Abstract
A number of studies in recent years have shown that members of the Roundabout (Robo) receptor family, Robo1 and Robo2, play significant roles in the formation of axonal tracks in the developing forebrain and in the migration and morphological differentiation of cortical interneurons. Here, we investigated the expression and function of Robo3 in the developing cortex. We found that this receptor is strongly expressed in the preplate layer and cortical hem of the early cortex where it colocalizes with markers of Cajal–Retzius cells and interneurons. Analysis of Robo3 mutant mice at early (embryonic day [E] 13.5) and late (E18.5) stages of corticogenesis revealed no significant change in the number of interneurons, but a change in their morphology at E13.5. However, preliminary analysis on a small number of mice that lacked all 3 Robo receptors indicated a marked reduction in the number of cortical interneurons, but only a limited effect on their morphology. These observations and the results of other recent studies suggest a complex interplay between the 3 Robo receptors in regulating the number, migration and morphological differentiation of cortical interneurons.
Keywords: interneuron, morphology, Robo
Introduction and overview
The molecular mechanisms that guide the migration of interneurons from the subpallium into the cortex are slowly being elucidated, and a number of molecules have been shown to play important roles (Marín and Rubenstein 2003; Métin et al. 2006; Nakajima 2007). These include Slit proteins and their receptors of the Robo family (Andrews et al. 2006, 2008). Four Robo family members have been identified so far in vertebrates, and all have been shown to bind to Slit: Robo1, Robo2, Robo3 (also known as Rig1), and Robo4 (Kidd et al. 1998; Yuan, Cox, et al. 1999; Yuan, Zhou, et al. 1999; Park et al. 2003; Liu et al. 2004; Camurri et al. 2005; Mambetisaeva et al. 2005). The first 3 receptors show strongest expression in the developing central nervous system, whereas Robo4 is specifically found in endothelial cells (Huminiecki et al. 2002; Park et al. 2003).
The expression of slit/robo genes in the developing nervous system has been investigated predominantly by in situ hybridization, and these studies have shown them to be dynamically expressed in complementary patterns during cortical development (Yuan, Cox, et al. 1999; Yuan, Zhou, et al. 1999; Bagri et al. 2002; Marillat et al. 2002; Whitford et al. 2002; Camurri et al. 2004). The dynamic expression of Robo proteins was subsequently confirmed in the developing mouse forebrain using pan-Robo (Robo1 and Robo2) antisera (Sundaresan et al. 2004) and, more recently, Robo1- and Robo2-specific antibodies (Andrews et al. 2006, 2007, 2008; López-Bendito et al. 2007; Plachez et al. 2008). Using these antibodies, investigators have demonstrated the expression of Robo1 and Robo2 in major axonal tracts in the forebrain (López-Bendito et al. 2007; Plachez et al. 2008) and in cortical interneurons (Andrews et al. 2006, 2008), prompting speculation that Robo receptors are involved in the formation of axonal pathways in the cortex and in the migration and differentiation of interneurons.
The Role of Slit-Robo Proteins in the Formation of Forebrain Axonal Tracts
Previous in vitro experiments have demonstrated that thalamocortical, corticofugal, and callosal axons are repelled by Slits (Shu and Richards 2001; Whitford et al. 2002), raising the possibility that these molecules play important roles in the development of these axons. This was subsequently confirmed in in vivo experiments using both gene mutations and antisense knockdown of the protein, where an absence or reduction in the level of Slit2 or both Slit1/Slit2 resulted in the malformation of the corpus callosum, and in aberrant corticothalamic and thalamocortical targeting (Bagri et al. 2002; Shu et al. 2003). More specifically, in Slit mutants, corticothalamic axons appear to form large fasciculated bundles that aberrantly cross the midline at the level of the hippocampal and anterior commissures, and more caudally at the medial preoptic area (POA). These results suggest that Slit proteins are involved in maintaining the positioning of axonal tracts, in preventing extension of axons toward and across the midline, and in channeling axons into particular cortical regions.
Subsequent analysis of Robo1 mutants revealed similar defects including dysgenesis of the corpus callosum and hippocampal commissure, and abnormalities in corticothalamic and thalamocortical targeting. In particular, in Robo1 knockouts, thalamocortical and corticothalamic axons reach their targets earlier than in control mice and callosal axons form tight bundles, contrasting with observations in Slit mutants where axons appear to defasciculate (Bagri et al. 2002; Andrews et al. 2006). These observations have recently been confirmed using independently generated Robo1, Robo2, and double Robo1/Robo2 mutants; these animals displayed prominent guidance errors in the development of corticofugal, thalamocortical, and callosal connections (López-Bendito et al. 2007). The defects observed in double Robo1/Robo2 mutants strongly resembled those reported for Slit1/Slit2 double mutants, suggesting Robo1 and Robo2 mediate the function of Slit1 and Slit2 in the formation of these connections within the developing cortex.
The Role of Slit-Robo Proteins in Interneuron Migration
Earlier studies have shown that the migration of γ-amino butyric acid (GABA)-containing interneurons from the ganglionic eminences (GEs) is mediated by the repulsive activity of Slit1 present in the ventricular zone (VZ) of the subpallium (Hu 1999; Wu et al. 1999; Zhu et al. 1999; Sang et al. 2002). However, it was subsequently reported that migration of cortical interneurons is normal in Slit1/Slit2 double mutants, prompting speculation that Slits do not play a major role in this process (Marín and Rubenstein 2003; Marín et al. 2003). These findings were surprising, given the strong effects of Slit in vitro and the subsequent observation that cortical interneurons express Robo receptors (Andrews et al. 2006, 2008). More recent analysis of Robo1 and Robo2 single mutants showed no change in the positions of the streams of migrating interneurons, similar to that reported for Slit mutants (Marín et al. 2003). However, an increased number of cells that persisted to adulthood were observed in the cortices of both groups of animals, although this was only significant for the Robo1 mutant mice (Andrews et al. 2006, 2008).
The Role of Slit-Robo Proteins on Interneuron Morphology
In addition to regulating axon guidance and cell migration, Slit-Robo signaling plays a role in process outgrowth and branching. Specifically, Slit has been reported to promote axonal elongation and branching in sensory neurons (Wang et al. 1999; Ozdinler and Erzurumlu 2002; Ma and Tessier-Lavigne 2007) and dendritic growth and branching in cortical cells (Whitford et al. 2002). Furthermore, Slit has been found to promote branching and elongation of neurites of GABA-containing interneurons in embryonic forebrain cultures (Sang et al. 2002), and more recently in fetal dopaminergic neuron cocultures (Lin and Isacson 2006). Recent analysis of the morphology of migrating interneurons in Slit and Robo mutants revealed that loss of Robo1 or both Slit1/Slit2 had a marked effect on neurite process length, number and branching. This suggested that absence of Robo1 or both Slit1/2 molecules, but not Robo2 and Slit1, has a pronounced effect on their morphology (Andrews et al. 2008).
Studies on the role of Robo receptors in the development of the mammalian forebrain have so far focused on the Robo1 and Robo2 subtypes. Previous expression studies have shown that Robo3 is expressed in the developing hindbrain and spinal cord and controls the development of the commissural tracts at this level (Yuan, Cox, et al. 1999; Camurri et al. 2004; Marillat et al. 2004; Sabatier et al. 2004). However, Robo3 expression and function in the developing forebrain have not been investigated. Here, we used immunohistochemistry to document the expression pattern of Robo3 in the developing forebrain. We found that this receptor protein is strongly expressed within the preplate layer (PPL) and cortical hem of the early cortex where it colocalizes to some extent with markers for Cajal–Retzius cells and cortical interneurons. This observation suggested that Robo3 might play a role in the formation of the cortex and in the migration of interneurons. Analysis of Robo3 mutant mice revealed no significant change in the number of interneurons, but an alteration in their morphology in the early developing cortex. Preliminary analysis of mice that lacked all 3 neuronal Robo receptors (Robo1/2/3 triple mutants) suggested a marked reduction in the number of interneurons in the developing cortex, but only a limited effect on their morphology. Based on these observations and the results of other recent studies, we conclude that Robo receptors differentially affect the migration and morphological differentiation of interneurons in the developing cortex.
Results
Robo3 Expression in the Developing Forebrain
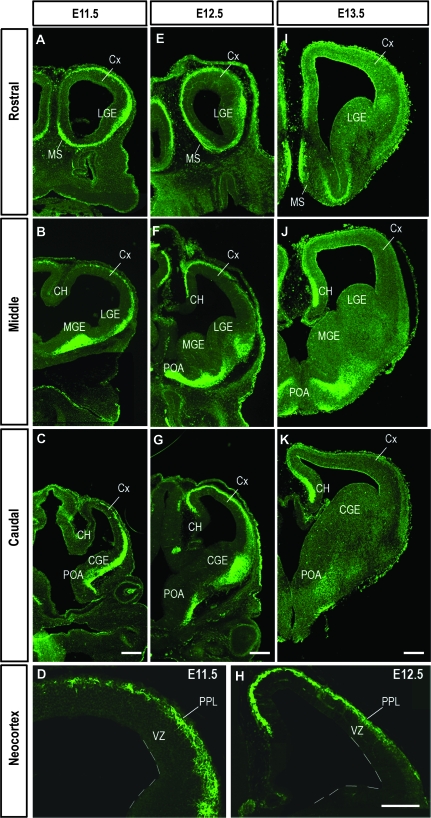
We used immunohistochemistry to investigate in detail the expression of the Robo3 receptor protein within the mouse forebrain during early (embryonic day 11.5 [E11.5] to E13.5) and later (E15.5, E18.5) stages of corticogenesis. We found that this receptor was expressed throughout the rostral–caudal extent of the early forebrain (Fig. 1). In the subpallium, the expression was distinctly localized within the medial septum (MS) and POA as well as throughout the differentiating fields of the lateral (LGE), medial (MGE), and caudal (CGE) ganglionic eminences at E11.5 (Fig. 1A–C). Distinctive stream-like patterns of Robo3 protein also extended dorsally from the MGE and LGE toward the corticostriatal boundary in rostral and middle levels of the forebrain (Fig. 1A,B), and from the CGE in more caudal regions (Fig. 1C).
Figure 1.
Expression of Robo3 protein at rostral (A, E, I), middle (B, F, J), and caudal (C, G, K) levels of the embryonic mouse forebrain during preplate stages of development (E11.5–13.5). (D, H) Higher magnification images taken through middle levels of the cortex at E11.5 (D) and E12.5 (H). Scale bar in (C), (G), and (K) is 200 μm and applies to panels of equivalent ages; scale bar in H is 200 μm and also applies to (D) (Cx, cortex; CH cortical hem).
In the developing pallium, Robo3 protein was expressed robustly throughout the rostral–caudal extent at E11.5 (Fig. 1A–C). Expression was localized predominantly in the PPL, and was contiguous with labeling that emanated from the GE. This stream of migrating cells extended dorsomedially from the level of the corticostriatal boundary and, in rostral and middle regions, reached the prospective cingulate cortex and cortical hem. Although Robo3 continued to be strongly localized throughout the developing PPL at E12.5, it now showed a reverse gradient of expression, with the protein most strongly expressed within the cortical hem and diminishing in a mediolateral gradient (Fig. 1E–G). This second band of Robo3 immunopositive cells extended in a direction opposite to the stream seen to arise from the ventral forebrain at E11.5 (Fig. 1D,H). Also at these early stages (E11.5–13.5), Robo3 staining was detected in the cortical VZ (data not shown).
Consistent with previous observations (Camurri et al. 2004), Robo3 expression was mostly down regulated within the embryonic forebrain by E15.5. However, some staining was maintained within the MS, the hippocampus, and the piriform and lateral cortices as late as E18.5 (data not shown). Our present findings and results of previous in situ hybridization studies (Camurri et al. 2004) contrast with the complete lack of Robo3 expression reported in Western blot analysis of mouse embryonic forebrain by Yuan, Cox, et al. (1999). This discrepancy may be attributed to differences in sensitivity between the assays used.
Expression of all Robo Receptors during Forebrain Development
We utilized immunohistochemistry to compare the expression profiles of all 3 Robo proteins in the developing forebrain at E11.5–13.5. At E11.5, they were all detected predominantly within the PPL throughout the rostral–caudal extent of the cortex (Supplementary Fig. 1A–C). In the subpallium, they showed distinct, but overlapping, patterns of expression throughout the LGE and CGE. Although Robo1 and Robo3 receptors were broadly expressed in overlapping patterns within the MGE, Robo2 expression was restricted to a narrow stream of cells in the dorsal-most region of the structure at this early stage (Supplementary Fig. 1B).
A day later (E12.5), Robo1 and Robo3 were clearly localized within the PPL in a decreasing mediolateral gradient; Robo2 was only weakly expressed in this layer. All 3 Robo proteins were localized in the GE at this stage (Supplementary Fig. 1D–F). Specifically, Robo1 and Robo3 showed similar patterns of expression both within the MGE and LGE, and overlapped in regions where Robo1 protein was most robustly localized. Interestingly, Robo2 expression appeared to be complementary to that of Robo3 within the subpallium, while overlapping with Robo1 in the LGE. All 3 Robo receptors were strongly localized within the CGE at this stage (data not shown).
All 3 receptors were also observed within the developing forebrain at E13.5 (Supplementary Fig. 1G–I). In the pallium, Robo1 and Robo3 proteins were strongly expressed within the cortical hem, and this extended in a decreasing mediolateral gradient throughout the PPL/MZ (marginal zone). Robo2 showed a more restricted expression in medial regions of the cortical hem, and diffuse staining within the PPL/MZ. Taken together, these immunohistochemical experiments showed that all 3 Robo receptors are expressed in the early forebrain (E11.5–13.5), with Robo1 and Robo3 overlapping to a high degree, both within the subpallium and pallium, suggesting that Robo3 may play similar roles to the other 2 receptors, and may be expressed by the same cell types.
Robo Receptors and Cajal–Retzius Cells
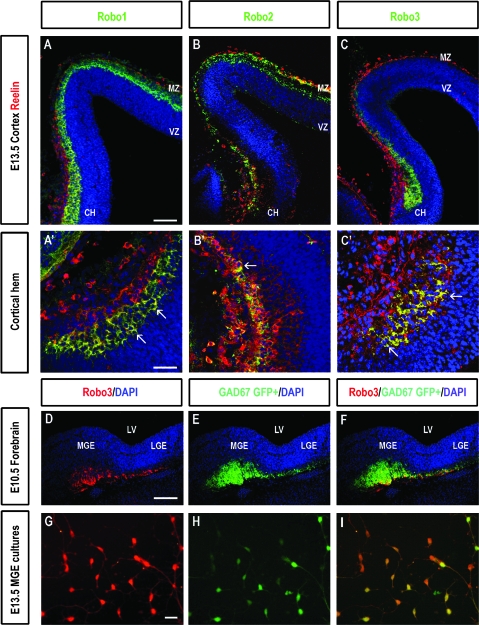
The robust expression of all 3 Robo receptors throughout the PPL and MZ at E11.5–13.5 was of interest, as this corresponds to the time when Cajal–Retzius cells are generated (E10.5–12.5) (Meyer at al. 1998; Garcia-Moreno et al. 2007). Robo1 and Robo3 proteins were also strongly expressed within the cortical hem (Supplementary Fig. 1G,I), a major source of these neurons (Takiguchi-Hayashi et al. 2004; Yoshida et al. 2006; Garcia-Moreno et al. 2007; Siegenthaler and Miller 2008). Double labeling experiments showed that most Robo-positive cells were located within the lower half of the PPL/MZ and below a single layer of strongly labeled reelin immunopositive cells, a marker of Cajal–Retzius cells (Ogawa et al. 1995; Frotscher 1997; Tissir and Goffinet 2003) (Fig. 2A, inset). Colocalization with reelin was also more prominent within the deeper layers of the cortical hem (Figs. 2A–C, 2A′–C′). The receptors were also noted in subpallial regions reported to give rise to a number of these distinctive cells: the MGE (Lavdas et al. 1999), the pallial–subpallial boundary, the POA and MS (Bielle et al. 2005). The distinct expression of Robos within the MZ and in the germinal regions of Cajal–Retzius cells suggests that these receptor proteins play a role in their development and migration.
Figure 2.
(A–C) Localisation of Robo- and reelin proteins in coronal sections through the developing mouse cortex at E13.5. Robo1 (A), Robo2 (B), and Robo3 (C) proteins (shown in green) colocalize with reelin (red) in some cells in the CH and MZ of the neocortex (yellow). Reelin labeling is predominantly present in the more superficial aspect of the MZ, whereas Robo expression is prevalent in the lower part of this layer, where some colocalization of the 2 proteins is evident (yellow). (A′–C′) Higher magnification images of the CH, in (A–C), respectively, illustrate colocalization in a number of cells (yellow) some of which are pointed with arrows. (D–F) Robo3 (red) colocalizes with GAD67 GFP+ (green) in the MGE and in a stream of cells extending toward the corticostriatal boundary in coronal sections through the forebrain at E10.5. (G–I) A number of dissociated GAD67 GFP+ MGE neurons (green) express Robo3 (red) (shown in yellow) (I). Scale bar in A is 100 μm and corresponds to A–C; in D is 100 μm and corresponds to D–F; in G is 30 μm and corresponds to G–I; scale bar in A′ is 75 μm and corresponds to A′–C′ (CH, cortical hem; LV, lateral ventricle).
We investigated the putative role of Robo receptors in the development of Cajal–Retzius cells by using transgenic mice that lacked functional Robo1 (Robo1−/−), Robo2 (Robo2−/−), or Robo3 (Robo3−/−) receptors. This was investigated during early (PPL; E12.5, E13.5) and later stages of development (E17.5, E18.5). Examination of Robo3 deficient cortices revealed no significant changes in the total number of reelin-positive cells within the PPL of the developing neocortex and hippocampus compared with heterozygote littermates (n = 3, Robo3+/−; n = 3, Robo3−/−) at E13.5. This was the case within both the rostral-middle (cortex, Robo3+/− 59.9 ± 3.2; Robo3−/− 63 ± 3.2; hippocampus, Robo3+/− 41.1 ± 2.07; Robo3−/− 46.5 ± 2.8) and caudal regions (cortex, Robo3+/− 61.3 ± 3.4; Robo3−/− 64.1 ± 2.8; hippocampus, Robo3+/− 37.8 ± 3.52; Robo3−/− 37.5 ± 3.54). Similar analysis at later stages of corticogenesis also revealed no significant changes in the total number of reelin-positive cells within the MZ of Robo3 mutants compared with wild-type littermates (E18.5; n = 3, Robo3+/+; n = 3, Robo3−/−). This applied to both rostral-middle (Robo3+/+ 62.1 ± 3.5; Robo3−/− 62.7 ± 2.2) and caudal levels (Robo3+/+ 123.3 ± 4.1; Robo3−/− 126.2 ± 5.4) of the neocortex, cingulate cortex (Robo3+/+ 43.6 ± 2.2; Robo3−/− 42.3 ± 1.5), hippocampus (Robo3+/+ 51.3 ± 3.8; Robo3−/− 53.7 ± 2.7), and cortical hem (Robo3+/+ 78.1 ± 4.9; Robo3−/− 76.9 ± 5.3) regions. Thus, removal of the Robo3 receptor appeared to have no significant effect on the overall number of Cajal–Retzius cells within the developing cortex.
The total number of Cajal–Retzius cells was also investigated within the cortices of Robo1 deficient mice (n = 3, Robo1 +/+; n = 3, Robo1−/−) during the PPL stage of development. Similar to Robo3 mutants, we found no significant differences in the total number of reelin immunopositive cells in Robo1 mutant mice compared with wild-type littermates within the neocortex (Robo1−/− 61.26 ± 2.34; Robo1+/+ 59.5 ± 1.90) and hippocampal region (Robo1−/− 43.32 ± 3.90; Robo1+/+ 36.85 ± 3.11) at E12.5. Cell counts in Robo2 deficient mice (n = 3, Robo2+/+; n = 3, Robo2−/−) at the same stage of development (E12.5) showed no significant changes in the number of reelin immunopositive cells within the neocortex compared with wild-type littermates (Robo2−/− 62.7 ± 4.33; Robo2+/+ 71.5 ± 5.84). However, a significant increase (38%) in reelin containing cells was observed within the hippocampal region of Robo2 mutant animals compared with wild-type littermates, especially in middle regions (Robo2−/− 47.2 ± 3.2; Robo2+/+ 34.0 ± 1.97; P < 0.05) at E12.5.
Robo3 and Cortical Interneurons
We sought to investigate whether cortical interneurons also express the Robo3 receptor, given its robust expression within the early developing MGE (Fig. 1). In these experiments, we stained for Robo3 coronal sections taken from E10.5 GAD67-GFP+ mice. We found that a proportion of GAD67-GFP+ MGE cells coexpressed Robo3 (Fig. 2D–F). This was confirmed by carrying out Robo3 labeling in dissociated cell cultures of MGE prepared from GAD67-GFP+ mice at E13.5 (Fig. 2G–I). Examination of coronal sections taken from such mice at E12.5–13.5 showed a number of cells in the MZ that expressed both GAD67-GFP and Robo3 (data not shown), further confirming that cortical interneurons express this receptor.
We have previously shown that interneurons robustly express both Robo1 and Robo2 receptors in the developing forebrain, and provided evidence that Robo1 plays a major role in the development and migration of cortical interneurons (Andrews et al. 2006, 2008). Given that an early population of interneurons also expresses Robo3, we wanted to investigate whether this receptor is also involved in the migration and morphological differentiation of interneurons. This was investigated within the cortices of Robo3 deficient mice during early (E13.5) and late phases of tangential migration (E18.5) (Fig. 3). Coronal sections were taken from the cortices of mutant and wild-type littermates, and processed immunohistochemically for the interneuron marker, calbindin (Anderson et al. 1997; Andrews et al. 2006) (Fig. 3A,B). The total number and distribution of interneurons was assessed throughout the rostral–caudal extent of the developing cortex. As interneurons follow distinct and developmentally regulated tangential migratory paths, the number of labeled cells within each cortical layer was further assessed for any changes in specific migratory routes.
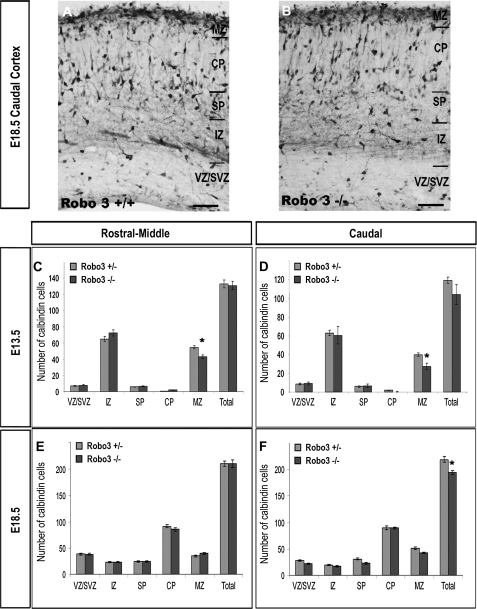
Figure 3.
(A, B) Photomicrographs of coronal sections through the caudal cortex of Robo3+/+ and Robo3−/− mice stained for calbindin. (C–F) Analysis of the number and distribution of calbindin-labeled cells in all layers of the cortex of Robo3+/− and Robo3−/− mice at E13.5 (C, D) and E18.5 (E, F). Counts were made in rostral-middle (C, E) and caudal (D, F) regions of the cortex. Scale bar in (A, B) is 150 μm (*P < 0.01) (SVZ, subventricular zone; IZ, intermediate zone; SP, subplate; CP, cortical plate).
Analysis of Robo3 deficient cortices (n = 3, Robo3−/−) during the early (E13.5) phase of tangential interneuron migration showed no significant changes in the total number of calbindin cells within rostral-middle (Robo3−/− 130.9 ± 5.3; Robo3+/− 132.7 ± 4.8) or caudal levels (Robo3−/− 119.4 ± 3.1; Robo3+/− 104 ± 10.4) of the cortex compared with Robo3 heterozygote littermates (n = 3 Robo3+/−) (Fig. 3C,D). When we assessed the distribution of calbindin positive cells within the different layers of the developing cortex, we observed a significant reduction in the number of cells within the MZ of Robo3 deficient mice compared with heterozygote littermates (Robo3−/− 42.9 ± 1.8; Robo3+/− 54.5 ± 1.8; P < 0.0001) in rostral-middle levels and caudal levels of the cortex (Robo3−/− 27.3 ± 3.4; Robo3+/− 39.7 ± 1.7; P < 0.01) (Fig. 3C,D) at E13.5. This decrease within the MZ of mutant cortex coincided with a small, but not significant, increase of cells in some of the other layers of these animals.
Analysis at a later phase of corticogenesis (E18.5) similarly revealed no differences in the number of calbindin cells in rostral-middle levels of the cortex (n = 3, Robo3−/−, 211.1 ± 4.75; n = 3, Robo3+/−, 211.4 ± 7.02) (Fig. 3E). However, a small but just significant decrease (11%) was observed within the caudal cortex of mutant animals relative to heterozygote littermates (Robo3−/− 194.4 ± 4.0; Robo3+/− 218.5 ± 5.5; P < 0.01) (Fig. 3F). Thus, Robo3 does not appear to have a major role in regulating the number and distribution of interneurons within the developing cortex.
Analysis of Interneurons in Triple Robo1−/−Robo2−/−Robo3−/− Mutant Mice
We have shown that the 3 Robo receptors are expressed in overlapping patterns both in the subpallium and pallium, suggesting that individual neurons in the GE and cortex express more than one receptor. We have also shown that the 3 receptors exert a differential effect on the number and positions of cortical interneurons. In view of these observations, it is possible that silencing one of the receptors may induce a compensatory response from the others. For this reason, we undertook a preliminary analysis on a limited number of animals of the number and morphology of cortical interneurons that lack all 3 Robo receptors. Coronal sections, taken from the cortex of triple Robo1−/−Robo2−/−Robo3−/− mice, were immunohistochemically processed for calbindin. The total number and distribution of interneurons was assessed throughout the rostral–caudal extent of the developing cortex.
Analysis at the early stages of corticogenesis was difficult due to lack of appropriate control littermate animals. However, a decrease in the total number of calbindin-labeled cells was observed within the cortices of triple Robo mutant animals (n = 2) relative to an animal which was heterozygote for both Robo1 and Robo2 receptors and fully deficient for the Robo3 receptor (Robo1+/−Robo2+/−Robo3−/−). Specifically, there was a 26% decrease in the total number of calbindin cells within rostral-middle regions of the cortex (Robo1−/−Robo2−/−Robo3−/−, 112.7 ± 5.62; Robo1+/−Robo2+/−Robo3−/−, 151.3 ± 3.96) in triple Robo mutants relative to the heterozygote littermate. A comparable 21% decrease in total calbindin cells was observed within caudal regions of the cortex of triple Robo mutant animals (Robo1−/−Robo2−/−Robo3−/−, 119.8 ± 6.48; Robo1+/−Robo2+/-Robo3−/−, 149.9 ± 6.21).
Cell counts at E18.5 were consistent with observations at E13.5. These showed that removal of all 3 Robo receptors resulted in approximately 38% decrease in the number of calbindin cells within rostral-middle regions of the cortex (n = 1, Robo1−/−Robo2−/−Robo3−/−, 167 ± 8.63; n = 1 Robo1+/+Robo2+/+Robo3+/−, 285.5 ± 28.4) compared with a single Robo3 heterozygote littermate. A similar (30%) decrease in total cell number was observed within caudal regions of the cortex in triple mutants (Robo1−/−Robo2−/−Robo3−/−, 184.5 ± 9.88; Robo1+/+Robo2+/+Robo3+/−, 260.2 ± 33.1) compared with Robo3 heterozygote littermates. Thus, removal of all 3 Robo receptors appears to result in a marked decrease in the total number of interneurons throughout the rostral–caudal extent of the developing cortex.
Robo3 and Interneuron Morphology
We have recently shown that, in addition to regulating interneuron numbers in the developing cortex, both Robo1 and Robo2 receptors play a role in the morphological differentiation of migrating cortical interneurons (Andrews et al. 2008). Thus, we wanted to determine whether Robo3 has a similar effect during development. This was investigated in Robo3 deficient transgenic mice during early (E13.5) (n = 3, Robo3−/−, n = 3, Robo3+/−) and late (E18.5) stages of tangential migration. The morphology of calbindin positive neurons was assessed by measuring the total length of their processes, and counting the number of processes and their branch points (Supplementary Fig. 2F). This analysis indicated that, at E13.5, interneurons in Robo3−/− cortices (n = 422 neurons Robo3−/−; n = 298 neurons Robo3+/−) showed significantly greater process length (Robo3−/−, 77.32 ± 1.72 μm; Robo3+/−, 43.56 ± 1.15 μm), and significantly more processes (Robo3−/−, 1.77 ± 0.04; Robo3+/−, 1.60 ± 0.04) and branch points (Robo3−/−, 0.41 ± 0.03; Robo3+/− 0.29 ± 0.03) than interneurons taken from heterozygote littermates (Supplementary Fig. 2). Interestingly, interneuron morphology was not found to differ significantly between Robo3−/− and Robo3+/− heterozygote cortices at later stages of development (E18.5). This data suggest that Robo3 regulates the morphology of early born cortical interneurons.
Discussion
Migrating interneurons are guided by intrinsic and extrinsic cues along their tortuous journey to the cortex, where they disperse in all layers and form functional circuits with their pyramidal counterparts. Much effort has recently been devoted to understanding the molecular mechanisms that regulate interneuron migration, as a deficiency of these cells in the cortex results in an imbalance of excitation and inhibition that underlies a number of neurological disorders (Powell et al. 2003; Cobos et al. 2005; Mallet et al. 2006; Di Cristo 2007). These efforts have identified a number of ligands and receptors that regulate the tangential migration of these cells from the subpallium to the cortex, such as semaphorins–neuropilins (Marín et al. 2001), neuregulin1-erb4 (Flames et al. 2004), and Slit-Robo (Andrews et al. 2006, 2008), as well as molecules that direct interneurons to their appropriate laminar and areal positions within the cortex (Métin et al. 2006; Nakajima 2007; López-Bendito et al. 2008).
Recent analyses have shown that during early development of the subpallium, the expression patterns of Robo1 and Robo2 are distinct and complementary to Slits (Andrews et al. 2007). It has also been shown that Robo1, in addition to semaphorin–neuropilin (Marín et al. 2001), plays a role in steering migrating interneurons around the striatum and into the neocortex, as well as regulating the number of these cells that enter the cortex (Andrews et al. 2006, 2008). Furthermore, interactions of Robo1 and Robo2 receptors with Slits appear to affect the morphological differentiation of cortical interneurons (Whitford et al. 2002; Sang et al. 2002; Sang and Tan 2003; Andrews et al. 2008). Thus, the interactions of Slit proteins with Robo1 and Robo2 receptors appear to have multiple roles in interneuron development. Here, we investigated whether the third Robo receptor, Robo3, is also involved in these processes.
Our detailed immunohistochemical analysis revealed that Robo3 protein was strongly localized within the superficial layers of the early cortex (PPL stage; E11.5-E13.5) as well as within the differentiating fields of the GE. Its distinct localization in the MGE, at a time when the first wave of these cells are generated, and in the PPL/MZ through which early born interneurons migrate, suggested that these cells may also express the Robo3 receptor. We confirmed that an early population of interneurons indeed express the receptor, as shown by the colocalization of Robo3 with GAD67 GFP+. Our analysis of mutants that lacked the receptor revealed that unlike Robo1 but, similar to Robo2, Robo3 does not appear to regulate the number of interneurons within the developing cortex (Andrews et al. 2008). However, closer analysis showed that the number of interneurons within the MZ of the cortex decreases in the absence of this receptor. This is interesting considering that Slit1- and Slit3 transcripts are strongly expressed within the MZ of the early cortex (Bagri et al. 2002; Marillat et al. 2002; Whitford et al. 2002), suggesting that removal of Robo3, but not the other 2 Robo receptors, alters the sensitivity of interneurons to chemorepulsion within this region. Such a role for Robo3 is plausible, as it has been shown to modulate Slit chemorepulsion in ventral spinal cord guidance systems (Jen et al. 2004; Marillat et al. 2004; Sabatier et al. 2004). This possibility is currently under investigation in cortical interneurons.
We have quantified the morphological parameters (number of neurites, total neurite length and number of branch points) of labeled migrating interneurons. We found a pronounced increase in all parameters in the cortices of Robo3 mutant mice compared with heterozygotes in the early (E13.5), but not later stages of development (E18.5), which is consistent with the timing of Robo3 expression. Comparable increases in process length and branching were documented in Robo1-, but more modest changes were noted in Robo2 mutants at E15.5. Taken together, these results indicate that Robo receptors differentially affect the morphological development of migrating cortical inteneurons.
Because cortical interneurons express all 3 Robo receptors, it is possible that silencing Robo3 may induce a compensatory response from the others. Thus, we examined the cortices of a small number of triple mutant animals in earlier (E13.5) and later (E18.5) stages of embryonic development. Interestingly, they contained markedly fewer interneurons throughout their rostral–caudal extent. This is contrary to the increased interneuron populations found in Robo2 and, especially, in Robo1 mutants. Furthermore, triple mutants only showed a modest change in morphology (data not shown), unlike the single receptor mutants that demonstrated striking changes. These observations point to a complex interplay between the 3 Robo receptors in regulating number, migration and morphology of cortical interneurons, and highlight the importance of understanding the relationship between these receptors.
Robo Receptors and Cajal–Retzius Cells
Cajal–Retzius cells constitute a morphologically conspicuous group of neurons in the MZ of the early developing cortex (Edmunds and Parnavelas 1982; Frotscher 1997; Marín-Padilla 1998). The reelin-producing members of the Cajal–Retzius family make up a large portion of this group (Alcantara et al. 1998; Meyer et al. 1999), and the secreted protein is thought to be crucial for the establishment of normal lamination of the cortical plate (D'Arcangelo et al. 1995; Ogawa et al. 1995; Super et al. 2000). Cajal–Retzius cells comprise changing populations of cells, with new neurons continuously added in the MZ, whereas others die having fulfilled their developmental role (Parnavelas and Edmunds 1983; Derer and Derer 1990; Meyer et al. 1999). Evidence suggests that the vast majority invade the MZ by tangential migration from cortical (Meyer et al. 1998; Takiguchi-Hayashi et al. 2004; Siegenthaler and Miller 2008) and extracortical sources (Lavdas et al. 1999; Shinozaki et al. 2002; Takiguchi-Hayashi et al. 2004; Bielle et al. 2005). It has recently been suggested that the cortical hem is the main source of Cajal–Retzius cells of cortical origin (Takiguchi-Hayashi et al. 2004; Yoshida et al. 2006; Garcia-Moreno et al. 2007; Siegenthaler and Miller 2008). The observation that Slit is expressed within the cortical hem and superficial MZ during early development (Bagri et al. 2002; Marillat et al. 2002; Whitford et al. 2002), together with our finding that groups of Cajal–Retzius cells express all 3 Robo receptors would suggest that Slit-Robo signalling plays a role in the development of this precocious neuronal population. However, silencing of Robo1, Robo2, or Robo3 receptors had no effect on the overall number of reelin-positive cells within the developing cortex.
Concluding Remarks
The complex development of the mammalian forebrain requires the production of a plethora of neuronal and non-neuronal cell types that often have to migrate over long distances to reach their final targets within the cortex, as highlighted by cortical GABAergic interneurons. Once correctly positioned, these cells differentiate and form a bewildering array of connections. Here, we have pointed to contributions of Slit-Robo signalling in some of these developmental processes. Recent clinical studies have highlighted a role for these molecules in the pathogenesis of a number of human disorders, including some that affect the nervous system (Jen et al. 2004; Anitha et al. 2008; Miranda et al. 2008). Thus, a better understanding of the functional roles of Slit-Robo in different developmental and disease processes may result in the development of treatments for these disorders.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
Wellcome Trust, Programme Grant (074549) to J.G.P.; Association pour la Recherche sur le Cancer and the Fondation pour la Recherche Médicale (Programme Equipe FRM) to A.C.; and Ministére de la Recherche et de la Technologie fellowship to T.D.M.
Supplementary Material
Acknowledgments
Conflict of Interest: None declared.
References
- Alcantara S, Ruiz M, D'Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, Murakami F, Parnavelas JG, Sundaresan V, Richards LJ. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Andrews WD, Barber M, Parnavelas JG. Slit-Robo interactions during cortical development. J Anat. 2007;211:188–198. doi: 10.1111/j.1469-7580.2007.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews W, Barber M, Hernandez-Miranda LR, Xian J, Rakic S, Sundaresan V, Rabbitts TH, Pannell R, Rabbitts P, Thompson H, et al. The role of Slit-Robo signalling in the generation, migration and morphological differentiation of cortical interneruons. Dev Biol. 2008;313:648–658. doi: 10.1016/j.ydbio.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Anitha A, Nakamura K, Yamada K, Suda S, Thanseem I, Tsujii M, Iwayama Y, Hattori E, Toyota T, Miyachi T, et al. Genetic analyses of roundabout (ROBO) axon guidance receptors in autism. Am J Med Genet B Neuropsychiatr Genet. 2008;147:1019–1027. doi: 10.1002/ajmg.b.30697. [DOI] [PubMed] [Google Scholar]
- Bagri A, Marín O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Neme N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal–Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Camurri L, Mambetisaeva E, Sundaresan V. Rig-1 a new member of Robo family genes exhibits distinct pattern of expression during mouse development. Gene Expr Patterns. 2004;4:99–103. doi: 10.1016/s1567-133x(03)00142-x. [DOI] [PubMed] [Google Scholar]
- Camurri L, Mambetisaeva E, Davies D, Parnavelas J, Sundaresan V, Andrews W. Evidence for the existence of two Robo3 isoforms with divergent biochemical properties. Mol Cell Neurosci. 2005;30:485–493. doi: 10.1016/j.mcn.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Miao CG, Chen S-C, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Derer P, Derer M. Cajal-Retzius cell ontogenesis and death in mouse brain, visualised with horseradish peroxidise and electron microscopy. Neuroscience. 1990;36:839–856. doi: 10.1016/0306-4522(90)90027-2. [DOI] [PubMed] [Google Scholar]
- Di Cristo G. Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin Genet. 2007;72:1–8. doi: 10.1111/j.1399-0004.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- Edmunds SM, Parnavelas JG. Retzius-Cajal cells: an ultrastructural study in the developing visual cortex of the rat. J Neurocytol. 1982;11:427–446. doi: 10.1007/BF01257987. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marín O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Frotscher M. Dual role of Cajal-Retzius cells and reelin in cortical development. Cell Tissue Res. 1997;290:315–322. doi: 10.1007/s004410050936. [DOI] [PubMed] [Google Scholar]
- Garcia-Moreno F, Lopez-Mascaraque L, De Carlos JA. Origins and migratory routes of murine Cajal-Retzius cells. J Comp Neurol. 2007;500:419–432. doi: 10.1002/cne.21128. [DOI] [PubMed] [Google Scholar]
- Hu H. Chemorepulsion of neuronal migration by Slit2 in the developing mammalian forebrain. Neuron. 1999;23:703–711. doi: 10.1016/s0896-6273(01)80029-5. [DOI] [PubMed] [Google Scholar]
- Huminiecki L, Gorn M, Suchting S, Poulsom R, Bicknell R. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of angiogenesis. Genomics. 2002;79:547–552. doi: 10.1006/geno.2002.6745. [DOI] [PubMed] [Google Scholar]
- Jen JC, Chan WM, Bosley TM, Wan J, Carr JR, Rub U, Shattuck D, Salamon G, Kudo LC, Ou J, et al. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304:1509–1513. doi: 10.1126/science.1096437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;23:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Lavdas A, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Isacson O. Axonal growth regulation of fetal and embryonic stem cell derived dopaminergic neurons by Netrin-1 and Slits. Stem Cells. 2006;24:2504–2513. doi: 10.1634/stemcells.2006-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Patel K, Schmidt H, Andrews W, Pini A, Sundaresan V. Extracellular Ig domains 1 and 2 of Robo are important for ligand (Slit) binding. Mol Cell Neurosci. 2004;26:232–240. doi: 10.1016/j.mcn.2004.01.002. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Flames N, Ma L, Fouquet C, Di Meglio T, Chedotal A, Tessier-Lavigne M, Marín O. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J Neurosci. 2007;27:3395–3407. doi: 10.1523/JNEUROSCI.4605-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Sanchez-Alcaniz JA, Pla R, Borrell V, Pico E, Valdeolmillos M, Marín O. Chemokine signalling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Tessier-Lavigne M. Dual branch-promoting and branch-repelling actions of Slit/Robo signaling on peripheral and central branches of developing sensory axons. J Neurosci. 2007;27:6843–6851. doi: 10.1523/JNEUROSCI.1479-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambetisaeva ET, Andrews W, Camurri L, Annan A, Sundaresan V. Robo family of proteins exhibit differential expression in mouse spinal cord and Robo-Slit interactions is required for midline crossing in vertebrate spinal cord. Dev Dyn. 2005;233:41–51. doi: 10.1002/dvdy.20324. [DOI] [PubMed] [Google Scholar]
- Marín O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphoring-neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- Marín O, Plump AS, Flames N, Sánchez-Camacho C, Tessier-Lavigne M, Rubenstein JL. Directional guidance of interneuron migration to the cerebral cortex relies on subcortical Slit1/2-independent repulsion and cortical attraction. Development. 2003;130:1889–1901. doi: 10.1242/dev.00417. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Marillat V, Cases O, Nguyen-Ba-Charvet KT, Tessier-Lavigne M, Sotelo C, Chedotal A. Spatiotemporal expression patterns of slit and robo genes in the rat brain. J Comp Neurol. 2002;442:130–155. doi: 10.1002/cne.10068. [DOI] [PubMed] [Google Scholar]
- Marillat V, Sabatier C, Failli V, Matsunaga E, Sotelo C, Tessier-Lavigne M, Chedotal A. The slit receptor Rig-1/Robo3 controls midline crossing by hindbrain precerebella neurons and axons. Neuron. 2004;43:69–79. doi: 10.1016/j.neuron.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Marín-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- Métin C, Baudoin JP, Rakic S, Parnavelas JG. Cell and molecular mechanisms involved in the migration of cortical interneurons. Eur J Neurosci. 2006;23:894–900. doi: 10.1111/j.1460-9568.2006.04630.x. [DOI] [PubMed] [Google Scholar]
- Meyer G, Soria JM, Martinez-Galan JR, Martin-Clemente B, Fairen A. Different origins and developmental histories of transient neurons in the marginal zone of the fetal and neonatal rat cortex. J Comp Neurol. 1998;397:493–518. [PubMed] [Google Scholar]
- Meyer G, Goffinet AM, Fairen A. What is a Cajal-Retzius cell? A reassessment of a classical cell type based on recent observations in the developing neocortex. Cereb Cortex. 1999;9:765–775. doi: 10.1093/cercor/9.8.765. [DOI] [PubMed] [Google Scholar]
- Miranda DM, Wigg K, Feng Y, Sandor P, Barr CL. Association study between Gilles de la Tourette Syndrome and two genes in the Robo-Slit pathway located in the chromosome 11q24 linked/associated region. Am J Med Genet B Neuropsychiatr Genet. 2008;147:68–72. doi: 10.1002/ajmg.b.30580. [DOI] [PubMed] [Google Scholar]
- Nakajima K. Control of tangential/non-radial migration of neurons in the developing cerebral cortex. Neurochem Int. 2007;51:121–131. doi: 10.1016/j.neuint.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamotot H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Ozdinler PH, Erzurumlu RS. Slit2, a branching-arborization factor for sensory axons in the Mammalian CNS. J Neurosci. 2002;22:4540–4549. doi: 10.1523/JNEUROSCI.22-11-04540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY. Robo4 is a vascular specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–267. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- Parnavelas JG, Edmunds SM. Further evidence that Cajal-Retzius cells transform to nonpyramidal neurons in the developing rat visual cortex. J Neurocytol. 1983;12:863–871. doi: 10.1007/BF01258156. [DOI] [PubMed] [Google Scholar]
- Plachez C, Andrews W, Liapi A, Knoell B, Drescher U, Mankoo B, Zhe L, Mambetisaeva E, Annan A, Bannister L, et al. Robos are required for the correct targeting of retinal ganglion cell axons in the visual pathway of the brain. Mol Cell Neurosci. 2008;37:719–730. doi: 10.1016/j.mcn.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region and GABA cell type-specific deficits, epilepsy, and behavioural dysfunction. J Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Le Ma, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Sang Q, Wu J, Rao Y, Hsueh YP, Tan SS. Slit promotes branching and elongation of neuritis of interneurons but not projection neurons from the developing telencephalon. Mol Cell Neurosci. 2002;21:250–265. doi: 10.1006/mcne.2002.1156. [DOI] [PubMed] [Google Scholar]
- Sang Q, Tan SS. Contact-associated neurite outgrowth and branching of immature cortical interneurons. Cereb Cortex. 2003;13:677–683. doi: 10.1093/cercor/13.6.677. [DOI] [PubMed] [Google Scholar]
- Shu T, Richards LJ. Cortical axon guidance by the glial wedge during the development of the corpus callosum. J Neurosci. 2001;21:2749–2758. doi: 10.1523/JNEUROSCI.21-08-02749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Sundaresan V, McCarthy MM, Richards LJ. Slit2 guides both precrossing and postcrossing callosal axons at the midline in vivo. J Neurosci. 2003;23:8176–8184. doi: 10.1523/JNEUROSCI.23-22-08176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Miller MW. Generation of Cajal-Retzius neurons in mouse forebrain is regulated by transforming growth factor beta-Fox signalling pathways. Dev Biol. 2008;313:35–46. doi: 10.1016/j.ydbio.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Miyagi T, Yoshida M, Miyata T, Ogawa M, Aizawa S, Suda Y. Absence of Cajal-Retzius cells and subplate neurons associated with defects of tangential cell migration from ganglionic eminence in Emx1/2double mutant cerebral cortex. Development. 2002;129:3479–3492. doi: 10.1242/dev.129.14.3479. [DOI] [PubMed] [Google Scholar]
- Sundaresan V, Mambetisaeva E, Andrews W, Annan A, Knöll B, Tear G, Bannister L. Dynamic expression patterns of Robo (Robo1 and Robo2) in the developing murine central nervous system. J Comp Neurol. 2004;468:467–481. doi: 10.1002/cne.10984. [DOI] [PubMed] [Google Scholar]
- Super H, Del Rio JA, Martinez A, Perez-Sust P, Soriano E. Disruption of neuronal migration and radial glia in the developing cerebral cortex following ablation of Cajal-Retzius cells. Cereb Cortex. 2000;10:602–613. doi: 10.1093/cercor/10.6.602. [DOI] [PubMed] [Google Scholar]
- Takiguchi-Hayashi K, Sekiguchi M, Ashigaki S, Takamatsu M, Hasegawa R, Migishima R, Yokoyama M, Nakanishi S, Tanabe Y. Generation of reelin positive marginal zone cells from the caudomedial wall of telencephalic vesicles. J Neurosci. 2004;24:2286–2295. doi: 10.1523/JNEUROSCI.4671-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Whitford KL, Marillat V, Stein E, Goodman CS, Tessier-Lavigne M. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002;33:47–61. doi: 10.1016/s0896-6273(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, Rao Y. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones KR, Grove EA. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- Yuan SS, Cox LA, Dasika GK, Lee EY. Cloning and functional studies of a novel gene aberrantly expressed in RB-deficient embryos. Dev Biol. 1999;207:62–75. doi: 10.1006/dbio.1998.9141. [DOI] [PubMed] [Google Scholar]
- Yuan W, Zhou L, Chen JH, Wu JY, Rao Y, Ornitz DM. The mouse SLIT family: secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 1999;212:290–306. doi: 10.1006/dbio.1999.9371. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Li H, Zhou L, Wu JY, Rao Y. Cellular and molecular guidance of GABAergic neuronal migration from an extracortical origin to the neocortex. Neuron. 1999;23:473–485. doi: 10.1016/s0896-6273(00)80801-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.