appearance and mitotic behavior of IPCs, in relation to the growth of the cortical vasculature, using Tbr2 expression to identify IPCs (Englund et al. 2005). Here we show that in mouse embryonic cortex, Tbr2 cells are temporally and spatially associated with cortical vasculature in the SVZ. We also show that Tbr2+ cells follow and mimic the pattern of nascent blood vessels and divide near blood vessels. Our findings present a new understanding of the local microenvironment and the cues that orchestrate neurogenesis and the division of IPCs in the SVZ. These data also suggest that vascular patterning coordinates cortical patterning during embryonic development.
Materials and Methods
Immunofluorescence and Imaging
For whole-mount immunofluorescence, cortical hemispheres were dissected from mouse embryos (Swiss Webster, Simonsen Labs, Santa Clara, CA), flattened on a membrane and fixed slowly from below by floating on 4% paraformaldehyde for 1 h on ice and fixed overnight. The tissue was permeabilized in block buffer (0.4% Triton-X 100 and 0.5% goat serum) overnight followed by antibody incubation in block buffer for 2 days at 4 °C on a nutator. The tissue was washed 3× in phosphate-buffered saline (PBS) and stained with secondary antibodies overnight and mounted between 2 coverslips in aquamount. Immunofluorescence on sections was carried out on 50-μm cryostat sections in a similar manner but incubated with primary antibodies overnight and secondary antibodies for 1 h at room temperature. Primary antibodies used: Rabbit anti Tbr2 (Chemicon, Billerica, MA), Mouse anti PH3 (eBiosciences, San Diego, CA), Mouse anti VEGF-R2 (Flk-1 A3, Santa Cruz Biotechnology, Santa Cruz, CA), Mouse anti RC2 (Hybridoma Bank, University of Iowa, donated by Miyuki Yamamoto, clone NS-1), Rat anti-PECAM-1 (a.k.a. CD31, Pharmingen, San Jose, CA, clone MEC13.3), rabbit anti Ki67 (Pharmingen). Secondary antibodies were conjugated with Alexa dyes, made in goat (Invitrogen, Carlsbad, CA). Imaging was carried out with a Leica (Germany) SP5 laser scanning confocal microscope. Dyes close in emission spectrum were imaged sequentially to prevent bleed-through. The distance of cell bodies to blood vessels was quantified using Leica imaging software. Quantification of Figure 2D was done by dividing images into parts with vascular plexus and parts without. The number of Tbr2 cells in each part (equal in area, mm2) was counted and a percentage was obtained by normalizing to the total number of Tbr2 cells for that image.
Figure 2.
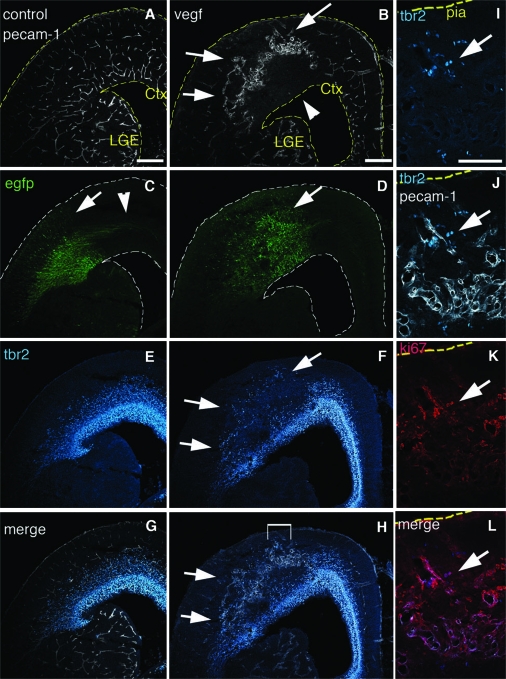
Confocal stack through the SVZ of Tbr2 transgenic at E13 shows Tbr2:EGFP cells (A) are aligned in a honeycomb pattern that resembles the vasculature in (B) labeled with Alexa-594-Lectin. (C) Merged image shows the GFP cells overlap with vasculature (arrows). (D) Same confocal stack as in (A–C) but rotated 90° to mimic a coronal view. Note that the majority of EGFP cells aligned with the vasculature (shown in F) are not adjacent to the blood vessels but have migrated 30–50 μm toward the cortical plate (arrows). (E) Confocal optical Z section of E14 lateral cortex stained for endogenous Tbr2 (blue), PECAM-1 (red), and PH3 (green). Tbr2/PH3 double positive cells are adjacent to blood vessels (arrows). Tbr2 density is increased in the basal region of the SVZ in lateral cortex at this age and Tbr2 cells are now also found in areas between blood vessels (blue, arrowhead). (F) Cartoon schematic of (E) emphasizes that Tbr2/PH3 double positive cells are near blood vessel branch points. (G) The vessel segments between branch points were divided into 4 quadrants. The distance of cell bodies to each segment was measured with quadrant 1 being nearest the cell. (G’) Quantification of Tbr2/PH3 cells shows that Tbr2 cells are preferentially located near branch points. (H–M) Tbr2 cells are associated with vasculature in VEGF electroporated brains. (H) Ventricular view shows region of electroporated at E13 and imaged at E17. (I) PECAM-1 staining shows blood vessels have formed a ring-like structure surrounding the electroporated area. (J) Tbr2 staining of the tissue in (H) and (I) shows that Tbr2 cell distribution matches the altered blood vessel geometry and forms a ring-like pattern (arrow). (K–M) Higher magnification shows Tbr2 cells associated with the leading edge of developing vasculature (arrow). Scale bars: (A–C, E, K–M) 100 μm, (D–G) 30 μm, (H, I) 200 μm.
Animals and In Utero Electroporation
Timed pregnant Swiss Webster mice (Simonsen Labs, CA) were anesthetized and their embryos were electroporated as described (Elias et al. 2007). VEGF120 vector was obtained through Addgene (Donated by Bob Weinberg, Addgene number: 10909) and were prepared endotoxin-free (Qiagen, Valencia, CA) and coelectroporated together with EGFP-N1 (Clontech, Mountain View, CA) in a 1:1 molar ratio. BAC transgenic Tbr2:EGFP (Eomesodermin:EGFP) were obtained from GENSAT (www.gensat.org). For live vascular labeling, embryos were perfused with mung bean lectin-Alexa-594 (Invitrogen) dissolved in PBS and incubated in oxygenated Hanks buffer at room temperature for 20 min. The cortex was dissected and imaged live using a Leica SP5 or an Olympus FV-1000 (Center Valley, PA) confocal microscope. Animal experiments were carried out in accordance with UCSF Institutional Animal Care and Use Committee animal protocols.
Results
In order to better observe the orientation of the vasculature throughout the cortex we prepared flattened whole-mounts of the embryonic mouse cortex, immunostained for the endothelial marker PECAM-1 (CD31), and used confocal microscopy to image the ventricular zone (VZ) and SVZ starting from the surface of the ventricle. This preparation allowed us to visualize the vast network of capillaries in the SVZ and obtain new spatial information about the vasculature and IPCs not obvious in coronal sections (Fig. 1A,D, Supplementary Fig. 1 and Movie 1).
Figure 1.
Tbr2 cells and blood vessels are temporally and spatially connected. (A–C) Collapsed confocal stacks of cortical E12 (A–C) and E14 (D–F) whole-mounts stained for PECAM-1 in (A, D) (red), Tbr2 in (B, E) (green), and merged in (C, F). Lateral is to the left. Note that there are more Tbr2 cells laterally at E12 (A, bracket) and that this area is also more vascularized (B, C, bracket). Tbr2 cells are also associated with the leading edge of growing vasculature (arrows). (D–F) At E14 Tbr2 cells are arranged in rows aligned with the pattern of developing blood vessels (arrows in F). Note that the typical linear arrangement of Tbr2 cells becomes obvious even without staining for the vasculature (E and F). (G) Quantification of Tbr2 cells at E12. Images similar to (C) were quantified. There are significantly more Tbr2 cells in areas that are vascularized (76 vs. 24%, paired Student's t-test P < 0.05, N = 2288 cells from 3 animals). l = lateral, m = medial. (H) Confocal stack of PECAM-1 stained E13 cortex. Note the tip cell filopodia are near Tbr2 cells. (I) Ninety degrees rotation of a confocal stack through E14 cortex showing staining for Tbr2 (green) and PECAM-1 (red) where only surface Tbr2 cells were imaged by turning off the laser imaging the Tbr2 stain during collection of optical stacks (arrow; also see main text). The laser imaging PECAM-1 staining was used to capture all blood vessels (bracket). (J) Collapsed confocal stacks of DAPI (white), Tbr2 (green), and PECAM-1 (red). Distance between surface Tbr2 cells and overlying vasculature was measured (arrows show example measurement bars: yellow lines from Tbr2 cell to blood vessels) and a similar measurement was made of all DAPI cells within the same region. (D3) Cumulative probability histogram of the proportion of Tbr2 cells (green) close to blood vessels as compared with proportion of DAPI nuclei (black). Tbr2 cells are significantly closer to blood vessels (P < 0.0001, Kolmogorov-Smirnov [KS] normality test). VZ = ventricular zone. Scale bars: (A–C) 50 μm, (D–F) 100 μm, (H) 20 μm, (I) 50 μm, (J) 10 μm.
Endothelial cells invade the VZ/SVZ by E13 and form a plexus of vasculature in a honeycomb pattern within the SVZ, with branches occasionally extending into the VZ (Supplementary Fig. 2 and Movie 1). Some endothelial cells form sparse radially oriented capillaries that extend into the cortical plate and meet the vasculature on the pial surface as has previously been shown (Vasudevan et al. 2008) (Supplementary Fig. 1 and Movie 1).
To visualize the spatial relationship between capillaries and IPCs, we double labeled E12 flattened cortical whole-mounts with antibodies for PECAM-1 and Tbr2. We imaged the VZ and SVZ from the ventricular surface and analyzed confocal projections. We found that in the dorsal cortex of E12 embryos, Tbr2 cell density was higher in the vascularized lateral regions (Fig. 1A–C; bracketed area) as compared with medial regions, which were nearly avascular at this time point. We also found Tbr2 cells associated with some of the leading endothelial tip cells (Fig. 1A–C, arrows). We counted the total number of Tbr2 cells in confocal images of whole-mounts and quantified the proportions of Tbr2 cells in high-vascularized versus low-vascularized regions. High-vascularized regions were areas where a vascular plexus had formed. Low-vascularized areas contained few sparse and scattered endothelial cells. We found that a significantly greater number of Tbr2 cells were present in regions that are highly vascularized at E12 (Fig. 1G; 76 vs. 24% of total Tbr2 cells per image—see methods for detail, paired student's t-test P < 0.05, N = 2288 cells from 3 animals). This indicates that the expansion of Tbr2 cells is temporally and spatially correlated with the appearance of cortical vasculature in the embryonic cortex.
Over embryonic days 12–14, the density of Tbr2 cells increased significantly with a lateral to medial gradient. In order to ask whether Tbr2 cells are spatially associated with the vasculature following its initial formation, we examined single optical sections through the lower parts of the SVZ focusing on medial parts of the dorsal cortex where Tbr2 cells are still relatively sparse at E14 (Fig. 1D–F). These images reveal that Tbr2 cells are highly associated with the vasculature in these regions and are often aligned in rows adjacent to developing blood vessels (Fig. 1D–F, arrows). Therefore, the spatial patterning of Tbr2 cells and blood vessels are correlated in the SVZ. This pattern however became less obvious as more Tbr2 cells appeared during the course of development. For example, in the SVZ of the lateral cortex at E14, Tbr2 cells were lined up on blood vessels but also filled the gaps between blood vessels (Fig. 2E).
We also found Tbr2 cells at the ventricular surface contacting endothelial tip cells (Fig. 1H and Supplementary Movie 2). Therefore, we examined whether surface Tbr2 cells are more likely to reside in spatial relation to overlying blood vessels. To address this, we imaged Tbr2 cells at the ventricular surface together with the overlying vascular plexus in whole mounts using confocal microscopy. We collected confocal stacks of emission channels corresponding to 4′,6-diamidino-2-phenylindole (DAPI), Tbr2, and PECAM-1 stains starting at the ventricular surface. In order to only image the surface cells stained with DAPI and/or Tbr2 but continue imaging the overlying vasculature in the Z direction, the lasers used for imaging DAPI and Tbr2 cells were turned off after 10 μm in the Z direction, whereas signal from PECAM-1 staining continued to be collected for another 30 μm. An example of a Tbr2 and PECAM-1 stained stack rotated 90 degrees in the Z direction is shown in Figure 1I. Confocal stacks were collapsed into projections and the distance from the center of each Tbr2 cell to the nearest blood vessel was measured and compared with the distance between other non-Tbr2 cells stained with DAPI and their nearby blood vessels (Fig. 1I–K). As shown in the cumulative probability histogram in Figure 1K, Tbr2 cell position is significantly correlated with the position of overlying vasculature as compared with other non-Tbr2 cells whose nuclei are labeled with DAPI (P < 0.0001, KS normality test, N = 699 for Tbr2 and 528 for DAPI cells).
We next asked whether cortical vasculature influences the position of differentiating Tbr2 cells as they migrate away from the ventricular surface past the vascular plexus toward the cortical plate. We obtained BAC transgenic mice where an EGFP reporter is under the control of the Tbr2 promoter (Tbr2:EGFP, a.k.a. Eomesodermin:EGFP; GENSAT) (Kwon and Hadjantonakis 2007). We perfused E14 Tbr2:EGFP live embryos with Alexa-594 conjugated lectin to label the vasculature. We found that Tbr2:EGFP cells are associated with the vasculature in the Tbr2 reporter animals similar to what we found with immunostaining for endogenous Tbr2 (Supplementary Fig. 3E–G). We postulated that because Tbr2 cells are spatially distributed in alignment with SVZ vasculature at the ventricular surface as well as within the SVZ, the vasculature might influence the position of radially migrating cells. In order to look at the position of migrating Tbr2:EGFP cells in relation to the vascular plexus, we acquired confocal stacks of the SVZ in Lecin-594 labeled Tbr2:EGFP cortex of E13 embryos (Fig. 2A–D, Supplementary Movie 3). When we examined projections of confocal stacks, we found that EGFP cells were often aligned in a honeycomb pattern resembling the vascular pattern (Fig. 2C). However, when we rotated confocal stacks 90° in the Z direction to mimic a coronal view, we found that many of the EGFP cells were oriented radially above the vasculature and were not adjacent to blood vessels, indicating that they were migrating away from the SVZ vascular plexus (Fig. 2D, Supplementary Movie 3). Therefore, it appears that when Tbr2 cells migrate radially toward the cortical plate they retain the honeycomb pattern of the vasculature they encountered within the SVZ. This indicates the SVZ vasculature affects the pattern of neurogenesis and migration during development and may influence the final position of excitatory neurons in the cortex.
The great majority of IPCs divide within the SVZ (Englund et al. 2005). We therefore asked whether mitotic Tbr2 cells are positioned differently than nonmitotic Tbr2 cells with respect to the vasculature in the SVZ. To address this, we prepared flattened whole mounts of E14 cortex and immunostained for phospho-histone-3 (PH3; a mitotic marker), endogenous Tbr2, and PECAM-1. We quantified and compared the distance between mitotic Tbr2 cells and capillaries vs. the distance between nonmitotic Tbr2 cells and capillaries in single confocal optical sections. We found that in general, PH3/Tbr2 cells were nearly always directly adjacent to a blood vessel as compared with all other Tbr2 cells in the regions imaged (Fig. 2E–G). Interestingly, we also observed that PH3/Tbr2 cells were often found near vascular branch points (Fig. 2F, the cartoon trace of Fig. 2E). In order to quantify this observation, we measured the distance between PH3/Tbr2 double positive cells and the closest branch point and the length of the branch that the cell was positioned on. We then divided the length of each branch into 4 quadrants, with quadrant 1 and 4 at each end of the branch near branch points, and quadrants 2 and 3 in the central region (Fig. 2G’). We asked whether each cell falls within a quadrant close to a branch point or a quadrant close to a branch center. For simplicity, we only measured the distance to one branch point and therefore analyzed the distribution of Tbr2 cells in quadrants 1 versus 2. We also limited our analysis to those cells positioned on branches that were more than 32 μm, 4 times longer than the width of an average Tbr2 cell (8 μm, data not shown) so that the length of each quadrant would allow at least one Tbr2 cell. If PH3/Tbr2 cells were distributed randomly, Tbr2 cells would fall equally near branch points and branch centers, quadrants 1 and 2. However, we found that a significantly higher proportion of cells were positioned near a branch point (62% in quadrant 1 vs. 38% in quadrant 2, P < 0.05 chi square, N = 82, 4 embryos) (Fig. 2G). These data suggest vascular branch points provide a niche for mitotic Tbr2 cells in the SVZ.
To determine if blood vessels influence the position of IPCs, we sought to alter the pattern of vasculature in the SVZ and ask whether this would alter the Tbr2 cell pattern. One molecule that has been shown to alter the pattern of vasculature and promote angiogenesis in the central nervous system (CNS) is vascular endothelial growth factor (VEGF) (Breier et al. 1992, 1995; Rosenstein et al. 1998; Louissaint et al. 2002; Gerhardt et al. 2003; Hogan et al. 2004). VEGF-A has been shown to alter and promote CNS angiogenesis by acting through VEGF-R2 (Rosenstein et al. 1998; Hogan et al. 2004). In the embryonic cortex, VEGF is only expressed by radial glia in the VZ, whereas the VEGF receptors VEGF-R1 (Flt1) and VEGF-R2 (Flk1) are expressed by cortical endothelial cells (Breier et al. 1992, 1995), (Supplementary Fig. 4). We therefore reasoned that by overexpressing VEGF-A in the cortex, we would only affect the IPCs indirectly through the alteration of blood vessel development. We used in utero injection and electroporation to introduce plasmids expressing either EGFP as control or EGFP together with VEGF-A into the embryonic cortex at E13 and we analyzed the brains 4 days later at E17.
We prepared flattened whole-mounts of VEGF electroporated brains and stained for Tbr2 and PECAM-1. Low magnification confocal stacks starting at the ventricular surface showed that the electroporated area was surrounded by anomalous ring-like overgrown blood vessels (Fig. 2H,I). Interestingly, the distribution of Tbr2 cells also seemed to follow the aberrant structure with more cells concentrated at the edges of the ring-like vascular structure (Fig. 2H–J, arrows). Endothelial cells did not invade the electroporated region uniformly probably because as it has been shown before, endothelial tip cells respond to a gradient of VEGF rather than an absolute concentration for migration (Gerhardt et al. 2003). We collected single optical sections in order to visualize the vasculature near the surface of the ventricle at higher magnification (Fig. 2K–M). We found that many Tbr2 cells were associated with the leading edge of invading ectopic blood vessels in the electroporated area and the pattern of Tbr2 cells followed the newly altered vascular pattern (Fig. 2 K-M, arrow).
We also analyzed VEGF overexpressing embryos by examining coronal sections. In control animals electroporated with only EGFP, we found an expected distribution of EGFP+ cells through the VZ, SVZ, and cortical plate with radially oriented EGFP labeled neurons in the cortex (Fig. 3C, arrow) and commissural axons leaving the electroporated region (Fig. 3C, arrowhead). However, in brains electroporated with EGFP and VEGF-A, cells in the electroporated region were dysplastic and disorganized with fewer radially oriented neurons in the cortical plate and a reduced number of axons (Fig. 3B, arrow). Compared with controls, the vasculature in the VEGF electroporated area was grossly abnormal with formation of large tangled clusters of blood vessels (Fig. 3B,D, arrows). The enlarged dome-like vessels are likely formed by endothelial tip cells that were stalled at a concentration gradient boundary formed by overproliferation of stalk cells. We found that Tbr2 cells were closely associated with aberrant vessels throughout the electroporated regions (Fig. 3B,D,F,H). Ectopic Tbr2 cells were located in the cortical plate (Fig. 3H, arrows), closely associated with aberrant vessels invading the cortical plate, and many were Ki67 positive, indicating that they were cycling cells (Fig. 3I–L).
Figure 3.
Tbr2 cells are associated with ectopic blood vessels. E13 animals were electroporated in utero with EGFP (A, C, E, G) or coelectroporated with EGFP and VEGF-A (B, D, F, H) and visualized at E17. (A) PECAM-1 immunofluorescence of control sections shows the cortex is vascularized throughout. (B) PECAM-1 staining of VEGF electroporated brain shows blood vessels are grossly abnormal and enlarged in the electroporated area (arrows), whereas the underlying region is less vascularized (arrowhead). (C) In controls EGFP labeled radially oriented cells are present in the VZ, SVZ, and cortical plate (arrow) with GFP axons leaving the electroporated region (arrowhead). (D) VEGF exposed brains have a dysplastic morphology in the electroporated region (arrow), and have fewer radially oriented cells and axons. (E) Tbr2 immunostaining of the same control sections shows Tbr2 cells distributed in the cortical SVZ. (F) Tbr2 staining of VEGF electroporated brains shows Tbr2 cells are ectopically present in the cortical plate where they are normally never found (arrows). (G, H) Merged images of Tbr2 and PECAM staining of controls and VEGF electroporated brains respectively. Bracketed area in (H) is magnified in (I–L). (I) Ectopic Tbr2 cells in VEGF treated brains. (J) Tbr2 and PECAM-1 staining merged. (K) Same section as (I) and (J) colabeled with the proliferative marker Ki67. (L) Tbr2 and Ki67 Merged. Arrow shows Tbr2 cells near the pia that are double labeled with Ki67. ctx = Cortex, lge = Lateral Ganglionic Eminence Scale bars: (A–H) 200 μm, (I–L) 100 μm.
Discussion
Here we show that the position of IPCs during mitosis, migration and differentiation is correlated with developing endothelial cells in the SVZ. The appearance of Tbr2 cells correlates with the appearance of vascularization, and Tbr2 cells are aligned with the honeycomb pattern of the SVZ vascular plexus when they are at the ventricular surface; within the SVZ where they divide, and as they migrate away from the SVZ radially toward the cortical plate. Tbr2 cells divide near vascular branch-points suggesting endothelial tip cells may contribute to a neurogenic niche for IPCs. Our experiments with ectopic overexpression of VEGF-A suggest that the pattern of Tbr2 expression follows the pattern of blood vessel development. Together these data indicate that the developing cortical vasculature provides a microenvironment within the SVZ in which IPCs accumulate and divide during neurogenesis.
The location of ventricular surface Tbr2 cells in association with overlying blood vessels suggests that endothelial cells may influence the location of IPCs. Although some Tbr2 cells may be generated at the ventricular surface in association with endothelial cells, others may have been born elsewhere in the VZ and migrated toward the vasculature. It is possible that endothelial cells at the pial surface may also influence neurogenesis or IPC production through their contact with radial glia endfeet and fibers at the pial surface before cortical vessels invade the SVZ.
Our VEGF overexpression experiments suggest that the spatial distribution of Tbr2 cells is influenced by the developing vasculature. However, some reports have shown that neural progenitors directly respond to VEGF through VEGF receptors potentially complicating the interpretation of the data presented here (Ogunshola et al. 2002; Le Bras et al. 2006; Li et al. 2006; Wada et al. 2006; Xiao et al. 2007). These experiments were either carried out with neural progenitors from regions other than embryonic cortex; or were based on embryonic stem cell-derived progenitors; or tested the response of progenitor cells to VEGF in dissociated brain cultures where endothelial cells had not been removed and an indirect action of VEGF on neural progenitors through contaminating endothelial cells could not be ruled out. Furthermore, culture conditions may promote VEGF receptor expression by neural progenitors that do not normally express them in vivo. Consistent with this, even though VEGF receptors are expressed by cells in vitro, VEGF receptors (flt1, flt3, flt4) are not expressed by embryonic cortical VZ or SVZ cells in vivo (Breier et al. 1992, 1995; Visel et al. 2004) and we do not find Flk-1 expression in cortical progenitors in vivo (Supplementary Fig. 4). Moreover, we find that although all electroporated GFP+ cells overexpress VEGF, the pattern of Tbr2 cells matches that of the newly invading vasculature: more Tbr2 cells are associated with the edges of the vasculature surrounding the electroporated region and fewer in the center of the electroporated region where the tissue is less vascularized (Fig. 2H–M). If the altered pattern of Tbr2 cells was due to a direct effect of VEGF on neural progenitors and independent of vasculature, the distribution would not match the circular pattern of the aberrant blood vessels (Fig. 2J).
One reason Tbr2 cells are associated with blood vessels may be that blood vessels are a source of circulating growth factors that the IPCs require. Although our observations do not rule this out, our data show that during angiogenesis at E12, Tbr2 cells are found associated with those regions where developing blood vessels are beginning to appear. The cells at the leading edge of the developing vasculature are primarily endothelial tip cells that do not form luminal structures and do not contain circulating blood (Gerhardt et al. 2003). This suggests that the interaction of IPCs with the vasculature may be due to an association with endothelial tip cells rather than factors in the circulation.
The great majority of IPCs are known to divide in the SVZ but the local microenvironment that may influence mitosis has not been fully described. Our data indicate that IPCs undergoing mitosis are near blood vessels and often associated with endothelial cells at blood vessel branch points. This suggests that the SVZ vasculature may serve as a niche for mitotic IPCs. It is possible that tip cells interact with mitotic IPCs at these sites during division. Interestingly, vascular branch-points have previously been observed to be sites of mitosis in glial tumors where glioma cells are able to migrate tangentially along blood vessels (Farin et al. 2006). The vasculature of tumors resembles that present during development in that both types of tissue are undergoing angiogenesis with and abundance of endothelial tip cells present (Calabrese et al. 2007). Considering that endothelial cells can promote the proliferation of cortical stem cells in culture through the release of one or more diffusible factor(s) (Shen et al. 2004), there may be common mechanisms by which endothelial cells promote the proliferation of stem and progenitor cells during development as well as glioma cells during tumorigenesis.
Although sparsely positioned IPCs are associated with endothelial cells during early development, at later stages when the density of IPCs increases this pattern is less visible. However, IPCs are multipolar with many dynamic branches and some of these branches may remain in contact with endothelial cells even though the IPCs may be at a distance (Noctor et al. 2004). Alternatively, different pools of Tbr2 expressing cells may exist later in development and some may not associate with blood vessels.
In conclusion, our data builds upon previous work regarding the role of vasculature in the stem cell niche and suggests a model for coordinated embryonic cortical angiogenesis and neurogenesis. Early in development, VEGF expressed and secreted by radial glia in the VZ may attract endothelial cells and promote angiogenesis (Rosenstein et al. 1998; Hogan et al. 2004). As endothelial cells migrate into the cortex from the ventral telencephalon, endothelial tip cells interact with IPCs and may provide them with attractive cues. Tbr2 cells migrate radially into the SVZ, and have also been observed to migrate tangentially within the SVZ (Noctor et al. 2004). While migrating, they may move toward blood vessels and contact endothelial cells in the SVZ. Although nearly all Tbr2 cells are in the cell cycle (Englund et al. 2005), those undergoing mitosis divide in the SVZ near vascular branch-points where they may encounter endothelial tip cells. Cells later detach as they differentiate into neurons. Some early-born deep layer neurons may establish a honeycomb pattern imposed by the vasculature as they migrate into the cortical plate and differentiate. Later in development, as the density of IPCs increases, the pattern of neurogenesis may also change. Thus, our observations indicate that cortical IP cell position, generation, and division are correlated with angiogenesis and that disrupting the normal pattern of angiogenesis affects IPCs. Together this suggests that the developing cortical vasculature plays a critical role in regulating cortical neurogenesis through its influence on IPCs in the SVZ.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Institutes of Health; and Autism Speaks.
Supplementary Material
Acknowledgments
We would like to thank William Walantus and Jeanelle Ariza Torres for excellent technical assistance with all animal surgery and histology. We also thank Tristan Sands, Doris Wang, Corey Harwell, David Hansen, Jan Lui, Laura Elias, Philip Parker, and Joy Mirjahangir for helpful discussions and comments on the manuscript. We would like to thank Frank Lie (Leica Microsystems) for technical assistance in preparation of Supplementary Movies 1 and 2. We are grateful to Dr Zoltan Molnar and Dr Robert Hevner for insightful discussions. Conflict of Interest: None declared.
References
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Bovetti S, Hsieh YC, Bovolin P, Perroteau I, Kazunori T, Puche AC. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J Neurosci. 2007;27:5976–5980. doi: 10.1523/JNEUROSCI.0678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Breier G, Clauss M, Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn. 1995;204:228–239. doi: 10.1002/aja.1002040303. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Cheung AF, Pollen AA, Tavare A, DeProto J, Molnar Z. Comparative aspects of cortical neurogenesis in vertebrates. J Anat. 2007;211:164–176. doi: 10.1111/j.1469-7580.2007.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8:438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53:799–808. doi: 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci USA. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma T, Nakajima Y, Nakamura H. Development of pharyngeal arch arteries in early mouse embryo. J Anat. 2002;201:15–29. doi: 10.1046/j.1469-7580.2002.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan KA, Ambler CA, Chapman DL, Bautch VL. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development. 2004;131:1503–1513. doi: 10.1242/dev.01039. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Kwon GS, Hadjantonakis AK. Eomes::GFP-a tool for live imaging cells of the trophoblast, primitive streak, and telencephalon in the mouse embryo. Genesis. 2007;45:208–217. doi: 10.1002/dvg.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras B, Barallobre MJ, Homman-Ludiye J, Ny A, Wyns S, Tammela T, Haiko P, Karkkainen MJ, Yuan L, Muriel MP, et al. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci. 2006;9:340–348. doi: 10.1038/nn1646. [DOI] [PubMed] [Google Scholar]
- Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res. 2006;84:1656–1668. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- McConnell SK. Constructing the cerebral cortex: neurogenesis and fate determination. Neuron. 1995;15:761–768. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Misson JP, Edwards MA, Yamamoto M, Caviness VS., Jr Mitotic cycling of radial glial cells of the fetal murine cerebral wall: a combined autoradiographic and immunohistochemical study. Brain Res. 1988;466:183–190. doi: 10.1016/0165-3806(88)90043-0. [DOI] [PubMed] [Google Scholar]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508:28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunshola OO, Antic A, Donoghue MJ, Fan SY, Kim H, Stewart WB, Madri JA, Ment LR. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277:11410–11415. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Rakic P. A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb Cortex. 2006;16(Suppl. 1):i13–17. doi: 10.1093/cercor/bhk036. [DOI] [PubMed] [Google Scholar]
- Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein JM, Mani N, Silverman WF, Krum JM. Patterns of brain angiogenesis after vascular endothelial growth factor administration in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:7086–7091. doi: 10.1073/pnas.95.12.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Smart IH. Proliferative characteristics of the ependymal layer during the early development of the mouse neocortex: a pilot study based on recording the number, location and plane of cleavage of mitotic figures. J Anat. 1973;116:67–91. [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr Early ontogeny of the secondary proliferative population of the embryonic murine cerebral wall. J Neurosci. 1995;15:6058–6068. doi: 10.1523/JNEUROSCI.15-09-06058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Nakamura K, Okamoto K, Kaneko T. Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci Res. 2001;41:51–60. doi: 10.1016/s0168-0102(01)00259-0. [DOI] [PubMed] [Google Scholar]
- Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;128:1983–1993. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- Vasudevan A, Long JE, Crandall JE, Rubenstein JL, Bhide PG. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat Neurosci. 2008;11:429–439. doi: 10.1038/nn2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–D556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Haigh JJ, Ema M, Hitoshi S, Chaddah R, Rossant J, Nagy A, van der Kooy D. Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival. J Neurosci. 2006;26:6803–6812. doi: 10.1523/JNEUROSCI.0526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Kong Y, Yang S, Li M, Wen J, Li L. Upregulation of Flk-1 by bFGF via the ERK pathway is essential for VEGF-mediated promotion of neural stem cell proliferation. Cell Res. 2007;17:73–79. doi: 10.1038/sj.cr.7310126. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Tiveron MC, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex. 2004;14:1408–1420. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.