Abstract
Ventral telencephalic progenitors expressing the homeodomain transcription factor Nkx6-2 have been shown to give rise to a multitude of cortical interneuron subtypes usually associated with origin in either the medial ganglionic eminence or the caudal ganglionic eminence. The function of Nkx6-2 in directing the fate of those progenitors has, however, not been thoroughly analyzed. We used a combination of genetic inducible fate mapping and in vivo loss-of-function to analyze the requirement of Nkx6-2 in determining the fate of cortical interneurons. We have found that interneuron subtypes are born with a characteristic temporal pattern. Furthermore, we extend the characterization of interneurons from the Nkx6-2 lineage through the application of electrophysiological methods. Analysis of these populations in Nkx6-2 null mice suggests that there is a small and partially penetrant loss of delayed non-fast spiking somatostatin/calretinin double positive cortical interneurons in the absence of Nkx6-2 gene function.
Keywords: genetic fate mapping, interganglionic sulcus, loss of function, mouse genetics, whole-cell physiology
Introduction
Based on their immunohistochemical, morphological, electrophysiological, and connectivity properties (Gonchar and Burkhalter 1997; Kawaguchi and Kubota 1997; Somogyi et al. 1998; Markram et al. 2004; Flames et al. 2007; Wonders et al. 2008), cortical interneurons comprise a heterogeneous and diverse array of γ-aminobutyric acidergic (GABAergic) neuronal populations. These populations arise from 2 primordial ventral telencephalic structures, the medial and caudal ganglionic eminences (MGE and CGE, respectively). We and others have used transplantation, in vivo fate mapping, and loss-of-function analysis to study cells derived from these ventral eminences (Wichterle et al. 2001; Nery et al. 2002; Xu et al. 2004; Butt et al. 2005; Flames et al. 2007; Miyoshi et al. 2007). Although all cortical interneurons likely arise solely from the ventral regions, distinct populations of cortical interneuron subtypes are derived from the MGE and CGE (Xu et al. 2004; Butt et al. 2005). Multiple lines of evidence demonstrate that the MGE gives rise to the parvalbumin (PV)- and somatostatin (SST)-expressing populations of interneurons, whereas the vasoactive intestinal peptide (VIP) and the majority of calretinin (CR)-expressing lineages are predominantly generated in the CGE (Nery et al. 2003; Xu et al. 2004; Butt et al. 2005; Cobos et al. 2006). This strongly suggests the existence of a spatial mechanism for the specification of interneuron subsets that distinguishes between these embryonic structures. In agreement with such a mechanism, a number of studies have observed the spatially restricted expression of particular transcription factors within the MGE and CGE. These factors may impart distinct properties to progenitors located in these different domains (Butt et al. 2005; Flames et al. 2007; Fogarty et al. 2007; Kanatani et al. 2008). Recently, we have been able to provide a more refined understanding of the function of Nkx2-1, which appears to be a central regulator of MGE-derived interneurons, by showing its temporal requirement in this specification process (Butt et al. 2008). Both this analysis and a previous study from our laboratory (Miyoshi et al. 2007) demonstrate that cortical interneuron heterogeneity in addition to a reliance on spatial cues also depends on temporally regulated mechanisms.
The notion that changing progenitor competence underlies neuronal diversity has been observed in both invertebrate and vertebrate systems. Work in the Drosophila nerve cord (Cleary and Doe 2006), the vertebrate retina (Cepko et al. 1996), as well as the mammalian cerebral cortex (Desai and McConnell 2000), has provided evidence that neuronal progenitors can attain specific identities through progressive restrictions of their differentiation potential during development. In addition, it has long been established that neurons born sequentially at different developmental time points occupy distinct final positions in an inside-out manner in the layered structure of the mature mammalian cortex (Rakic 1974). Previous work has also found a similar correlation between the birthdate of cortical interneurons and their laminar position (Miller 1985; Fairen et al. 1986; Valcanis and Tan 2003; Xu et al. 2004; Yozu et al. 2004; Butt et al. 2005). Through in vivo genetic fate mapping studies, the time of origin has also been shown to play a role in determining the immunocytochemical and physiological properties of cortical interneurons (Miyoshi et al. 2007). Consistent with previous studies, those temporally dependent interneuron properties also correlate with their laminar position within the mature cortex (Miyoshi et al. 2007).
To further understand the spatial and temporal mechanisms of cortical interneuron determination, we have analyzed the homeodomain transcription factor Nkx6-2. Nkx genes and their homologs in other species are known to regulate neuronal cell fate. Both the ventral nervous system defective (vnd) gene in Drosophila and the Nkx proteins in the mouse spinal cord act to direct progenitors to adopt specific ventral neural identities, while repressing the fates of neighboring regions (Chu et al. 1998; McDonald et al. 1998; Weiss et al. 1998; Briscoe et al. 1999; Sander et al. 2000). Specifically, in the spinal cord, Nkx6-2 was shown to be required for specification of V1 interneurons and its absence causes progenitors to adopt a more dorsal V0 identity (Vallstedt et al. 2001).
In the ventral telencephalon, Nkx6-2 is expressed in a small subset of ventral neural progenitors in a restricted spatial pattern at the border between the MGE and CGE (Stenman et al. 2003). Despite its relatively small region of expression, the Nkx6-2 region has been shown to give rise to both PV and SST cortical interneurons and in particular be enriched in a subpopulation of SST cortical interneurons that coexpress CR (Fogarty et al. 2007). Our laboratory has recently demonstrated that the generation of these populations is at least partially dependent on the homologous protein Nkx2-1 (Butt et al. 2008).
The analysis of the function of Nkx6-2 provides, therefore, an excellent opportunity to further understand the contribution of spatial and temporal determination mechanisms in the telencephalon. In this study, we have used a combination of genetic inducible fate mapping (GIFM) (Zinyk et al. 1998) and in vivo loss-of-function to analyze the temporal requirement of Nkx6-2 for cortical interneuron specification.
Materials and Methods
Generation of Nkx6-2CreER Mutant Mice
Mouse Nkx6-2 genomic DNA was obtained from a 129/Ola mouse genomic library (Vallstedt et al. 2001) and by polymerase chain reaction (PCR) of genomic DNA from the Nkx6-2 locus. The targeting vector was designed to replace the first 2 exons of Nkx6-2 with an inducible form of the Cre recombinase, CreERT2 (Feil et al. 1997). The coding sequence for CreERT2 was inserted between a 5-kb 5′ HindIII–NcoI and a 3.3 kb 3′ SacI–AccI genomic fragments. Three targeted W4 (Auerbach et al. 2000) embryonic stem (ES) cell clones were identified by Southern blot analysis using BamHI and a 200 bp AccI fragment as 3′ external probe. ES cell chimeric founders were generated by injection of C57Bl/6 blastocysts (Skirball Transgenic facility). Chimeras were bred to C57BL/6 mice, and heterozygous offspring were maintained in a Swiss Webster genomic background. Homozygote Nkx6-2CreER/CreER mice were generated in normal Mendelian ratios and survived to adulthood as described (Cai et al. 2001).
Generation of RCE:loxP Reporter Mice
To generate a multipurpose sensitive reporter mouse with strong enhanced green fluorescent protein (EGFP) expression (Supplementary Fig. 1), we inserted a CAG sequence (Niwa et al. 1991) after promoter sequences for the Rosa locus (Soriano 1999; Farago et al. 2006). To generate pRCE:dual, we inserted an EGFP coding sequence into a modified pBS302 (Sauer 1993) cassette flanked by FLP recognition target sites and then inserted into the multiple cloning site of a pBigT vector (Srinivas et al. 2001). This dual stop–EGFP cassette was cloned downstream of CAG sequences, and the entire CAG-driven cassette was cloned into a pRosa26-PA-M1 targeting vector (Farago et al. 2006). W4 ES cells were electroporated as described above, and targeted clones were identified by Southern blot analysis using a 362-bp 5′ external probe amplified by PCR from genomic DNA using primers RP1 (5′-GGGACTAGGGCTGCGTGAG-3′) and RP2 (5′-.ATCCCCGCAAACGCACCAAGC-3′). ES cell chimeric founders were generated by injection of C57Bl/6 blastocysts. Chimeras were bred to C57BL/6 mice and the resulting line (RCE:dual) maintained in a Swiss Webster background. RCE:dual heterozygotes were bred to an FLPe-deleter line, flipper (Farley et al. 2000), resulting in the generation of the RCE:loxP reporter line that was maintained in a Swiss Webster background.
Animal Methods and Genotyping
All mice used in these studies were maintained according to protocols approved by the Institutional Animal Care and Use Committee of the New York University School of Medicine. Double homozygous male mice (Nkx6-2CreER/CreER; RCE:loxP) were intercrossed with Nkx6-2LacZ heterozygote females (Nkx6-2LacZ/+) (Vallstedt et al. 2001) to generate experimental control (Nkx6-2CreER/+; RCE:loxP) and mutant (Nkx6-2CreER/LacZ; RCE:loxP) mice. Pregnant females were administered 4 mg tamoxifen (Sigma, St Louis, MO) (20 mg/mL dissolved in corn oil [Sigma]) at 12:00–2:00 PM on either E10.5 or E12.5 by gavaging with silicon-protected needles. When pregnant mothers did not deliver pups by noon of E19.5, a cesarean section was performed and pups were fostered. PCR genotyping of the Nkx6-2WT, Nkx6-2LacZ, and RCE:loxP alleles was performed using the following sets of primers: N6.2wt-S (5′-GAGATGAAGACGTCGCTGTTCC-3′), N6.2wt-AS (5′-CTGCCAAATACTTGGTCTGCTCG-3′), N6.2wt-S2 (5′-GAGATGAAGACGTCGCTGTTCC-3′), bTAU (5′-CCTGGATAACATCACACACGTCC-3′), LacZ129 (5′-ACGACTGTCCTGGCCGTAACCGACCC-3′), RCE-Rosa1 (5′-CCCAAAGTCGCTCTGAGTTGTTATC-3′), RCE-Rosa2 (5′-GAAGGAGCGGGAGAAATGGATATG-3′), and RCE-Cag3 (5′-CCAGGCGGGCCATTTACCGTAAG-3′). The Nkx6-2CreER allele was genotyped by using primers specific to the CRE recombinase.
In Situ Hybridization
Embryos at all analyzed ages were dissected in cold phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde (PFA)/PBS solution overnight at 4 °C. Embryos were rinsed with PBS and cryoprotected using 30% sucrose/PBS solution overnight at 4 °C. Tissues were embedded in Tissue Tek, frozen on dry ice, and sectioned at 20 μm. Section in situ hybridizations were performed as previously described (Hanashima et al. 2002) using nonradioactive DIG-labeled probes. The cDNA probes used included Nkx6-2 and Cre.
Immunohistochemistry
E12.5 embryos were dissected in cold PBS and fixed in 4% PFA/PBS solution 40 min at 4 °C. Postnatal P21 brains (for molecular expression profile analysis) were fixed by transcardiac perfusion followed by 1 h postfixation with 4% PFA/PBS solution at 4 °C. Embryos and brain tissue were rinsed with PBS and cryoprotected using 30% sucrose/PBS solution overnight at 4 °C. Tissues were embedded in Tissue Tek, frozen on dry ice, and sectioned at 16 (for molecular expression profile analysis) and 30 μm (for cell imaging). Sections for immunohistochemistry analysis were processed using 3% normal goat serum/0.1% Triton X-100 in all procedures except washing steps where only PBS was used. Sections were blocked for 30 min followed by incubation with the primary antibodies overnight at 4 °C. After one wash with PBS, secondary antibodies (Molecular probes, Inc., Eugene, OR) were applied for 60 min at room temperature. Nuclear counterstaining was performed with 100 ng/mL 4′,6-diamidino-2-phenylindole (DAPI) solution in PBS for 15 min. The immunohistochemical profiles of interneurons were analyzed in the somatosensory and motor neocortices of 4 pairs of mutant (Nkx6-2CreER/LacZ) and control (Nkx6-2CreER/+) littermates from distinct litters. In each brain, cells were counted from 3 independent slides, and sampling was done from the entire anterior–posterior axis of the areas analyzed. The following antibodies were used in distinct combinations: rabbit anti-GFP (1:1000; Molecular Probes), rat anti-GFP (1:1000; Nacalai Tesque, Kyoto, Japan), mouse anti-PV (1:1000; Sigma), rat anti-SST (1:500; Chemicon, Temecula, CA), rabbit anti-Neuropeptide Y (1:500; Incstar, Stillwater, MN) mouse anti-CR (1:1000; Chemicon), rabbit anti-VIP (1:300; Incstar), mouse anti-Nkx2-1 (TTF-1) (1:200; Progen, Heidelberg, Germany), and rabbit anti-Nkx6-2 (1:1000; J. Ericson, Karolinska Institute, Stockholm, Sweden). Fluorescent images were captured using a cooled charge coupled device camera (Princeton Scientific Instruments, Trenton, NJ) or a Zeiss LSM 510 META laser-scanning microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY).
Acute In Vitro Cortical Slice Electrophysiology and Morphology
Whole-cell patch clamp electrophysiology recordings were performed on EGFP+ cells in acute in vitro slices from control (Nkx6-2CreER/+; RCE:loxP) animals (age: postnatal day (P)14-P18) similar to that previously published (Butt et al. 2005; Miyoshi et al. 2007) with the exception that biocytin (50 mg/ml; Sigma) was used in the pipette solution for filling the cells and the slices was incubated in Alexa Fluor 488 conjugated streptavidin (4 μg/ml; Invitrogen, Carlsbad, CA) overnight at 4 °C to visualize the biocytin. After the fluorescent imaging, the cells were developed using the ABC-peroxidase/DAB reaction (Vector Laboratories), and subsequent tracing was performed using a Neurolucida setup (MBF Biosciences, Williston, VT). The interneurons recorded were located in the primary somatosensory neocortex or more medial cortices.
Results
Expression of Nkx6-2 in the Ventral Ganglionic Eminences
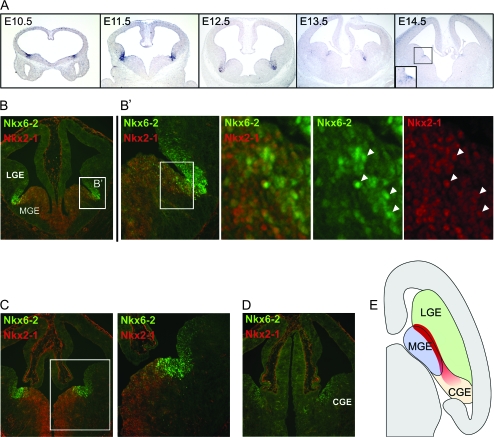
The persistent expression of Nkx6-2 throughout neurogenesis in the ventral telencephalon is suggestive of it playing a functional role during cortical interneuron development. As previously reported (Stenman et al. 2003), Nkx6-2 mRNA was detected in the ventricular zone (VZ) at the border region between the MGE and lateral ganglionic eminence (LGE) in the anterior ventral telencephalon—the interganglionic sulcus. Nkx6-2 can be detected in the primordium of this domain before E10.5 and expression continues until at least E14.5 (Fig. 1A). At E12.5 when expression of Nkx6-2 reaches its highest levels, the domain of Nkx6-2 expression has a small overlap in the most dorsal part of the MGE with the domain expressing Nkx2-1 (Fig. 1B,B′). This suggests that analogous to its function in the spinal cord (Vallstedt et al. 2001), Nkx6-2 may have a role in the specification of progenitors giving rise to cortical interneurons. Given that interneuron subtypes that arise from this region (Fogarty et al. 2007) are similar to the subtypes arising from the Nkx2-1-expressing portion of the MGE, the roles of Nkx6-2 and Nkx2-1 (Butt et al. 2008) may be similar and potentially redundant. Indeed, Nkx6-2 is coexpressed with Nkx2-1 in many of the progenitors from which cortical interneurons arise (Fogarty et al. 2007), and these 2 proteins are homologous in structure.
Figure 1.
Temporally and spatially regulated expression of Nkx6-2 in the ventral telencephalon. (A) In situ hybridization reveals expression of Nkx6-2 in the primordium of the interganglionic sulcus before E10.5. Expression of Nkx6-2 continues at strong levels in this region until E12.5 becoming restricted to a narrow domain during later stages of development. (B) Symmetrical expression of an Nkx6-2 gradient in the interganglionic sulcus at rostral levels of the telencephalon shows a partial overlap with Nkx2-1 in the MGE. Discrete progenitors of the overlap region in the dorsal MGE (B′) coexpress Nkx6-2 and Nkx2-1 (white arrowheads). (C) At intermediate levels of the telencephalon, the Nkx6-2 gradient becomes assymetrical with stronger levels of the protein detected in the ventral side of the expression domain. In addition, this domain is shifted ventrally from the anatomical sulcus accompanying the same shift in the expression of Nkx2-1 in the MGE. (D) The gradient of Nkx6-2 expression persists at caudal levels in the ventral side of the CGE although at weaker levels. CGE MGE, medial ganglionic eminence; LGE, lateral ganglionic eminence; and CGE, caudal ganglionic eminence. (E) Schematic view of a horizontal section of an E12.5 embryo depicting the domain of Nkx6-2 expression (red).
The strongest expression of Nkx6-2 occurs at anterior levels and coincides anatomically with the interganglionic sulcus. In this region, the most ventral Nkx6-2-expressing progenitors also express Nkx2-1 (Fig. 1B,B′). Nkx6-2 expression diminishes at more posterior levels. Although in this region the expression domains of Nkx2-1 and Nkx6-2 continue to partially overlap (Fig. 1C), at more posterior levels, the expression of both proteins shifts ventrally toward the MGE. Moreover, Nkx6-2 expression extends more posteriorly than Nkx2-1. As a result, Nkx6-2 is also observed in the most ventral portion of the CGE (Fig. 1D).
Genetic Fate Mapping of Nkx6-2-Expressing Neurons
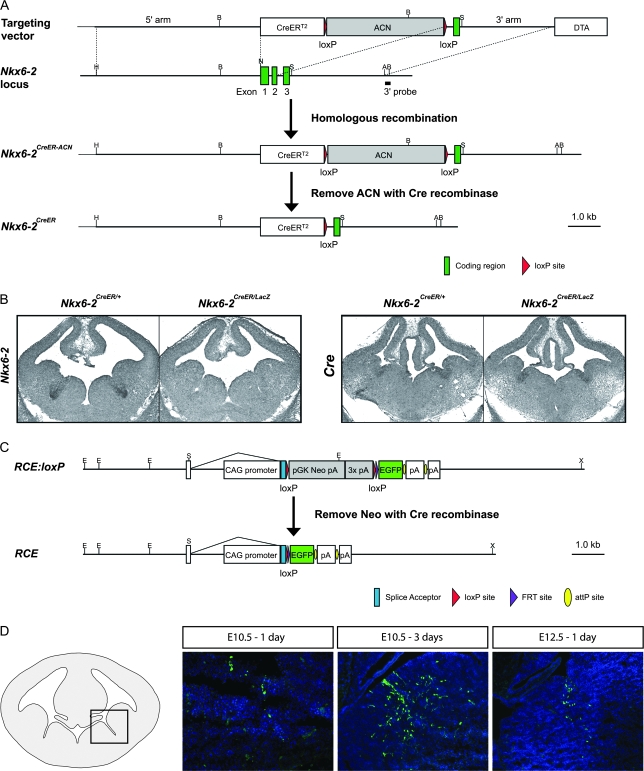
To determine the neurons arising from the Nkx6-2 expression domain at different developmental time points, we utilized GIFM (Zinyk et al. 1998). This was achieved by placing a tamoxifen-inducible form of the Cre recombinase (Feil et al. 1997) under the control of the endogenous Nkx6-2 locus (Nkx6-2CreER). In addition, in order to produce a loss-of-function allele, we deleted portions of the Nkx6-2 gene required for the production of a functional protein (Fig. 2A,B). By using this Cre driver line in the context of a novel Cre-responsive fluorescent reporter (RCE:loxP; Fig. 2C and Suppl. Fig. 1), we were able to genetically label Nkx6-2-derived precursors (Fig. 2D). By combining the Nkx6-2CreER driver line with an Nkx6-2 loss-of-function allele (Vallstedt et al. 2001), we were also able to label Nkx6-2-derived precursors in which the function of Nkx6-2 was removed.
Figure 2.
Nkx6-2 loss-of-function and temporally regulated genetic fate mapping in Nkx6-2 precursors. (A) Schematic representation of the “knock-in” gene targeting strategy for Nkx6-2. The first 2 exons of Nkx6-2 were replaced by a CreERT2 cassette and the self-excising positive selection ACN cassette (Bunting et al. 1999) by homologous recombination. Targeted ES cell clones were screened by Southern blot hybridization of BamHI digested DNA with a 3′ external probe. (B) In situ hybridization reveals absence of Nkx6-2 expression in the sulcus of E12.5 Nkx6-2CreER/LacZ embryos demonstrating a loss of Nkx6-2 function in the Nkx6-2CreER allele. Expression of Cre in the sulcus demonstrates correct expression of the site-specific recombinase under control of the Nkx6-2 locus regulatory sequences in both Nkx6-2CreER/+ control and Nkx6-2CreER/LacZ mutant mice. (C) Schematic representation of the recombination event that activates the new Rosa locus targeted EGFP reporter RCE:loxP (Supplementary Fig. 1). The ROSA26 locus boosted by insertion of a hybrid CAG promoter drives strong EGFP expression in cells where Cre activity has promoted excision by recombination of a loxP-flanked pGK-Neo-pA cassette. (D) Schematic view and fluorescent immunohistochemistry showing EGFP labeling of cells after tamoxifen-induced activation of CreER in Nkx6-2-expressing cells at different time points in development. With tamoxifen induction at E10.5, EGFP-labeled cells are seen in the sulcus after 1 day. A population of ventrally labeled and migratory cells are seen in this region after 3 days, indicating that in addition to precursors, a few radial glial progenitors are labeled. One day after activation at E12.5, a population of ventrally located and migratory labeled cells is seen in proximity to the sulcus.
This experimental strategy had a number of benefits. First, it allowed us to label precursors that activate the Nkx6-2 locus at different times during development (Fig. 2D). Second, as we used a single Nkx6-2CreER allele in all experiments, it permitted us to compare the fates of the same precursor population in control and mutant mice by avoiding any quantitative differences in the kinetics of Cre recombination. Third, the addition of a hybrid CAG promoter to the Rosa locus (Soriano 1999) in the RCE:loxP reporter resulted in strong EGFP labeling of the mature offspring of temporally distinct cohorts of Nkx6-2-derived precursors. In addition, the very efficient recombination of the RCE:loxP reporter allowed us to assess the effects of Nkx6-2 removal on the cellular phenotype of progenitors that express Nkx6-2 at specific developmental time points (Figs 1 and 2D).
Genetic Inducible Fate Mapping of the Nkx6-2 Lineage Labels Distinct Temporal Cohorts of Cortical Interneuron Precursors
To analyze the populations of cortical interneurons that arise from Nkx6-2-expressing precursors during development, we performed a mosaic fate-mapping analysis by transiently activating CreER through tamoxifen administration to pregnant mothers at 2 embryonic time points, E10.5 and E12.5. To identify the initial precursors that give rise to our fate-mapped population, we analyzed EGFP expression 24 and 72 h after tamoxifen administration and found that cells in the telencephalon arise exclusively from the spatially restricted interganglionic domain in both control and mutant mice. Notably, 72 h after tamoxifen administration was performed at E10.5, most EGFP-expressing neurons were located in the mantle of the subpallium with a number of cells appearing to be migrating toward the cortex (data not shown). The presence of limited but persistent labeling within the progenitor zone of both control and mutant embryos 3 days after tamoxifen induction indicates that in addition to nonproliferating precursors, Nkx6-2 appears to be expressed in some radial glial stem cells (Fig. 2D).
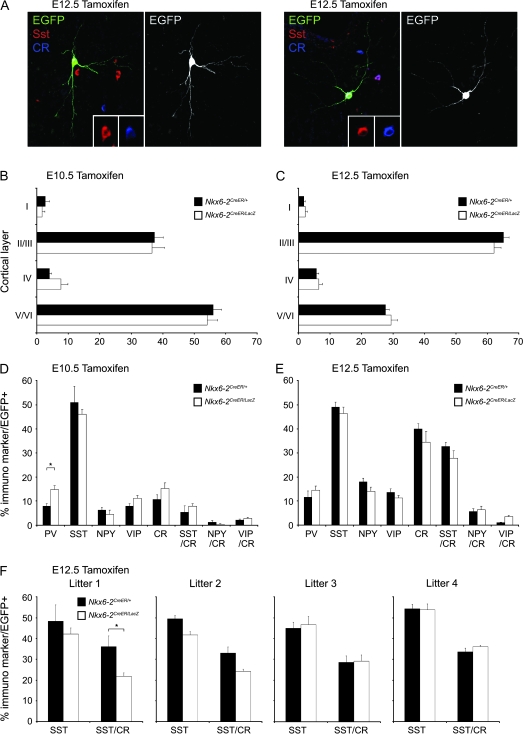
Previous analyses observed a correlation between birthdate and the final laminar position of cortical interneurons, with early-born interneurons occupying deeper layers and late-born interneurons occupying more superficial layers in the mature cortex. (Miller 1985; Fairen et al. 1986; Valcanis and Tan 2003; Xu et al. 2004; Yozu et al. 2004; Butt et al. 2005; Miyoshi et al. 2007). We therefore examined the laminar distribution of interneurons labeled at different embryonic time points in the somatosensory cortex of P21 mice (Fig. 3). This could be done in both wild-type and mutant mice as Nkx6-2 nulls live to adulthood (Cai et al. 2001; Southwood et al. 2004). We observed that both in control and Nkx6-2 mutant mice, cells labeled at the early developmental time point (E10.5) preferentially occupied deep cortical layers and interneurons born at the later time point populated more superficial layers (Fig. 3B,C). These observations are consistent with our previous genetic fate mapping of the ventral MGE (Miyoshi et al. 2007). Moreover, it suggests that in both control and Nkx6-2 null mice, GIFM results in labeling of distinct populations at the 2 time points examined. This indicates that Nkx6-2 is predominantly expressed in more mature precursors rather than progenitors that retain their proliferative potential.
Figure 3.
Temporal fate mapping of Nkx6-2 lineage cortical interneurons. (A) A majority of cells arising from Nkx6-2-expressing precursors after tamoxifen induction at E12.5 are SST+/CR+ and typically display multipolar morphologies. Insets show the cell body region of the EGFP-labeled interneurons. (B, C) Fate mapping from early to late time points gives rise to EGFP-labeled interneuron populations with a layer distribution that follows an “inside-out” pattern. Early-born populations tend to reside in the deeper layers, whereas cells born at later stages predominantly populate more superficial laminae. (D, E) An evaluation of the immunoprofiles of fate-mapped cortical interneurons from Nkx6-2-expressing precursors shows that the majority of cells born at E10.5 express SST but are CR negative. At E12.5, a substantial population of SST+/Cr+ double positive cortical interneurons are observed to arise from the Nkx6-2-expressing precursors. (F) The differentiation of the SST+/CR+ double-labeled population is partly dependent on Nkx6-2 as their numbers are reduced in a subset of Nkx6.2 null mutant mice. Histograms show a comparison in the number of total SST+ and double SST+/CR+ colabeled EGFP interneurons in 4 different Nkx6-2CreER/LacZ mutants from distinct litters and corresponding control littermates. Error bars in the histograms of (B–F) represent standard error of the mean. A single asterisk above the histogram indicates P < 0.05 as evaluated by a Student's t-test.
Diversity of Cortical Interneurons from Nkx6-2 Precursors Is Generated in a Temporally Dependent Manner
To assess the effect of Nkx6-2 removal on cortical interneuron diversity, we analyzed the genetically labeled EGFP populations after tamoxifen administration at E10.5 and E12.5. This was accomplished by utilizing immunohistochemistry with markers of interneuron subtypes in both control (Nkx6-2CreER/+) and Nkx6-2 null (Nkx2-1CreER/LacZ) animals. Consistent with their dorsal MGE origin (Flames et al. 2007; Fogarty et al. 2007), EGFP-expressing cells in Nkx6-2CreER/+ mice colabeled with a diversity of immunomarkers usually associated with interneurons born in the MGE (PV and SST). In addition, we also observed that a subpopulation of the labeled neurons expressed either CR or vasoactive intestinal peptide at both time points (Fig. 3D,E), suggesting that some of the labeled cells had characteristics similar to CGE-derived cortical interneurons (Xu et al. 2004; Butt et al. 2005). Regardless of their expression of these markers, the production of all populations that we could classify as specific subtypes were biased toward the later time point (E12.5). The most numerous group of interneurons generated at E10.5 and E12.5 colabeled with SST. However, distinct SST-expressing subpopulations were born at each time point. At E10.5, the SST population was primarily SST positive (SST+) but CR negative (CR−; Fig. 3D). This is consistent with our previous finding demonstrating that burst-spiking Martinotti neurons, which are SST+ and CR−, are predominantly an early-born population (Miyoshi et al. 2007). By contrast, at E12.5, SST+/CR+ double-labeled interneurons (Fogarty et al. 2007) predominated (Fig. 3E). Interestingly, this later group of interneurons in some Nkx6-2 null mice appears to be partially affected by the loss of Nkx6-2. In a subset of mutant mice, the number of EGFP fate-mapped cells that colabeled with SST and CR was decreased when compared with a wild-type littermate. However, the reduction in SST+/CR+ cells was not fully penetrant as it was only observed in a subset of the Nkx6-2CreER/LacZ mutants analyzed (Fig. 3F).
Analysis of the Intrinsic Physiological Properties of Cortical Interneurons from the Nkx6-2 Lineage
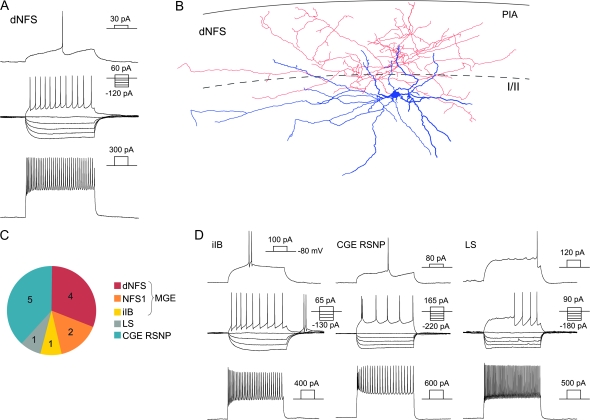
A percentage of EGFP-labeled populations in the E10.5 fate mapping did not express any of the immunohistochemical markers analyzed. In addition, these markers are usually also expressed in an overlapping fashion and in multiple interneuron subtypes (as identified by morphological or electrophysiological analysis [Miyoshi et al. 2007]). This reinforces the notion that immunohistochemical analysis alone provides a relatively incomplete description of the diversity encountered in the cerebral cortex. In order to characterize the specific subtypes produced from the Nkx6-2 lineage, we performed whole-cell current clamp recordings on 13 EGFP-positive cells from 5 different mice whose mothers had been administered tamoxifen at E12.5, an age where the entire interneuron diversity is better represented based on the immunohistochemical data (Fig. 4). The cells were randomly chosen, and the first healthy EGFP-positive neuron that was encountered after locating the somatosensory cortex was recorded. In all, 93% of cells recorded exhibited electrophysiological and morphological characteristics of one of the discrete interneuron subtypes that our laboratory has previously described (Butt et al. 2005; Miyoshi et al. 2007). In accordance with the immunohistochemical data, we identified cells from classes that we have previously found to be both CGE-and MGE-derived (6 and 7, respectively). Consistent with the MGE-derived populations being SST-expressing, 6 of the cells showed the typical physiological profile of the non-fast spiking (NFS) cells (Fig. 4A; see also Miyoshi et al. 2007). These correspond to populations that have been previously described as regular spiking nonpyramidal cells (RSNPs; Kawaguchi 1995; Butt et al. 2005) but can be further subdivided based on the presence of a delayed spike at threshold or the spike half- width (Miyoshi et al. 2007). Following the same classification protocol, we found 4 NFS cells with a delay to the threshold spike (dNFS) and 2 larger NFS cells with a short spike half width (0.9 ms), which identifies them as NFS1. Of the 4 dNFS cells recorded, we recovered 2 morphologies that were consistent with the dNFS2 subtype. These cells were located in superficial layer II and were multipolar with lateral dendrites extending along the I/II border and an axon projecting up into layer I (Fig. 4B). The morphologies of the other 2 dNFS cells could not be recovered, but their superficial location suggests that these might also be of the dNFS2 subtype. The seventh cell exhibited rebound bursting from −100 mV, as well as a burst at threshold when kept at −80 mV which is typical for the initial intrinsic bursting (iIB) subtype, indicating that it likely expressed SST and calbindin (CB) (Ma et al. 2006; Miyoshi et al. 2007).
Figure 4.
Nkx6.2+/− EGF+ cortical interneurons recorded in the somatosensory cortex. (A) An example of the firing properties of a dNFS cell. (B) Reconstruction of the cell referred to in (A). In blue is the soma and dendrites and in red is the axon. (C) Overall chart of the distribution of the cell types recorded in this study. (D) Examples of the firing patterns of different classes of interneurons recorded.
Five of the Nkx6-2-derived recorded cells have previously been described as CGE-derived RSNP subtypes. Two cells exhibited firing properties that resemble those previously described as VIP expressing bitufted or fusiform RSNPs (Cauli et al. 2000; Butt et al. 2005). These cells fire a burst of spikes followed by regular firing and show a pronounced spike height adaptation after the initial spike that recovers (Fig. 4). The fourth cell was similar to the subtype previously named “dense plexus” cell, and 2 cells were similar to ones derived from a dorsal CGE transplant exhibiting CR and VIP immunoreactivity (Rozov et al. 2001; Butt et al. 2005; Caputi et al. 2008). In addition to the subtypes known to be derived from either the MGE and the CGE, we also recorded one late spiking cell showing the typical firing characteristics of neurogliaform cells such as subthreshold oscillations and a steady state depolarization leading to the initial spike at lower depolarizing steps (Fig. 4D) with stereotypic morphology (data not shown).
Discussion
In this study, we combined GIFM with loss-of-function analysis to ascertain the temporal specification of GABAergic cortical interneurons derived from the Nkx6-2 lineage. The results from our immunohistochemical analysis confirm the array of interneuron subtypes previously seen to arise from the Nkx6-2 domain (Flames et al. 2007; Fogarty et al. 2007). In addition, we extended these previous analyses through temporal fate mapping and whole-cell physiology to provide a better understanding of the interneuron diversity derived from the Nkx6-2-expressing lineage.
Nkx6-2 Lineage Includes an Extensive Diversity of Cortical Interneurons
We find that cortical interneurons derived from the Nkx6-2 domain colabel with all the immunohistochemical markers analyzed suggesting that interneuron progenitors in this small region have an extensive differentiation potential. Interneurons that are thought to arise exclusively from both the MGE and the CGE were obtained from our fate-mapping analysis. This is consistent with the fact that the Nkx6-2 expression domain spans both parts of the dorsal MGE and ventral LGE/CGE (Fig. 1). These include PV- and SST-labeled interneurons from the MGE and VIP-expressing interneurons from the CGE (Butt et al. 2005). In agreement with the spatial expression, we observed that most types of interneurons born in this region appear in frequencies that are intermediate between what has been seen for the MGE and CGE (Miyoshi et al. 2007). Interestingly, the exception to this general trend is the subset of interneurons that labels for SST, which is the dominant class of interneuron derived from the Nkx6-2 domain. In fact, the observed frequency of SST-expressing interneurons from the Nkx6-2 lineage is comparable to that documented when the entire MGE is fate mapped (Miyoshi et al. 2007; Xu et al. 2008). This suggests a dorsal enrichment of progenitors that give rise to SST-expressing interneurons, whereas progenitors giving rise to other MGE subtypes (PV) are biased to more ventral regions (Flames et al. 2007; Wonders et al. 2008). Notably, a subset of SST-expressing interneurons appeared to be affected by the loss of Nkx6-2 gene function suggesting that Nkx6-2 may be a determination factor for this population.
Temporal Determination of Interneuron Subsets from the Nkx6-2 Lineage
In fate mapping the Nkx6-2 lineage at different time points, we observed that distinct interneuron subtypes appeared to be generated at the 2 developmental ages analyzed. In addition to the broad diversity of interneurons detected, a sizable percentage of those generated at E10.5 did not stain with any of the known interneuron markers that we examined.
Interneurons that express SST but not CR were preferentially born at early ages, whereas the majority of SST-expressing interneurons born at E12.5 colabeled with CR and seem to have multipolar morphologies. From our electrophysiological analysis, we identify the early-born SST+/CR− interneurons as iIB Martinotti cells and the later-born SST+/CR+ as dNFS2 interneurons. The properties of the iIB subtype are consistent with cells reported to be SST+ and CB+ but CR− (Ma et al. 2006; Xu et al. 2006), whereas the laterally biased morphologies and the firing patterns of the dNFS2 cells are consistent with those previously reported to be SST and CR double positive (Xu et al. 2006). Based on the immunohistochemical analysis, 14–28% of Nkx6-2-expressing precursors were SST+/CR+ double positive compared with the 3% that were found in an MGE-fate mapping using Olig2-CreER; Z/EG analysis (Miyoshi et al. 2007). Although we obtained good physiological and morphological confirmation that 2 of the neurons analyzed were of dNFS2 subtype, 2 others although not definitively classified also likely belonged to this category. This enrichment of dNFS cell types is consistent with the high levels of SST+/CR+ double expressing neurons observed both in the present analysis as well as in previous studies (Flames et al. 2007; Fogarty et al. 2007).
The present study extends our previous observations that birthdate is a strong predictor of interneuron subtype. However, the present findings are not consistent with Nkx6-2-expressing progenitors having progressive alterations in progenitor competence akin to that seen in Drosophila (Cleary and Doe 2006). If this were the case, populations of Nkx6-2 progenitors labeled after E12.5 tamoxifen administration would be a subpopulation of those fate mapped at the E10.5 time point. The fact that fate mapping at these time ages results in the labeling of distinct interneuron populations indicates that Nkx6-2 is preferentially expressed in precursors rather than proliferating progenitors. This is somewhat surprising as Nkx6-2 expression is within the VZ itself and yet appears to be largely absent from the radial glial stem cells (Noctor et al. 2004). In the future, it will be interesting to determine through lineage analysis whether radial glia in the subpallium sequentially give rise to distinct cortical interneuron subtypes. However, to accomplish, this will require identification of a gene expressed earlier than Nkx6-2 in the cortical interneuron lineage.
Nkx6-2 as a Transcription Factor in Interneuron Specification
Although we found that cortical interneurons from the Nkx6-2 lineage are heterogeneous, distinct populations arise from Nkx6-2-expressing progenitors at different time points. Although Nkx6-2 does not seem to be absolutely required for proper specification of most interneuron populations, dNFS cells which we propose are SST+/CR+ and born at the later time point analyzed (E12.5) were reduced in number in a subset of Nkx6-2 null mice. As such, this study suggests that Nkx6-2 may be required in the regulatory cascade for cortical interneurons generated from this population at E12.5. Nevertheless, this study does not exclude an additional function for Nkx6-2 at earlier time points. Nkx6-2 is expressed in the ventral telencephalon well before the appearance of dNFS cells, and a requirement for the related Nkx transcription factor Nkx2-1 has been found in the ventral telencephalon (Butt et al. 2008). In addition, our inability at present to definitely classify a substantial proportion of the interneuron subtypes born of Nkx6-2-expressing precursors at E10.5 using immunohistochemistry precludes us from knowing if the fate of these neurons is altered by the loss of this gene function.
Although dNFS populations are reduced in a number of mutant mice, the persistence of a substantial number of these cells in all mutants demonstrates that this interneuron subtype is not solely dependent on Nkx6-2 for its specification. This suggests that additional transcription factors are involved normally in the specification of this interneuron subtype or at very least can partially compensate for the loss of Nkx6-2. One possibility for such a factor is Nkx2-1, which was shown to be required for determination of specific SST-expressing interneurons at early time points (Anderson et al. 2001; Xu et al. 2004; Butt et al. 2008; Du et al. 2008). In agreement with that possibility, SST+ interneurons are known to arise from the MGE (Butt et al. 2005; Miyoshi et al. 2007) where the expression domains of Nkx2-1 and Nkx6-2 partially overlap and some neuronal progenitors express both factors.
In conclusion, using a combination of immunohistochemistry and electrophysiology analysis, we have genetically fate-mapped Nkx6-2 precursors at different embryonic time points. We have shown that dNFS cells, a subpopulation of SST+ interneurons that also express CR and have multipolar morphologies, are preferentially derived from E12.5 precursors within the Nkx6-2 expression domain. We demonstrate that this subgroup of later-born interneurons appears to be affected by the loss of Nkx6-2 gene function, suggesting that this gene may be functional during this phase of neurogenesis for specification of this interneuron subtype. Additional work will be required to clarify the earlier role of this gene and to determine the extent to which other transcription factors, particularly Nkx2-1, compensate for the loss of Nkx6-2.
Supplementary Material
Supplementary Figure 1 can be found at: http://www.cercor.oxfordjournals.org/.
Funding
Research in the Fishell laboratory is supported by National Institutes of Health—National Institute of Mental Health—National Institute of Neurological Disorders and Stroke (R01MH068469 and R01NS039007) and generous support from the Simons Foundation. Swedish Brain Foundation (Hjärnfonden) to J.H.L.
Supplementary Material
Acknowledgments
We wish to thank Dr J. Ericson for providing the Nkx6-2LacZ mouse and Nkx6-2 antibody. cDNA in situ probe templates were kindly provided by J. Ericson (Nkx6-2) and A. Joyner (Cre). We would also like to thank Lihong Yin and Jiali Deng for excellent technical help. Conflict of Interest: None declared.
References
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Auerbach W, Dunmore JH, Fairchild-Huntress V, Fang Q, Auerbach AB, Huszar D, Joyner AL. Establishment and chimera analysis of 129/SvEv- and C57BL/6-derived mouse embryonic stem cell lines. Biotechniques. 2000;29:1024–1028. doi: 10.2144/00295st04. 1030, 1032. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O'Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR. Targeting genes for self-excision in the germ line. Genes Dev. 1999;13:1524–1528. doi: 10.1101/gad.13.12.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Qi Y, Wu R, Modderman G, Fu H, Liu R, Qiu M. Mice lacking the Nkx6.2 (Gtx) homeodomain transcription factor develop and reproduce normally. Mol Cell Biol. 2001;21:4399–4403. doi: 10.1128/MCB.21.13.4399-4403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputi A, Rozov A, Blatow M, Monyer H. Two calretinin-positive GABAergic cell types in layer 2/3 of the mouse neocortex provide different forms of inhibition. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn175. Advance Access published October 8, doi:10.1093/cercor/bhn175. [DOI] [PubMed] [Google Scholar]
- Cauli B, Porter JT, Tsuzuki K, Lambolez B, Rossier J, Quenet B, Audinat E. Classification of fusiform neocortical interneurons based on unsupervised clustering. Proc Natl Acad Sci USA. 2000;97:6144–6149. doi: 10.1073/pnas.97.11.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Parras C, White K, Jimenez F. Formation and specification of ventral neuroblasts is controlled by vnd in Drosophila neurogenesis. Genes Dev. 1998;12:3613–3624. doi: 10.1101/gad.12.22.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary MD, Doe CQ. Regulation of neuroblast competence: multiple temporal identity factors specify distinct neuronal fates within a single early competence window. Genes Dev. 2006;20:429–434. doi: 10.1101/gad.1382206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Long JE, Thwin MT, Rubenstein JL. Cellular patterns of transcription factor expression in developing cortical interneurons. Cereb Cortex. 2006;16(Suppl 1):i82–i88. doi: 10.1093/cercor/bhk003. [DOI] [PubMed] [Google Scholar]
- Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- Du T, Xu Q, Ocbina PJ, Anderson SA. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–1567. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- Fairen A, Cobas A, Fonseca M. Times of generation of glutamic acid decarboxylase immunoreactive neurons in mouse somatosensory cortex. J Comp Neurol. 1986;251:67–83. doi: 10.1002/cne.902510105. [DOI] [PubMed] [Google Scholar]
- Farago AF, Awatramani RB, Dymecki SM. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 2006;50:205–218. doi: 10.1016/j.neuron.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex. 1997;7:347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- Hanashima C, Shen L, Li SC, Lai E. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J Neurosci. 2002;22:6526–6536. doi: 10.1523/JNEUROSCI.22-15-06526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatani S, Yozu M, Tabata H, Nakajima K. COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. J Neurosci. 2008;28:13582–13591. doi: 10.1523/JNEUROSCI.2132-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci. 1995;15:2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Holbrook S, Isshiki T, Weiss J, Doe CQ, Mellerick DM. Dorsoventral patterning in the Drosophila central nervous system: the vnd homeobox gene specifies ventral column identity. Genes Dev. 1998;12:3603–3612. doi: 10.1101/gad.12.22.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW. Cogeneration of retrogradely labeled corticocortical projection and GABA-immunoreactive local circuit neurons in cerebral cortex. Brain Res. 1985;355:187–192. doi: 10.1016/0165-3806(85)90040-9. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Corbin JG, Fishell G. Dlx2 progenitor migration in wild type and Nkx2.1 mutant telencephalon. Cereb Cortex. 2003;13:895–903. doi: 10.1093/cercor/13.9.895. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rozov A, Jerecic J, Sakmann B, Burnashev N. AMPA receptor channels with long-lasting desensitization in bipolar interneurons contribute to synaptic depression in a novel feedback circuit in layer 2/3 of rat neocortex. J Neurosci. 2001;21:8062–8071. doi: 10.1523/JNEUROSCI.21-20-08062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Paydar S, Ericson J, Briscoe J, Berber E, German M, Jessell TM, Rubenstein JL. Ventral neural patterning by Nkx homeobox genes: Nkx6.1 controls somatic motor neuron and ventral interneuron fates. Genes Dev. 2000;14:2134–2139. doi: 10.1101/gad.820400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Manipulation of transgenes by site-specific recombination: use of Cre recombinase. Methods Enzymol. 1993;225:890–900. doi: 10.1016/0076-6879(93)25056-8. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Southwood C, He C, Garbern J, Kamholz J, Arroyo E, Gow A. CNS myelin paranodes require Nkx6-2 homeoprotein transcriptional activity for normal structure. J Neurosci. 2004;24:11215–11225. doi: 10.1523/JNEUROSCI.3479-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Wang B, Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci. 2003;23:10568–10576. doi: 10.1523/JNEUROSCI.23-33-10568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. J Neurosci. 2003;23:5113–5122. doi: 10.1523/JNEUROSCI.23-12-05113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallstedt A, Muhr J, Pattyn A, Pierani A, Mendelsohn M, Sander M, Jessell TM, Ericson J. Different levels of repressor activity assign redundant and specific roles to Nkx6 genes in motor neuron and interneuron specification. Neuron. 2001;31:743–755. doi: 10.1016/s0896-6273(01)00412-3. [DOI] [PubMed] [Google Scholar]
- Weiss JB, Von Ohlen T, Mellerick DM, Dressler G, Doe CQ, Scott MP. Dorsoventral patterning in the Drosophila central nervous system: the intermediate neuroblasts defective homeobox gene specifies intermediate column identity. Genes Dev. 1998;12:3591–3602. doi: 10.1101/gad.12.22.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol. 2006;499:144–160. doi: 10.1002/cne.21101. [DOI] [PubMed] [Google Scholar]
- Yozu M, Tabata H, Nakajima K. Birth-date dependent alignment of GABAergic neurons occurs in a different pattern from that of non-GABAergic neurons in the developing mouse visual cortex. Neurosci Res. 2004;49:395–403. doi: 10.1016/j.neures.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Zinyk DL, Mercer EH, Harris E, Anderson DJ, Joyner AL. Fate mapping of the mouse midbrain-hindbrain constriction using a site-specific recombination system. Curr Biol. 1998;8:665–668. doi: 10.1016/s0960-9822(98)70255-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.