Abstract
Thalamocortical axons (TCAs) originate in dorsal thalamus, extend ventrally along the lateral thalamic surface, and as they approach hypothalamus make a lateral turn into ventral telencephalon. In vitro studies show that hypothalamus releases a chemorepellent for TCAs, and analyses of knockout mice indicate that Slit chemorepellents and their receptor Robo2 influence TCA pathfinding. We show that Slit chemorepellents are the hypothalamic chemorepellent and act through Robos to steer TCAs into ventral telencephalon. During TCA pathfinding, Slit1 and Slit2 are expressed in hypothalamus and ventral thalamus and Robo1 and Robo2 are expressed in dorsal thalamus. In collagen gel cocultures of dorsal thalamus and Slit2-expressing cells, axon number and length are decreased on the explant side facing Slit2-expressing cells, overall axon outgrowth is diminished, and axons turn away from the Slit2-expressing cells. Thus, Slit2 is an inhibitor and chemorepellent for dorsal thalamic axons. Collagen gel cocultures of dorsal thalamus with sections of live diencephalon, with and without the hypothalamus portion overlaid with Robo2-fc-expressing cells to block Slit function, identify Slits as the hypothalamic chemorepellent. Thus, Slits are chemorepellents for TCAs endogenous to hypothalamus and steer TCAs from diencephalon into ventral telencephalon, a critical pathfinding event defective in Slit and Robo2 mutant mice.
Keywords: axon guidance, axon inhibition, dorsal thalamus, hypothalamus, internal capsule, neocortex, robos
Introduction
The directed growth of axons along stereotypic pathways is a crucial early step in establishing the appropriate axonal connections that underlie the proper functioning of the adult nervous system. The projection of thalamocortical axons (TCAs), which originates in dorsal thalamus and relays sensory information from the periphery to the neocortex, forms a prominent axon tract in the mammalian forebrain called the internal capsule that passes through the mammalian ventral telencephalon. During development, TCAs extend ventrally from dorsal thalamus along the lateral surface of the diencephalon. As TCAs approach the hypothalamus, they make a sharp lateral turn in ventral thalamus to enter the ventral telencephalon and extend dorsolaterally through the internal capsule toward their major target, the neocortex.
Both axonal scaffolds and secreted molecular activities have been implicated in the control of TCA pathfinding. For example, neurons in the medial aspect of the ventral telencephalon have been proposed to form axonal scaffolds that direct TCAs into the ventral telencephalon (Metin and Godement 1996; Molnar et al. 1998; Braisted et al. 1999; Tuttle et al. 1999). In support of this proposal is the finding in mice deficient for the basic helix-loop-helix transcription factor Mash-1 that a failure of TCAs to extend into the ventral telencephalon is correlated with the absence of a discrete population of ventral telencephalic cells that extends axons into dorsal thalamus early in development (Tuttle et al. 1999). Other studies have provided evidence that secreted molecular activities have a prominent role in guiding TCAs. For example, in vitro axon guidance assays and analyses of mutant mice have provided evidence that the ventral telencephalon releases a diffusible attractant activity for dorsal thalamic axons (Braisted et al. 1999) and that the chemoattractant netrin-1 accounts at least in part for this activity (Braisted et al. 2000). In vitro studies have also shown that the hypothalamus, a ventral diencephalic structure, releases a robust chemorepellent activity for dorsal thalamic axons that is proposed to help deviate TCAs from their ventrally directed trajectory and steer them laterally into the ventral telencephalon (Braisted et al. 1999).
Here we provide evidence from in vitro axon guidance assays and in vivo expression analyses that members of the Slit family of secreted chemorepellents, acting through their receptors, Robo1 and Robo2, are the chemorepellent activity for TCAs secreted by ventral diencephalon (hypothalamus). Three mammalian Slit genes have been identified, Slit1, Slit2, and Slit3 (Itoh et al. 1998; Nakayama et al. 1998; Brose et al. 1999; Li et al. 1999; Wang et al. 1999), as well as 2 Robo genes, Robo1 and Robo2. Slit2 has been shown to inhibit or repel several axonal populations, including those from olfactory bulb (Nguyen Ba-Charvet et al. 1999), hippocampus (Nguyen Ba-Charvet et al. 1999; Wu et al. 1999), spinal motoneurons (Brose et al. 1999), and retinal ganglion cells (Erskine et al. 2000; Niclou et al. 2000; Ringstedt et al. 2000). Our results help provide a definitive mechanism for the findings that TCA pathfinding is defective in mice deficient in Slits (Bagri et al. 2002; Andrews et al. 2006) or Robo2 (López-Bendito et al. 2007).
Materials and Methods
Animals
Rat embryos were obtained from timed-pregnant females (Harlan Sprague–Dawley) and staged. The day of insemination is designated as embryonic day 0. All animal works were done in accordance with the regulations governing the use of animals in research and were carried out under the auspices of animal protocols approved by our institutions animal use and care committee.
In Situ Hybridization
Timed-pregnant rats were anesthetized, and embryo heads were fixed by immersion in 4% paraformaldehyde (Pfa). After cryoprotection, the heads were sectioned coronally at 20 μm in a cryostat and processed for in situ hybridization using S35 radiolabeled riboprobes, as previously described (Ringstedt et al. 2000).
Preparation of Explants
E15 embryos were removed by Cesarean section from timed-pregnant rats anesthetized with sodium pentobarbital and placed in cold L-15 medium supplemented with 0.6% glucose (Sigma, St Louis, MO; L15-glucose). To prepare explants of dorsal thalamus and ventral diencephalon, brains were removed, embedded in 3% low melting point agar in L15-glucose, and then sectioned coronally at 200 μm using a vibratome. Sections in which the internal capsule bridges the diencephalon and ventral telencephalon were collected, and the ventral telencephalon was removed. The ventral portion of the diencephalic piece contains the ventral thalamus and hypothalamus and was used as the “hypothalamus” explant. Dorsal thalamus was isolated from sections similar to those used for the hypothalamic explants as previously described (Braisted et al. 1999), and its lateral–medial axis was positioned parallel to the test tissue.
Preparation of Aggregates of Slit2-Expressing Cells
The 293T cells were transfected with a pSecTagB expression vector containing cDNA for human Slit2 tagged with a myc epitope Slit2-myc or Robo2-fc using SuperFect Transfection Reagent (Qiagen, Valencia, CA). The pSecTagB without the insert was used for mock transfections. Subconfluent cells were transfected, agar blocks containing cells were prepared, and small cubes were cut from the agar and used for cocultures as described in Braisted et al. (2000).
Preparation of Collagen Gel Cocultures
Slit2-myc Expressing Cells
Collagen was prepared from adult rat tails, and cocultures were suspended in collagen in 4-well dishes (Nunc, Rochester, NY) as described (Braisted et al. 1999). Dorsal thalamic explants and Slit2-myc or vector (mock)-transfected 293T cell aggregates were positioned approximately 150–300 μm apart.
Slit Function Blocking Experiments
Dorsal thalamic explants were placed adjacent to the lateral aspect of hypothalamic explants in collagen gel cocultures as described above. To block Slit function, the hypothalamus was overlaid with a block of Robo2-fc-expressing 293T cells or mock-transfected cells as a control.
DiI Labeling of Cocultures
Collagen gel cocultures were fixed with 4% Pfa plus 1% glutaraldehyde at 4 °C, rinsed and then crystals of the axonal tracer 1,1′-dioctadecyl 3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes, Carlsbad, CA; Honig and Hume 1989a, 1989b) were placed into the dorsal thalamus. Cultures were stored to allow the DiI to diffuse along the full length of the axons.
Immunocytochemistry
Collagen gel cocultures were fixed and incubated 3–4 days at 4 °C in mouse monoclonal anti-ß-tubulin antibody (Amersham, Piscataway, NJ), prior to secondary processing.
Analysis of Axon Outgrowth in Cocultures
All analyses were done blind to the nature of the test explant.
Semiquantitative Analysis
After 1.5 days in vitro, cocultures were photographed using phase-contrast optics. Axon outgrowth was assigned to 1 of 3 categories: explants that had more axon outgrowth 1) “toward,” 2) “away” from the test explant or 293 cell aggregates, or 3) explants that had similar amounts of axon outgrowth on both these sides and were therefore scored as “symmetric.”
Quantitative Analysis
After fixation and axonal labeling with DiI or an anti-ß-tubulin monoclonal antibody (see above), cocultures were photographed. From the digital images, the number and length of axons on the sides of the explant facing toward and away from the 293T cells or hypothalamus were measured from digitized images. In some experiments, the total number of axons emanating from all sides of the dorsal thalamic explants was counted.
To determine whether dorsal thalamic axons turn in response to Slit2, the “angle of deviation” of axons away from the 293T cells was measured in octants T1 and T2 on the half of the explant facing toward the cells as follows: In Photoshop, a line was drawn through the center of the explant and the base of the neurite. This equals the expected angle of growth if the neurite had grown out radial from the explant. Another line, equaling the actual angle of growth, was drawn through the base and the tip of the neurite. The difference between these angles was determined, using the expected angle of growth as a baseline. A neurite growing more toward the Slit2-expressing cells than predicted by the expected angle of growth resulted in a positive value, respective a negative when it grew away from the cells. The differences were then averaged and the data analyzed using the Student's t-test.
Results
Expression of Slits Relative to the TCA Pathway
TCAs project ventrally out of dorsal thalamus along the lateral surface of ventral thalamus (Braisted et al. 1999; Tuttle et al. 1999). As they approach hypothalamus, they make a sharp lateral turn, enter ventral telencephalon, and extend dorsolaterally toward neocortex. To determine whether Slits may be involved in TCA pathfinding and, in particular, whether they can account for a hypothalamic repellent activity proposed to steer TCAs into the ventral telencephalon (Fig. 1), we first examined the expression patterns of the mammalian Slit homologues Slit1, Slit2, and Slit3, relative to the developing TCA pathway. In rat, dorsal thalamic projection neurons that form the TCA pathway are generated between E12 and E17 (Altman and Bayer 1979a, 1979b, 1979c, 1989a, 1989b, 1989c). Based on these birth dates of dorsal thalamic neurons in rat and early axon tracing studies in rat and in mice (De Carlos and O'Leary 1992; Braisted et al. 1999; Tuttle et al. 1999), one can conclude that at E13 in rat, few if any TCAs have extended ventrally out of the dorsal thalamus, and none have turned from diencephalon to enter the ventral telencephalon. Therefore, in situ hybridizations were performed on coronal sections of E13, E15, and E17 rat brains (Fig. 2), ages when TCAs are pathfinding from dorsal thalamus to telencephalon in vivo.
Figure 1.
Summary of findings in this study on expression patterns of Slits and Robos related to the pathfinding of TCAs through diencephalon and ventral telencephalon (vTel) viewed in a schematic coronal section. Dorsal is to the top, medial to the left. Robo expression is shown in dorsal thalamus (dTh) only. The Slits act as chemorepellents for TCAs that originate from dTh and induce the turning of TCAs as they approach ventral thalamus (vTh) and hypothalamus (Hy) to steer them laterally into the vTel. Ctx, cortex; Epi, epithalamus; GE, ganglionic eminence; and ic, internal capsule.
Figure 2.
Expression of Slits in embryonic rat diencephalon. Coronal sections through diencephalon of E13 (A, D, G), E15 (B, E, H), and E17 (C, F, I) rat brains showing Slit1 (A–C), Slit2 (D–F), and Slit3 (G–I) expression detected by in situ hybridization using S35 labeled riboprobes. Sections are counterstained with bisbenzimide. Photos are single exposures using dark field and ultraviolet illumination. Dorsal is up. Arrows mark the ventral extent of the TCA pathway in diencephalon at the level shown. For details, see Results. dTh, dorsal thalamus; Epi, epithalamus; Hy, hypothalamus; and vTh, ventral thalamus. Scale bars: 200 mm.
At E13, both Slit1 and Slit2 are expressed in the lateral aspect of dorsal thalamus, where the postmitotic of early generated dorsal thalamic neurons are positioned (Fig. 2A,D). Slit1 is also expressed strongly in the laterodorsal aspect of ventral thalamus and weakly in the parenchyma of dorsolateral hypothalamus and ventral part of ventral thalamus (Fig. 2A). Slit2 is strongly expressed in ventral hypothalamus, with particularly high levels in the ventricular zone, and in epithalamus, which is dorsal to dorsal thalamus (Fig. 2D). In contrast, Slit3 exhibits low expression in limited parts of ventral thalamus and hypothalamus (Fig. 2G).
At E15, Slit1 and Slit2 are expressed in ventral thalamus and dorsal hypothalamus. Particularly, striking is the expression of Slit1 and Slit2 medial and ventral to the path of TCAs as it exits diencephalon and enters ventral telencephalon (Fig. 2B, E). Slit1 and Slit2 are also expressed in epithalamus and adjacent to the ventricular zone in dorsal thalamus. In contrast, Slit3 is only expressed weakly, if at all, in diencephalon (Fig. 2H).
At E17, Slit1 continues to be expressed strongly in ventral thalamus and dorsal hypothalamus, just medial and ventral to TCA pathway, as well as more weakly throughout hypothalamus (Fig. 2C). Slit2 is strongly expressed in ventral hypothalamus, in both the ventricular zone and the mantle zone, just lateral to the presumptive arcuate nucleus (Fig. 2B). Slit1 and Slit2 are also expressed in epithalamus and the dorsal thalamic ventricular zone. Slit3 is weakly expressed throughout much of diencephalon (Fig. 2I). Thus, during the period that TCAs turn from diencephalon into ventral telencephalon, Slit1 and Slit2 are highly expressed medial and ventral to the TCA pathway within diencephalon, locations that position them appropriately to deviate TCAs from their ventrally directed trajectory within diencephalon, and steer them into ventral telencephalon.
Because TCAs turn into ventral telencephalon and extend dorsolaterally through it to neocortex, we examined at E13, E15, and E17 Slit expression within ventral telencephalon relative to the TCA pathway, the internal capsule (data not shown). Slit1 is moderately expressed within the ganglionic eminence and in a domain just dorsal to the internal capsule, the path of TCAs through the ventral telencephalon. Slit2 expression is nondetectable, and Slit3 expression is very low or nondetectable at the level of the TCA path through the ventral telencephalon. These expression patterns are consistent with the proposed role for Slit1 and Slit2 in TCA pathfinding.
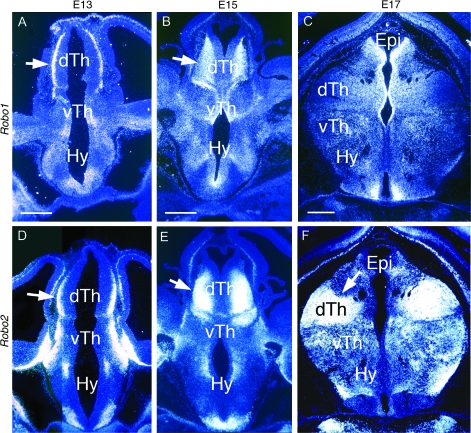
Expression of Slit Receptors in Dorsal Thalamus
If Slit1 and Slit2 act as chemorepellents for TCAs, then their receptors, Robo1 or Robo2, should be expressed in dorsal thalamus at the appropriate ages. Therefore, we performed in situ hybridizations on coronal sections of rat diencephalon at E13, E15, and E17. At each age, Robo2 expression was more robust than Robo1 (Fig. 3). At E13 and E15, both Robo1 (Fig. 3A,B) and Robo2 (Fig. 3D,E) are highly expressed throughout the lateral part of the dorsal thalamus, the location of postmitotic dorsal thalamic neurons that give rise to the TCA projection (Schlaggar et al. 1993; De Carlos et al. 1995). At E17, both Robo1 (Fig. 3C) and Robo2 (Fig. 3F) continue to be expressed in dorsal thalamus. Robo1 expression is highest medially, whereas Robo2 is expressed at high levels throughout most of dorsal thalamus. In addition, Robo1 and Robo2 are expressed in discrete domains elsewhere in the diencephalon at each age (Fig. 3). Thus, the early expression of Robo1 and Robo2 in dorsal thalamus is consistent with a role for them in mediating a repellent action of Slit1 and Slit2 on developing TCAs.
Figure 3.
Expression of Robos in embryonic rat diencephalon. Coronal sections through diencephalon of E13 (A, D), E15 (B, E), and E17 (C, F) rat brains showing Robo1 (A–C) and Robo2 (D–F) expression detected by in situ hybridization using S35 labeled riboprobes. Sections are counterstained with bisbenzimide. Photos are single exposures using dark field and ultraviolet illumination. Dorsal is up. Arrows mark Robo expression in domains of TCA projection neurons in nascent dTh nuclei. For details, see Results. dTh, dorsal thalamus; Epi, epithalamus; Hy, hypothalamus; and vTh, ventral thalamus. Scale bars: 200 mm.
Slit2 Is a Chemorepellent for Dorsal Thalamic Axons In Vitro
To test whether Slits act as growth inhibitors or chemorepellents for TCAs, we cocultured in 3-dimensional collagen gels explants of E15 rat dorsal thalamus at a distance from aggregates of 293T cells transiently transfected either with the human slit2-myc cDNA or with the vector cDNA as a control (referred to as mock). When dorsal thalamus is cocultured with mock-transfected cells, 27% of the explants have more outgrowth on the side facing toward the cells and none have more outgrowth on the side facing away from the cells (Fig. 4A). In contrast, when dorsal thalamus is cocultured with Slit2-expressing cells, axon outgrowth is strongly biased away from the cells (Fig. 4B): 61% of the explants have more axon outgrowth on the side facing away from the cells and only 22% have more axon outgrowth on the side facing toward the cells (Fig. 4C,D; differences between cocultures with mock-transfected or Slit2-expressing cells are statistically significant, P < 0.0001). These results indicate that Slit2 inhibits and/or repels dorsal thalamic axons in vitro.
Figure 4.
Slit2 repels and inhibits dorsal thalamic axon outgrowth in vitro. (A, B) Phase-contrast images of axon outgrowth from explants of E15 rat dorsal thalamus (dTH) cocultured in collagen gels at a distance (150–300 μm) from 293T cells transfected with either the parental expression vector (A; dTh-mock) or a human Slit2-myc expression vector (B; dTh-Slit2). (E–G) Quantitation of cocultures after labeling with DiI or a ß-tubulin antibody (not shown)—all done blind to whether the 293T cells do or do not express Slit2. Scale bar is 150 μm. (C) Analysis scheme where the number and the length of axon fascicles extended from explants of dTh were quantified in the quadrants facing away or toward from the Slit2-expressing cells or in control cultures from the mock-transfected cells. (D) Percentage of explants exhibiting growth biased toward or away from the transfected cells, or equal outgrowth (symmetric), scored by visual impression. Blind qualitative scoring of outgrowth preference exhibited by explants. (E) The ratio of the numbers of axon fascicles in the quadrants facing toward (T) and away (A) from the transfected cells is decreased in the dTh-Slit2 cocultures (n = 23) compared with the dTh-mock cocultures (n = 27) (P < 0.0001, Student's t-test). The overall bias in dorsal thalamic axon outgrowth toward versus away from mock-transfected aggregates of 293T cells is apparently due to an unidentified activity released by 293T cells that enhances axon outgrowth (also see Fig. 5D). (F) The ratio between the average length of axon fascicles in the quadrants facing toward and away from the transfected cells is decreased in dTh-Slit2 cocultures (n = 10 cocultures, 270 axon fascicles) compared with the dTh-mock cocultures (n = 10 cocultures, 490 axon fascicles) (P < 0.0003, Student's t-test). (G) The total number of axon fascicles extended by dorsal thalamic explants (all 4 quadrants were included in these counts) is decreased by 49% in the dTh-Slit2 cocultures (n = 10 cocultures, 491 axon fascicles) compared with the dTh-mock cocultures (n = 10 cocultures, 961 axon fascicles) (P < 0.003, Student's t-test). (H) Scheme for quantifying axon turning. In the 2 “toward side” octants, the angles between a line drawn through the center of the explant and the base of the axon fascicle (i.e., the expected direction of growth, Gx, angle measured is VGx) and a line through the base and the tip of the axon fascicle (i.e., the actual direction of growth, Ga, angle measured is VGa) were calculated. This “angle of deviation” (Vdev) is positive when the axon fascicle deviated toward the explant and negative when it deviated away from the explant. (I) Axon fascicles growing out from dorsal thalamic explants deviated on average 15 degrees away from mock-transfected cells (n = 10 cocultures, 163 axon fascicles), but they deviated on average 37 degrees away from the Slit2-transfected cells (n = 10 cocultures, 69 axon fascicles) (P < 0.01, Student's t-test).
To further quantify the effects of Slit2 on dorsal thalamic axons and assess the possibility that it inhibits their growth, we measured the number and length of axons on the explant sides facing toward or away from the transfected cells. When dorsal thalamus is cocultured with Slit2-transfected cells, 65% fewer axons extend from the side facing toward the cells (Fig. 4E), and these axons are 48% shorter than those on the side facing away from the cells (Fig. 4F). In contrast, when dorsal thalamus is cocultured with mock-transfected cells, 11% more axons extend from the side facing toward the cells, and they are only 8% shorter than those on the side facing away from the cells (the 8% decrease in length is likely an artifact because many axons on the toward side have reached the mock-transfected cells and, therefore, limiting their maximum length to the distance between the explant and cells). The total number of axons extending from the dorsal thalamic explants is also decreased by 51% in cocultures with Slit2-transfected cells compared with mock-transfected cells (Fig. 4G). All comparisons between cocultures with mock-transfected or Slit2-expressing cells are statistically significant (Fig. 4). Taken together, these findings indicate that Slit2 inhibits dorsal thalamic axon growth in vitro.
To determine whether Slit2 also repels dorsal thalamic axons, we measured the angle of deviation of axons away from the transfected cells (Fig. 4H; also Materials and Methods). The angle of axon turning away from Slit2-transfected cells is significantly greater than their angle of turning away from mock-transfected cells (Fig. 4I). Taken together, these findings indicate that Slit2 acts as a chemorepellent for dorsal thalamic axons in vitro.
Slit-Dependent Repulsion of Dorsal Thalamic Axons by Hypothalamus
The findings presented above suggest that Slits are components of the hypothalamic repellent activity. Because the original experiments demonstrating that hypothalamus releases a repellent activity for dorsal thalamic axons were done using mouse tissues (Braisted et al. 1999), we performed similar experiments to verify that rat hypothalamus has a similar repellent effect. Living vibratome sections of the ventral diencephalon of E15 rats were cocultured in collagen gels with explants of E15 rat dorsal thalamus placed at a distance from the lateral surface of hypothalamus. Similar to mouse, rat dorsal thalamic axon outgrowth is biased away from hypothalamus (Fig. 5A): 50% of the explants have more axon outgrowth on the side facing away from hypothalamus, whereas only 12.5% had more outgrowth on the side facing toward hypothalamus (Fig. 5D).
Figure 5.
Hypothalamic releases in vitro a repellent activity for dorsal thalamic axons that can diminished by Robo2-fc. (A) Phase-contrast photo of axon outgrowth from explants of E15 rat dorsal thalamus (dTh) cocultured in collagen gels with living vibratome sections of E15 rat ventral diencephalon; the dorsal thalamic explants were placed at a distance (150–300 μm) from the lateral surface of the hypothalamus (dTh-Hy). Dorsal thalamic axon outgrowth is biased away from the hypothalamus. Scale bar is 150 μm. (B, C) Same coculture setup as in (A), but the sections of ventral diencephalon were overlaid with an agarose block containing 293T cells transfected with either a Robo2-fc expression vector (B; dTh-Hy + R2fc) or with the parental vector (C; dTh-Hy + mock). (B) Dorsal thalamic axon outgrowth is biased toward from the hypothalamus when overlaid with Robo2-fc-expressing cells. (C) Dorsal thalamic axon outgrowth toward and away from hypothalamus is relatively equal when overlaid with mock-transfected 293T cells. (D) Quantification of dorsal thalamic axon outgrowth. Axonal outgrowth from the dorsal thalamic explants was rated blind to culture type as being biased away or toward the hypothalamus or no bias (symmetric) in the cocultures types shown in (A) (Hy; n = 24), (B) (Hy + Rb2Fc; n = 43), and (C) (Hy + mock; n = 37). Significantly, more explants have outgrowth biased toward the hypothalamus in the Hy + Rb2Fc cocultures than in the Hy + mock cocultures (P < 0.05, Student's t-test) or in the Hy cocultures (P < 0.002, Student's t-test). However, the difference in outgrowth bias between the Hy + mock cocultures and Hy cocultures was not significant (P = 0.3). The relative symmetry in overall axon outgrowth of hypothalamus overlaid with mock-transfected 293T cells compared with both hypothalamus without cell overlays, and those overlaid with Robo2-fc transfected 293T cells, is apparently due to an unidentified activity released by 293T cells that enhances axon outgrowth—a similar effect on the bias in growth is observed in cocultures of dTh with mock-transfected aggregates of 293T cells (Fig. 4D). Counts of the number of dorsal thalamic axon fascicles show that the ratio between the number of axon fascicles in the quadrants facing toward and away from the hypothalamus is 23% greater in the Hy + Rb2Fc cocultures (n = 31 quantified) than in the Hy + mock cocultures (n = 24 quantified) (P < 0.04, Student's t-test).
To determine whether the endogenous hypothalamic repellent activity is due to Slits, we repeated these experiments but overlaid hypothalamus with an agarose block containing Robo2-fc-expressing 293T cells to block Slit function. First, we confirmed that Robo2-fc itself has no significant effect on the outgrowth bias because the distribution of axon outgrowth from explants of dorsal thalamus cocultured with mock-transfected 293T cells (Fig. 5B) is similar to that which we observe in cocultures with aggregates of Robo2-fc-expressing 293T cells (data not shown). However, exposure of hypothalamus to Robo2-fc results in a statistically significant reversal of the biased directional outgrowth of dorsal thalamic axons compared with hypothalamus alone (P < 0.002)—instead of being biased away from hypothalamus, dorsal thalamic axon outgrowth is biased toward hypothalamus overlaid with Robo2-fc-expressing cells (Fig. 5B): 54% of explants have more outgrowth toward hypothalamus and only 16% have more outgrowth away from hypothalamus (Fig. 5D). When hypothalamus is overlaid with mock-transfected cells, the overall outgrowth of dorsal thalamic axons is symmetric and shows a statistically significant (P < 0.002) difference compared with that in cocultures of hypothalamus overlaid with Robo2-fc (Fig. 5C,D).
In conclusion, these coculture experiments, and in particular the finding of a substantial, statistically significant block of the chemorepellent effect of hypothalamus by the Slit function blocking reagent, Robo2-fc, show that Slits are responsible for the chemorepellent activity endogenous to hypothalamus.
Discussion
The TCA projection originates in dorsal thalamus and relays sensory information from the periphery to neocortex. As TCAs navigate from dorsal thalamus to cortex, they make a sharp lateral turn near the ventral thalamic–hypothalamic border to enter the ventral telencephalon. These findings suggest that hypothalamus is repulsive and ventral telencephalon is attractive for TCAs. Supporting these hypotheses, dorsal thalamic axons are repelled at a distance in vitro by hypothalamus and attracted by ventral telencephalon (Braisted et al. 1999). We have previously provided evidence that netrin-1 is the chemoattractant for TCAs associated with ventral telencephalon (Braisted et al. 2000). Here we have investigated the chemorepellent activity for TCAs that is secreted by hypothalamus and provided evidence that the chemorepellent Slit2, and likely the related chemorepellent Slit1, induces this turning behavior exhibited by TCAs. The expression patterns of Slit1 and Slit2 and of their receptors, Robo1 and Robo2, are appropriate for this role (Fig. 1). We also find that in vitro, dorsal thalamic axons are both inhibited and repelled at a distance by aggregates of Slit2-expressing cells. Reagents that block Slit function, the Robo2-fc, also block the repellent activity endogenous to hypothalamus. These findings indicate that the Slits are ventral diencephalic/hypothalamic chemorepellents for TCAs and control a crucial phase of TCA pathfinding by deviating TCAs from their ventral trajectory and steering them laterally into the telencephalon.
We show that the distribution of mRNAs encoding Slits and Robos in the diencephalon would fit a model where the Slits exert a chemorepellent effect on TCAs. During the period of TCA pathfinding, Robo1 and Robo2 are expressed by dorsal thalamic projection neurons that form the TCA projection. Over the same time frame, when TCAs extending from dorsal thalamus make a sharp turn in response to a hypothalamic chemorepellent activity, the chemorepellents Slit1 and Slit2 are expressed in hypothalamus as well as in ventral thalamus. TCAs that extend ventrally toward these ventral telencephalic structures, do not enter them, but instead follow a path along the lateral surface of ventral thalamus as they turn sharply into ventral diencephalon.
In cocultures of explants from dorsal thalamus with aggregates of Slit2-expressing 293T cells, dorsal thalamic explants show a strong bias in axon outgrowth away from the Slit2 source. Further, dorsal thalamic axons are longer and more numerous on the explant side away from the Slit-secreting cells. Because Slit2 also significantly reduced the total number of axons, this effect might be due to an inhibition of axon growth. Therefore, to demonstrate the chemorepellent activity of Slit2, we also measured the angle of deviation of the paths of axons from a hypothetical path of growth that would extend radially from the explant and find that on average the axons exhibit a significant turning away from the Slit-secreting cell aggregates. Thus, TCAs exhibit a true chemorepellent response to Slit2.
In cocultures of explants of hypothalamus and dorsal thalamus, we show that Robo2 receptor bodies (Robo2-fc), which bind Slits and prevent their function (Nguyen Ba-Charvet et al. 2001), strongly diminish the chemorepellent effect exerted by hypothalamus on dorsal thalamic axons. In addition, we find that overlaying hypothalamus with Robo2-fc-expressing cells diminishes its inhibitory effect on axon outgrowth from dorsal thalamic explants. Thus, taken together, these studies demonstrate that Slits are chemorepellents for dorsal thalamic axons and are responsible for the chemorepellent activity endogenous to hypothalamus that we observe in vitro.
Recent studies have analyzed mice deficient for Slits and Robos to assess their requirements for TCA pathfinding. Mice deficient in Slit2 show defects in TCA pathfinding, characterized by a failure of TCAs to turn properly from diencephalon into ventral telencephalon; instead many TCAs continue on a ventral trajectory and abnormally enter the hypothalamus. This aberrant phenotype is exaggerated in mice deficient in both Slit1 and Slit2 (Bagri et al. 2002). Mice deficient in Robo2 exhibit similar defects in TCA pathfinding as Slit deficient mice (López-Bendito et al. 2007). Mice deficient in Robo1 do not exhibit these TCA pathfinding defects, and in fact, TCAs reach cortex earlier in Robo1 knockouts than in wild-type mice (Andrews et al. 2006) but fewer TCAs reach cortex in mice deficient in both Robo1 and Robo2 (López-Bendito et al. 2007). Thus, our in vitro axon guidance assays demonstrate that Slit2 is a chemorepellent and growth inhibitor for TCAs and that the Slits are responsible for the chemorepellent activity on TCA axons that is endogenous to hypothalamus. These in vitro findings corroborate and extend the analyses of Slit and Robo mouse mutants and provide definitive support for the mechanism underlying defects in TCA pathfinding by definitely identifying Slits as the hypothalamic chemorepellent. In conclusion, the Slits acting through the Robos have a prominent role as chemorepellents in TCA pathfinding from dorsal thalamus to cortex and cooperate with other diffusible and substrate-associated molecules, as well as guidepost cells and the scaffolds they establish, to ensure the proper development of TCA input.
Funding
National Institutes of Health (R37 NS31558 and R01 MH086147).
Acknowledgments
We thank M. Tessier-Lavigne and C. Goodman for the Robo2-fc expression vector. Conflict of Interest: None declared.
References
- Altman J, Bayer SA. Development of the diencephalon in the rat. IV. Quantitative study of the time of origin of neurons and the internuclear chronological gradients in the thalamus. J Comp Neurol. 1979a;188:455–471. doi: 10.1002/cne.901880308. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the diencephalon in the rat. V. Thymidine-radiographic observations on internuclear and intranuclear gradients in the thalamus. J Comp Neurol. 1979b;188:473–499. doi: 10.1002/cne.901880309. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the diencephalon in the rat. VI. Re-evaluation of the embryonic development of the thalamus on the basis of thymidine–radiographic datings. J Comp Neurol. 1979c;188:501–524. doi: 10.1002/cne.901880310. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the rat thalamus: IV. The intermediate lobule of the thalamic neuroepithelium, and the time and site of origin and settling pattern of neurons of the ventral nuclear complex. J Comp Neurol. 1989a;284:534–566. doi: 10.1002/cne.902840405. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the rat thalamus: V. The posterior lobule of the thalamic neuroepithelium and the time and site of origin and settling pattern of neurons of the medial geniculate body. J Comp Neurol. 1989b;284:567–580. doi: 10.1002/cne.902840406. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the rat thalamus: VI. The posterior lobule of the thalamic neuroepithelium and the time and site of origin and settling pattern of neurons of the lateral geniculate and lateral posterior nuclei. J Comp Neurol. 1989c;284:581–601. doi: 10.1002/cne.902840407. [DOI] [PubMed] [Google Scholar]
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, Murakami F, Parnavelas JG, Sundaresan V, Richards LJ. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Bagri A, Marín O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Braisted JE, Catalano SM, Stimac R, Kennedy TE, Tessier-Lavigne M, Shatz CJ, O'Leary DDM. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J Neurosci. 2000;20:5792–5801. doi: 10.1523/JNEUROSCI.20-15-05792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braisted JE, Tuttle R, O'Leary DDM. Thalamocortical axons are influenced by chemorepellent and chemoattractant activities localized to decision points along their path. Dev Biol. 1999;208:430–440. doi: 10.1006/dbio.1999.9216. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- De Carlos JA, O'Leary DDM. Growth and targeting of subplate axons and establishment of major cortical pathways. J Neurosci. 1992;12:1194–1211. doi: 10.1523/JNEUROSCI.12-04-01194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carlos JA, Schlaggar BL, O'Leary DDM. Development of acetylcholinesterase-positive thalamic and basal forebrain afferents to embryonic rat neocortex. Exp Brain Res. 1995;104:385–401. doi: 10.1007/BF00231974. [DOI] [PubMed] [Google Scholar]
- Erskine L, Williams SE, Brose K, Kidd T, Rachel RA, Goodman CS, Tessier-Lavigne M, Mason CA. Retinal ganglion cell axon guidance in the mouse optic chiasm: expression and function of robos and slits. J Neurosci. 2000;20:4975–4982. doi: 10.1523/JNEUROSCI.20-13-04975.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig MG, Hume RI. Carbocyanine dyes. Novel markers for labelling neurons. Trends Neurosci. 1989a;12:336–338. [PubMed] [Google Scholar]
- Honig MG, Hume RI. Dil and diO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 1989b;12:333–335. [PubMed] [Google Scholar]
- Itoh A, Miyabayashi T, Ohno M, Sakano S. Cloning and expressions of three mammalian homologues of Drosophila slit suggest possible roles for Slit in the formation and maintenance of the nervous system. Brain Res Mol Brain Res. 1998;62:175–186. doi: 10.1016/s0169-328x(98)00224-1. [DOI] [PubMed] [Google Scholar]
- Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, et al. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Flames N, Ma L, Fouquet C, Di Meglio T, Chedotal A, Tessier-Lavigne M, Marín O. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J Neurosci. 2007;27:3395–3407. doi: 10.1523/JNEUROSCI.4605-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metin C, Godement P. The ganglionic eminence may be an intermediate target for corticofugal and thalamocortical axons. J Neurosci. 1996;16:3219–3235. doi: 10.1523/JNEUROSCI.16-10-03219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Adams R, Blakemore C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci. 1998;18:5723–5745. doi: 10.1523/JNEUROSCI.18-15-05723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Nakajima D, Nagase T, Nomura N, Seki N, Ohara O. Identification of high-molecular-weight proteins with multiple EGF-like motifs by motif-trap screening. Genomics. 1998;51:27–34. doi: 10.1006/geno.1998.5341. [DOI] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Ma L, Wang KH, Marillat V, Sotelo C, Tessier-Lavigne M, Chedotal A. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J Neurosci. 2001;21:4281–4289. doi: 10.1523/JNEUROSCI.21-12-04281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Marillat V, Kidd T, Goodman CS, Tessier-Lavigne M, Sotelo C, Chedotal A. Slit2-mediated chemorepulsion and collapse of developing forebrain axons. Neuron. 1999;22:463–473. doi: 10.1016/s0896-6273(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Niclou SP, Jia L, Raper JA. Slit2 is a repellent for retinal ganglion cell axons. J Neurosci. 2000;20:4962–4974. doi: 10.1523/JNEUROSCI.20-13-04962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstedt T, Braisted JE, Brose K, Kidd T, Goodman C, Tessier-Lavigne M, O'Leary DDM. Slit inhibition of retinal axon growth and its role in retinal axon pathfinding and innervation patterns in the diencephalon. J Neurosci. 2000;20:4983–4991. doi: 10.1523/JNEUROSCI.20-13-04983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, De Carlos JA, O'Leary DDM. Acetylcholinesterase as an early marker of the differentiation of dorsal thalamus in embryonic rats. Brain Res Dev Brain Res. 1993;75:19–30. doi: 10.1016/0165-3806(93)90061-e. [DOI] [PubMed] [Google Scholar]
- Tuttle R, Nakagawa Y, Johnson JE, O'Leary DDM. Defects in thalamocortical axon pathfinding correlate with altered cell domains in Mash-1-deficient mice. Development. 1999;126:1903–1916. doi: 10.1242/dev.126.9.1903. [DOI] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian Slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, Rao Y. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]