Abstract
In mammalian brain development, neuroepithelial cells act as progenitors that produce self-renewing and differentiating cells. Recent technical advances in live imaging and gene manipulation now enable us to investigate how neural progenitors generate the 2 different types of cells with unprecedented accuracy and resolution, shedding new light on the roles of epithelial structure in cell fate decisions and also on the plasticity of neurogenesis.
Keywords: asymmetric division, neural progenitor, neuroepithelial cell, self-renewal, spindle orientation
In mammalian cortical development, neuroepithelial cells function as neural progenitor cells. These cells initially proliferate to expand their population and subsequently give rise to both neurons and neural progenitors (Fig. 1). Although the highly elongated neuroepithelial cells called radial glia were identified as the major neural progenitors in 2001 (Hartfuss et al. 2001; Miyata et al. 2001; Noctor et al. 2001), the question of how these neuroepithelial cells generate self-renewing and differentiating daughters remains a central issue in mammalian neurogenesis. Neuroepithelial cells extend from the apical to basal surface of the cortex and exhibit dynamic cell cycles, during which they exhibit interkinetic nuclear movement (INM; Baye and Link 2008). These progenitors (designated “apical progenitors” in this review) divide at the apical surface. After division, their nuclei migrate away from the apical position through the elongated cytoplasm during the G1 phase of mitosis and remain near the boundary between the ventricular zone and subventricular zone during the S phase. They subsequently descend to the apical surface upon entering the G2 phase, which is accomplished more quickly than in the upward migration. Recent studies have also revealed an intermediate type of progenitors, which are born as daughters of the apical divisions (Haubensak et al. 2004; Miyata et al. 2004; Noctor et al. 2004). These cells, designated “basal progenitors,” do not undergo INM but migrate into the subventricular zone where they divide into a pair of postmitotic neurons, whereas a small population of basal progenitors appear to undergo multiple cell divisions during late neurogenesis (Noctor et al. 2004). Although the apical progenitor population gradually decreases in late neurogenesis, they continue to self-renew and generate neurons. As these dynamic behaviors take place in structurally complex developing mammalian cortex, it has been difficult to accurately assess how the apical progenitor both self-replicates and generates a neuron, and different approaches to this question have given rise to different views. Here we will discuss recent progress in the field, with a particular emphasis on perspectives gained from the study of cellular architecture.
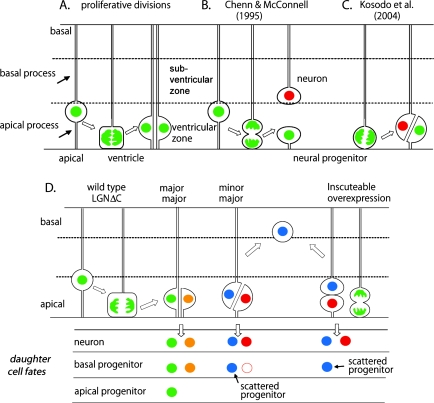
Figure 1.
Schematic illustration of models for the division patterns of apical progenitors. (A) At proliferative stages, apical divisions produce 2 equivalent daughters. (B) Chenn and McConnell (1995) proposed that neurogenic divisions have the cleavage plane perpendicular or oblique to the epithelial surface so as to give rise to a neuron as the basal daughter and a self-renewing progenitor as the apical daughter. (C) Kosodo et al. (2004) proposed that a slight tilt or displacement of mitotic spindles enables the cleavage plane to bypass the apical membrane because of the narrow apical membrane area during apical division. (D) Konno et al. (2008) proposed that one daughter inherits both the apical membrane and the basal process, whereas the other inherits only the apical membrane during normal apical divisions. The self-renewable progenitor (apical progenitor) appears to be formed only from the daughter that inherits both apical and basal processes at mid-neurogenic stages. Loss of LGN function or overexpression of Inscuteable induces asymmetric partition of the apical membrane and the basal process into different daughters and generates an apical neuron and an ectopic progenitor (or a neuron) as the basal daughter, which might self-renew and produce neurons.
Signaling that Maintains Apical Progenitors
Of the many extracellular signals that control the expansion and/or maintenance of apical progenitors, Notch signaling has been shown to be crucial for the maintenance of this population (Yoon and Gaiano 2005). In general terms, the gain and loss of Notch signaling regulate the decision between self-renewal and differentiation of apical progenitors. The intracellular domain of Notch directly transduces Notch signaling in the nucleus together with RBPJ-kappa (Selkoe and Kopan 2003), resulting in the activation of basic helix-loop-helix proteins, including Hes1 and Hes5. These factors in turn negatively regulate proneural genes such as Neurogenin 2 (Ngn2) and Mash1 as well as Notch ligands (Delta-like1 and Jagged). There are 2 regulatory features in the Notch signaling cascade. The first is the negative feedback regulation of Hes1 on its own transcription (Hirata et al. 2002; Lewis 2003; Shimojo et al. 2008), which can generate oscillations in the expression of Notch downstream components. A second feature is intercellular competition of Notch signaling (Heitzler and Simpson 1991). These features of Notch signaling can confer to cells’ stochastic responses to Notch as discussed later. On the other hand, neuronally committed cells (basal progenitors and neurons) strongly upregulate Delta-like 1 expression to maintain the pool of the self-renewing apical progenitors (Kawaguchi, Ikawa, et al. 2008). Overall robustness of neurogenesis thus seems to be achieved by this feedback.
Asymmetric Daughter Cell Fates
A typical mechanism, by which cells simultaneously self-renew and generate more differentiated cells, is asymmetric division that gives rise to daughters of different cell fates. Molecular mechanisms underlying the asymmetric division of progenitor cells are best understood in invertebrate model organisms, such as Drosophila and Caenorhabditis elegans (Knoblich 2008). In one typical scheme, cell fate determinants are asymmetrically distributed in the dividing mother cell. This intracellular biased distribution of determinants is often achieved by cell-intrinsic polarity, as seen in Drosophila neuroblasts. Alternatively, extracellular signals may act on a cell asymmetrically, causing a local bias in intracellular responses that results in cell polarization (cell nonautonomous). In both of these situations, if the division axis that is defined by the axis of the mitotic spindle is coordinated parallel to the axis of cell polarity, the 2 daughters receive determinants or extracellular signals differentially, causing them to assume different fates. A polarized cell gives rise to equivalent daughters only when the cell polarity axis is orthogonal to the mitotic spindle orientation (that is parallel to the cleavage plane). Thus, the relationship of the polarity axis and the mitotic orientation (or the cleavage plane) provides a cell biological cue toward determining whether a polarized cell divides symmetrically or asymmetrically. This general scheme is thought to hold true for mammalian neuroepithelial cells, including apical progenitors in the cortex, as well, as these cells are highly polarized along the radial axis and they give rise to daughter cells of heterogeneous fates.
Various Observations on the Variation of Mitotic Orientation
Pioneering works in 1970s recorded the mitotic orientation of apical divisions in the cerebral cortex by electron microscopy (Smart 1973). A study on ferret embryos in 1995 opened a new window into this question by directly visualizing apical divisions using live DNA staining of cortical slices from developing brains (Chenn and McConnell 1995). This work proposed a model in which horizontal divisions where the axis of the mitotic spindle is parallel to the apical surface of the neuroepithelium are symmetric and proliferative, giving rise to 2 apical progenitors, whereas vertical or oblique divisions are asymmetric and neurogenic, generating a basal neuron and an apical progenitor (Fig. 1B). As it mirrors the typical mode of asymmetric divisions seen in Drosophila neuroblasts, this has been the prevailing model to date. Since this epoch-making study, time-lapse observations of living slices and examinations of fixed sections have become standard techniques used in investigating this problem. Subsequent studies using these same techniques have generated results that were consistent with the above model in some cases, but in others, not. Immediately following the above study, monitoring of the open book slices from the apical side demonstrated that chromosomal separation parallel to the apical surface follows dynamic rotations of the metaphase plate in most apical divisions (Adams 1996), and a claim arose that most divisions in the neuroepithelium were horizontal in both proliferative and neurogenic stages as in the ordinary epithelium (Huttner and Brand 1997). It was reported that the frequency of vertical or oblique divisions peaked around E14 in mice (Haydar et al. 2003). Those divisions were assumed to be asymmetric, generating a neuron and a progenitor, whereas horizontal divisions were either symmetrically proliferative in early stages or symmetrically neurogenic in late stages. In contrast, in vivo observations in zebra fish indicated that neuroepithelial cell divisions remain horizontal during neurogenesis (Das et al. 2003). It was proposed, based on observations of fixed sections of developing mouse brains, that whether the cleavage plane bypasses or bisects the apical membrane was more correlated with proliferative or neurogenic divisions in the apical divisions that were mostly horizontal (Kosodo et al. 2004 and see below). Two recent studies (Konno et al. 2008; Noctor et al. 2008) carefully inspected the mitotic orientation of the apical divisions in both fixed sections and living slices from rodent cortex (Fig. 1D). These works revealed that approximately 90% of apical divisions show mitotic orientation within a range of 30 degrees from the epithelial plane throughout embryonic neurogenesis.
This raises the question of why observations vary from study to study. One reason seems to be the technological advances in the live imaging of cultured slices. Improvements in microscope performance and the use of green fluorescent protein (GFP) now enable us to assign the orientation of cleavage planes in series of 3-dimensional images at far better spatial and temporal resolutions. Improved resolutions also enable us to detect in vitro artifacts such as increases in apical divisions at positions that are near, but detached from, the apical surface, which are observed in cultured slices under suboptimal conditions (often showing relatively inactive INM). Such divisions frequently show skewed cleavage planes, and their daughters often subsequently undergo apoptosis.
It should be noted that the discrepancies among these works do not represent an “all or none” absoluteness. Variations or fluctuations in mitotic orientations at mitosis have been observed in all previous works dealing this problem. The questions are, therefore, 1) whether the degree of variations in spindle orientation makes a difference in the determination of daughter cell fates and 2) whether those variations are frequent enough to account for the population of a daughter cell type. These points affect the interpretation of how mitotic spindle orientation is connected to the daughter cell properties.
Structural Properties Associated with Asymmetric and Symmetric Divisions
Because highly elongated neuroepithelial cells (including apical progenitors) consist of distinct subcellular domains which, at interphase, are the apical end foot, cell body, and basal end foot, cleavage planes that deviate significantly from the radial axis will result in an exclusive partition of the apical domain or the basal end foot into one daughter, generating daughters lacking the complete epithelial morphology. In the extreme case (Fig. 1B), a horizontal cleavage plane will segregate the entire apical half of the cell (including the apical surface) into one daughter and the basal half (including the basal end foot) into the other (Chenn and McConnell 1995). Such cleavages are predicted to reduce the population of neuroepithelial cells unless the missing end foot regenerates, whereas oblique cleavage planes may partition different fractions of the apical and basal surfaces into both daughters, preserving the neuroepithelial population.
An obvious question arises in regard to models that suggest that both neurogenic and proliferative divisions are horizontal. How are differential fates conferred to the daughters arising from parallel divisions? Huttner and his colleagues have proposed a possible mechanism to answer this question (Huttner and Brand 1997; Kosodo et al. 2004). The apical membrane area represents a tiny fraction of the cell body surface, and a slight tilt of cleavage planes is sufficient for dividing apical progenitors to bisect the cell outside the apical membrane (Fig. 1C), leading to one daughter lacking the apical area that includes the apical junctional complex containing the Partition defective–atypical protein kinase C complex (Ohno 2001). This hypothesis was reinforced by their observations that deviation of spindle orientation from the horizontal plane, or lateral displacement of the vertical cleavage plane, is increased when progenitors become neurogenic (Kosodo et al. 2004); neurogenic progenitors were identified by the expression of the enhanced GFP (EGFP) gene knocked into the Tis21 gene, which appears to be correlated with neurogenic progenitors. These observations led to their model that asymmetric inheritance of the apical membrane is central to the assumption of differential cell fates by the daughters of apical divisions. This model is in clear contrast to the McConnell model (Chenn and McConnell 1995) in the respect that the division axis is nearly parallel to the plane for neurogenic divisions, although both models share the idea that the inheritance of the apical aspect is important for a daughter to acquire the ability to self-renew.
Genetic Tests of the Role for the Mitotic Orientation
Because spindle orientation depends on interactions between the cell cortex and astral microtubules, the roles of mitotic spindle orientation can be studied by perturbing microtubule-dependent processes. Several studies have investigated the effects of impaired function of molecules regulating the formation of mitotic spindles or microtubules. Lis1 and Nde1 form a complex with dynein to regulate spindle assembly and possibly cortical–astral interactions (Tai et al. 2002; Feng and Walsh 2004; Vallee and Tsai 2006). Lis1 mutations are known to cause lissencephaly in human. Abnormal spindle-like microcephaly associated is a centrosomal protein whose mutations cause microcephaly in humans (Bond et al. 2002). Elimination of the function of these molecules by knockout or knockdown induces abnormal spindle orientations and affects progenitor population and neuronal formation in mice (Feng and Walsh 2004; Fish et al. 2006), suggesting that proper spindle orientation is critical for neuronal fate decisions. Because other microtubule processes, such as interkinetic nuclear migration, often involve these molecules, careful evaluation of cell fate defects is needed to determine to what extent they result solely from impaired spindle orientations (Yingling et al. 2008).
Studies of Drosophila and C. elegans as well as mammalian cells have revealed that receptor-independent G-protein signaling is evolutionarily conserved in the regulation of mitotic spindle orientation (Schaefer et al. 2000; Yu et al. 2000; Gotta and Ahringer 2001). This pathway involves the cortical complex of Gαi and Goloco proteins: Drosophila Pins (Yu et al. 2000) and G protein-coupled receptor 1/2 in C. elegans (Colombo et al. 2003). This complex contains a component that interacts with astral microtubules directly or indirectly via the dynein–dynactin complex (Du et al. 2001; Srinivasan et al. 2003; Bowman et al. 2006; Izumi et al. 2006; Siller et al. 2006). If this cascade indeed functions in neuroepithelia, genetic manipulation or disruption of the complex should allow us to test the role of mitotic orientation in neurogenesis. There are 2 mammalian homologues for Drosophila Pins: activators of G-protein signaling 3 (Cismowski et al. 1999; Takesono et al. 1999) and LGN (Mochizuki et al. 1996; Yu et al. 2003). Sanada and Tsai (2005) tested the function of AGS3 and proposed that AGS3 destabilized the default planar mitotic orientation in the epithelia, allowing asymmetric divisions. Both knockdown of mouse AGS3 and interruption of its downstream Gβ–Gγ complex caused more planar spindle orientation than in the wild-type brain that frequently showed oblique spindle orientations in this study and also resulted in premature neurogenesis at the expense of the apical progenitors. To account for these phenotypes, this study postulates that determinants that promote differentiation are localized to the basal cortex in neurogenic progenitors (but not proliferative ones). Under this assumption, these determinants will segregate into one daughter in oblique divisions, but horizontal divisions as induced by the loss of AGS3 will partition these determinants into both daughters, which then assume the same neuronal fates. Recently, an AGS3 knockout mouse was reported to show no significant gross changes in brain morphology (Blumer et al. 2008). The role of AGS3 in spindle orientation in the cerebral cortex should be reexamined using this AGS3-mutant mouse.
In contrast to AGS3, LGN functions to orient mitotic spindles in a planar orientation, as revealed in both the chicken spinal cord (Morin et al. 2007) and mouse telencephalon (Konno et al. 2008). The C-terminal region of chicken LGN, which binds to Gαi, is dominant negative when overexpressed and induces a more random orientation of mitotic spindles in chick spinal cord. LGN knockdown produces the same outcome. Knockout of the LGN gene in mouse also randomized the orientation of apical divisions, indicating that LGN is necessary for the planar orientation of mitotic spindles in neuroepithelial cells. LGN and AGS3 may function antagonistically in the regulation of mitotic orientations in neuroepithelial progenitors, although it should be noted that the mitotic orientations in the control state in the AGS3 study (frequently oblique) and those for LGN (mostly horizontal) differed.
Interestingly, elimination of LGN function induces ectopic Paired box gene 6 (Pax6+) progenitors that are scattered in more basal regions along the radial axis in mouse brains (the midzone and sometimes even mantle zone) at the expense of the apical progenitors (Fig. 1D), suggesting that a transformation from apical progenitors to ectopically distributed progenitors occurs upon randomization of mitotic spindles (Konno et al. 2008). This is also the case for the spinal cord of chicken (Morin et al. 2007) and mouse (Konno et al. 2008). These observations provide evidence that randomization of mitotic spindle orientation does not occur in normal apical divisions because such ectopic mitoses are observed only as a minor fraction of divisions during mid-neurogenesis in wild type. Planar orientation of the apical division thus appears to be necessary for the maintenance of the apical progenitor population.
Progenitor Cells in Ectopic Positions: Plasticity of Neurogenesis
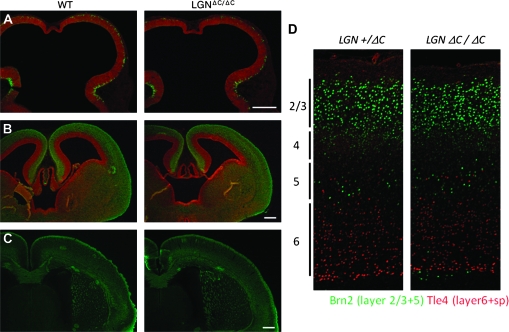
LGN-mutant mice are viable (Konno et al. 2008) and show no gross morphological changes in the brain during development (Fig. 2A). Stratification in the adult brain also looks normal (Fig. 2B). This apparent absence of abnormality is surprising, given that the population of apical progenitors is rapidly reduced in the mutant brain. One possible explanation is that decreases in the number of apical progenitors in the ventricular zone (and neuronal production) are compensated for by the formation of ectopically scattered progenitors, which resemble apical progenitors in their high Notch activity and Pax6 expression. In the chicken LGN knockdown study (Morin et al. 2007), ectopic progenitors were indeed shown to be self-renewable and neurogenic. The same may be true for the mouse cortex as well because the overall rate of neuronal production was indistinguishable between LGN-mutant brains and wild-type ones. These results suggest that the apical environment or niche is dispensable for the survival of progenitors. It would be interesting to know how progenitors can be self-renewable and neurogenic in such ectopic positions. Further study is needed to clarify whether the neuronal network functions normally in these mutant mice.
Figure 2.
The overall brain morphology of LGN mutants does not differ from that of wild type. (A) E10.5 and (B) E14.5, green; betaIII-tubulin, red; SRY-box containing gen2 (SOX2) and (C) E18.5, green; betaIII-tubulin. In the E14.5 LGN-mutant brain, SOX2-expressing cells distribute outside the ventricular zone, but it is not visible in (B) as betaIII-tubulin staining overrides the SOX2 staining. Scale bar, 200 μm. (D) Stratification of the adult cortex does not appear to be significantly affected by the absence of LGN function.
The Apical Membrane Domain in Daughter Cells of Apical Divisions
Models for neurogenic divisions often suppose that the asymmetric partition of the apical membrane domain (including the apical complex) determines differential daughter fates, assuming the apical domain as the site where determinants for undifferentiated or differentiated states reside (Chenn and McConnell 1995; Shen et al. 2002; Kosodo et al. 2004). Cell labeling studies using slice culture have, however, found a different mode of asymmetric divisions among apical progenitors at mid-neurogenesis; both daughters at the majority of apical divisions inherit the apical end foot (Miyata et al. 2001, 2004; Ochiai et al. 2007), and the neuronally committed daughter subsequently retracts the apical end foot (Ochiai et al. 2007). The expression of a ZO1–EGFP fusion protein enabled us to directly monitor apical membrane partition from the apical side of the slice culture, revealing that the exclusive asymmetric partition of the apical membrane normally occurred in no more than 15% of the apical divisions at E14–E14.5 (Konno et al. 2008). This frequency coincides with the proportion of neurogenic progenitors estimated by Tis21–GFP expression at E13.5, consistent with the concept that neurogenic progenitors partition the apical membrane asymmetrically. On the contrary, this numerical value does not account for observations that more than 50% of apical divisions generate a pair of daughters showing asymmetric fates (an apical progenitor and a basal progenitor or an apical progenitor and a neuron) in slice culture (Miyata et al. 2004; Noctor et al. 2004, 2008; Konno et al. 2008).
Randomization of cleavage orientation (by LGN knockout) increased frequency of the cleavage plane bypassing the apical membrane and decreased the population of apical progenitors (Konno et al. 2008). The concomitant appearance of ectopically scattered progenitors suggests that the loss of apical membrane at division causes the daughter to be an abnormally scattered progenitor due to lack of the apical anchor, raising the possibility that the inheritance of the apical domain is rather important for both daughters in order for them to assume ordinary fates. On the other hand, the overexpression of mouse Inscuteable (Zigman et al. 2005) artificially induces vertical divisions at the apical surface (Konno et al. 2008). The basal daughter of these vertical divisions, which was supposed to inherit the basal process, frequently took a progenitor fate (basal or scattered progenitors), whereas most of the apical daughters became postmitotic, suggesting that the inheritance of the apical membrane is not sufficient for the daughter to be an apical progenitor. It has not been directly examined what fate is adopted by the daughters that have lost the apical membrane at wild-type and LGN-mutant apical divisions. To clarify the role of the apical membrane domain, it would be worth examining the segregation of the apical membrane and junctional complex at division and following the fate of such daughters in living brain slices.
Partition of the Basal Process
In contrast to the apical membrane, imaging techniques with relatively high resolutions are needed to observe the elongated basal process, which becomes thinner during the mitosis of apical divisions. Recently, Kosodo et al. (2008) demonstrated that the basal process can divide into thin processes during apical divisions at early stages (E10.5) in mice and that such split processes did not necessarily segregate into different daughters in zebra fish apical divisions. Observations made using fluorescent dyes or GFP indicate that this process appears to segregate into one daughter at apical divisions during mid-neurogenic stages (Miyata et al. 2001; Noctor et al. 2001; Weissman et al. 2003), whereas a very thin process may remain in the daughter that does not apparently inherit the basal process. Although more sophisticated analyses will be needed to clarify the process of partition of the basal process, our current knowledge provides some insight into the morphological aspects of apical divisions during mid-neurogenic stages; the majority of the apical divisions give rise to one daughter that inherits the complete epithelial structure with a radial morphology, whereas the other daughter inherits only the apical process. It is intriguing to speculate whether a particular mechanism underlies the segregation of the basal process into a single daughter or whether it happens just within the natural range of fluctuations in cleavage plane position. Comparison of the neurogenic and proliferative progenitors at early stages will give insight about this issue. More fluctuations in presumptive cleavage planes were observed in Tis21-positive neurogenic progenitors than in proliferating progenitors (Kosodo et al. 2004). In this context, it would be interesting to see how the basal process is partitioned in the absence of AGS3 (Sanada and Tsai 2005).
Structural Constraints of Daughter Cell Fates of Apical Divisions
Are there any correlation between fate and structural features of cells that are born at the apical divisions? There are not many studies that have followed both the morphology and fate of daughters, due to technical difficulties in maintaining slice cultures sufficiently intact through multiple cell cycles. The prevailing view is that the daughter that does not possess the basal process will become either a postmitotic neuron or a basal progenitor, but not an apical progenitor as shown in Figure 1D (Miyata et al. 2001, 2004; Noctor et al. 2001, 2002). DiI labeling studies, however, show that cells having both an apical and a basal process can adopt either an apical progenitor fate or neuronally committed ones (Miyata et al. 2001, 2004). Although it has been observed that the basal process is regenerated in early neurogenic stages (Miyata et al. 2001), the missing apical or basal process is rarely regenerated in mid-neurogenesis (A.S. and F.M. unpublished observations). Thus, daughters of apical divisions, when they inherit both the apical and the basal process, appear to be able to assume a bona fide apical progenitor fate (Fig. 1D), suggesting that having a complete epithelial structure is necessary (but not sufficient) for self-renewable progenitors in the ventricular zone at mid-neurogenesis stages.
The uncertainty of the fate of a newly born cell with the full epithelial structure (apical progenitor, basal progenitor, or neuron) may be apparent due to limited resolutions in currently available methods for detection, meaning that it remains possible that some undiscovered structural variations affect neuroepithelial cell fate. It is also equally possible that the mechanism underlying cell fate determination may involve a stochastic nature. Indeed, it was recently shown that the fate of ventricular cells depends on their level of Delta-like 1 relative to that of neighboring cells (Kawaguchi, Yoshimatsu, et al. 2008). This leads to the situation where cell fate decisions include Notch-dependent competition, which has previously been shown genetically in the Drosophila peripheral nervous system (Heitzler and Simpson 1991). This competitive mechanism will introduce a stochastic nature into cell fate decisions, in contrast to the deterministic manner of Notch-mediated fate decisions, which require additional signaling or other factors, such as asymmetric segregation of Numb (Zhong et al. 1996). This Notch-dependent competition among neighboring cells may confer variations to the fate of daughter cells that inherit a full epithelial structure at apical divisions.
The Role of the Epithelial Structure in Self-Renewal and Differentiation of Neural Progenitors
In this review, we have looked at the role of spindle orientation and epithelial cell structure in neural cell fate determination in cortical neurogenesis, with a focus on the dorsal telencephalon. Currently available knowledge leads us to the view that the self-renewability of neural progenitors in the ventricular zone requires the inheritance of the full epithelial structure—the apical membrane and the basal process—although this alone is not sufficient. How is the inheritance of the full epithelial structure related with the maintenance of self-renewal? The study of a conditional aPKC knockout mouse revealed that a global defect in epithelial polarity does not significantly affect the progenitor cell population or the rate of neuronal production (Imai et al. 2006). This result seems to contradict with the predicted necessity of the epithelial structure for the self-renewability. However, the virus-mediated knockdown of Par3, a major component of the apical complex, induced premature neurogenesis in vivo (Costa et al. 2008). Furthermore, the disruption of cell polarity in sparsely distributed cells in the cerebral cortex causes these cells to differentiate (our unpublished observations), suggesting that the effect of defective cell polarity depends on the density of the affected cells in the ventricular zone. These results are explainable if the epithelial structure is a prerequisite for the ability to self-renew when progenitors are integrated into the pseudostratified neuroepithelium.
Can the ability to self-renew be attributed to a particular part of the epithelial cell structure? The epithelial polarity requires the apical complex as an organizing signal complex (Ohno 2001). It has been shown that loss of Par3 function results in premature neurogenesis, whereas overexpression of Par3 (and Par6) promotes proliferation of progenitors (Costa et al. 2008), suggesting that the apical complex has a role in the self-renewal of apical progenitors. This study also raises the possibility that the apical complex may have an activity to promote self-renewal in a way other than the formation or the maintenance of cell polarity because overexpressed Par6 increases symmetric proliferative divisions without changing a proportion of asymmetric divisions (Costa et al. 2008). On the other hand, the results from the observation of vertical divisions induced by mouse Inscuteable overexpression support the idea that the basal domain of the mother cell is also important in maintaining progenitor properties (Konno et al. 2008). This does not necessarily contradict the work showing that the basal membrane attachment is dispensable for radial glial cell fate (Haubst et al. 2006), as this work did not address the role of basal processes in toto, only that of basal attachment. A simple possibility is that the basal process includes something important for the self-renewal of neural progenitors, such as components promoting cell cycle progression. It may also be that the basal process is necessary to receive extrinsic signals important for the self-renewability. Alternatively, the epithelial structure itself might be important for self-renewal. Indeed, a recent work in zebra fish indicated that a dynactin mutant showed a similar phenotype to those of Notch mutants in neurogenesis (Del Bene et al. 2008), raising the possibility that the microtubule-dependent motility, such as INM, is important to the ability to self-renew. These possibilities are not mutually exclusive, and resolving them will throw light on a central problem of neural development; how the brain itself is shaped.
Acknowledgments
We thank Takaki Miyata, Ayano Kawaguchi, Yoichi Kosodo, Wieland Huttner, and Magdalena Gotz for discussions and Doug Sipp for revising the manuscript. We are also grateful to the organizers of the meeting “cortical development” for providing us with the opportunity to write a review of this field. We apologize if we have not cited all relevant studies and publications. Conflict of Interest: None declared.
References
- Adams RJ. Metaphase spindles rotate in the neuroepithelium of rat cerebral cortex. J Neurosci. 1996;16:7610–7618. doi: 10.1523/JNEUROSCI.16-23-07610.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baye LM, Link BA. Nuclear migration during retinal development. Brain Res. 2008;1192:29–36. doi: 10.1016/j.brainres.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer JB, Lord K, Saunders TL, Pacchioni A, Black C, Lazartigues E, Varner KJ, Gettys TW, Lanier SM. Activator of G protein signaling 3 null mice: I. Unexpected alterations in metabolic and cardiovascular function. Endocrinology. 2008;149:3842–3849. doi: 10.1210/en.2008-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J, Roberts E, Mochida GH, Hampshire DJ, Scott S, Askham JM, Springell K, Mahadevan M, Crow YJ, Markham AF, et al. ASPM is a major determinant of cerebral cortical size. Nat Genet. 2002;32:316–320. doi: 10.1038/ng995. [DOI] [PubMed] [Google Scholar]
- Bowman SK, Neumuller RA, Novatchkova M, Du Q, Knoblich JA. The Drosophila NuMA homolog Mud regulates spindle orientation in asymmetric cell division. Dev Cell. 2006;10:731–742. doi: 10.1016/j.devcel.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Cismowski MJ, Takesono A, Ma C, Lizano JS, Xie X, Fuernkranz H, Lanier SM, Duzic E. Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nat Biotechnol. 1999;17:878–883. doi: 10.1038/12867. [DOI] [PubMed] [Google Scholar]
- Colombo K, Grill SW, Kimple RJ, Willard FS, Siderovski DP, Gonczy P. Translation of polarity cues into asymmetric spindle positioning in Caenorhabditis elegans embryos. Science. 2003;300:1957–1961. doi: 10.1126/science.1084146. [DOI] [PubMed] [Google Scholar]
- Costa MR, Wen G, Lepier A, Schroeder T, Gotz M. Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development. 2008;135:11–22. doi: 10.1242/dev.009951. [DOI] [PubMed] [Google Scholar]
- Das T, Payer B, Cayouette M, Harris WA. In vivo time-lapse imaging of cell divisions during neurogenesis in the developing zebrafish retina. Neuron. 2003;37:597–609. doi: 10.1016/s0896-6273(03)00066-7. [DOI] [PubMed] [Google Scholar]
- Del Bene F, Wehman AM, Link BA, Baier H. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell. 2008;134:1055–1065. doi: 10.1016/j.cell.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Q, Stukenberg PT, Macara IG. A mammalian partner of inscuteable binds NuMA and regulates mitotic spindle organization. Nat Cell Biol. 2001;3:1069–1075. doi: 10.1038/ncb1201-1069. [DOI] [PubMed] [Google Scholar]
- Feng Y, Walsh CA. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron. 2004;44:279–293. doi: 10.1016/j.neuron.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Fish JL, Kosodo Y, Enard W, Paabo S, Huttner WB. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc Natl Acad Sci USA. 2006;103:10438–10443. doi: 10.1073/pnas.0604066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotta M, Ahringer J. Distinct roles for Galpha and Gbetagamma in regulating spindle position and orientation in Caenorhabditis elegans embryos. Nat Cell Biol. 2001;3:297–300. doi: 10.1038/35060092. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci USA. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubst N, Georges-Labouesse E, De Arcangelis A, Mayer U, Gotz M. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development. 2006;133:3245–3254. doi: 10.1242/dev.02486. [DOI] [PubMed] [Google Scholar]
- Haydar TF, Ang E, Jr, Rakic P. Mitotic spindle rotation and mode of cell division in the developing telencephalon. Proc Natl Acad Sci USA. 2003;100:2890–2895. doi: 10.1073/pnas.0437969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzler P, Simpson P. The choice of cell fate in the epidermis of Drosophila. Cell. 1991;64:1083–1092. doi: 10.1016/0092-8674(91)90263-x. [DOI] [PubMed] [Google Scholar]
- Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, Kageyama R. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science. 2002;298:840–843. doi: 10.1126/science.1074560. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Brand M. Asymmetric division and polarity of neuroepithelial cells. Curr Opin Neurobiol. 1997;7:29–39. doi: 10.1016/s0959-4388(97)80117-1. [DOI] [PubMed] [Google Scholar]
- Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–1744. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Ohta N, Hisata K, Raabe T, Matsuzaki F. Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization. Nat Cell Biol. 2006;8:586–593. doi: 10.1038/ncb1409. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A, Ikawa T, Kasukawa T, Ueda HR, Kurimoto K, Saitou M, Matsuzaki F. Single-cell gene profiling defines differential progenitor subclasses in mammalian neurogenesis. Development. 2008;135:3113–3124. doi: 10.1242/dev.022616. [DOI] [PubMed] [Google Scholar]
- Kawaguchi D, Yoshimatsu T, Hozumi K, Gotoh Y. Selection of differentiating cells by different levels of delta-like 1 among neural precursor cells in the developing mouse telencephalon. Development. 2008;135:3849–3858. doi: 10.1242/dev.024570. [DOI] [PubMed] [Google Scholar]
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosodo Y, Toida K, Dubreuil V, Alexandre P, Schenk J, Kiyokage E, Attardo A, Mora-Bermudez F, Arii T, Clarke JD, et al. Cytokinesis of neuroepithelial cells can divide their basal process before anaphase. EMBO J. 2008;27:3151–3163. doi: 10.1038/emboj.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. Autoinhibition with transcriptional delay: a simple mechanism for the zebrafish somitogenesis oscillator. Curr Biol. 2003;13:1398–1408. doi: 10.1016/s0960-9822(03)00534-7. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Cho G, Wen B, Insel PA. Identification and cDNA cloning of a novel human mosaic protein, LGN, based on interaction with G alpha i2. Gene. 1996;181:39–43. doi: 10.1016/s0378-1119(96)00456-8. [DOI] [PubMed] [Google Scholar]
- Morin X, Jaouen F, Durbec P. Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat Neurosci. 2007;10:1440–1448. doi: 10.1038/nn1984. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508:28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai W, Minobe S, Ogawa M, Miyata T. Transformation of pin-like ventricular zone cells into cortical neurons. Neurosci Res. 2007;57:326–329. doi: 10.1016/j.neures.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Ohno S. Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13:641–648. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- Sanada K, Tsai LH. G protein betagamma subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell. 2005;122:119–131. doi: 10.1016/j.cell.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Shevchenko A, Shevchenko A, Knoblich JA. A protein complex containing Inscuteable and the Galpha-binding protein Pins orients asymmetric cell divisions in Drosophila. Curr Biol. 2000;10:353–362. doi: 10.1016/s0960-9822(00)00401-2. [DOI] [PubMed] [Google Scholar]
- Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- Shen Q, Zhong W, Jan YN, Temple S. Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development. 2002;129:4843–4853. doi: 10.1242/dev.129.20.4843. [DOI] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Siller KH, Cabernard C, Doe CQ. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat Cell Biol. 2006;8:594–600. doi: 10.1038/ncb1412. [DOI] [PubMed] [Google Scholar]
- Smart IH. Proliferative characteristics of the ependymal layer during the early development of the mouse neocortex: a pilot study based on recording the number, location and plane of cleavage of mitotic figures. J Anat. 1973;116:67–91. [PMC free article] [PubMed] [Google Scholar]
- Srinivasan DG, Fisk RM, Xu H, van den Heuvel S. A complex of LIN-5 and GPR proteins regulates G protein signaling and spindle function in C. elegans. Genes Dev. 2003;17:1225–1239. doi: 10.1101/gad.1081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CY, Dujardin DL, Faulkner NE, Vallee RB. Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J Cell Biol. 2002;156:959–968. doi: 10.1083/jcb.200109046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesono A, Cismowski MJ, Ribas C, Bernard M, Chung P, Hazard S, 3rd, Duzic E, Lanier SM. Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem. 1999;274:33202–33205. doi: 10.1074/jbc.274.47.33202. [DOI] [PubMed] [Google Scholar]
- Vallee RB, Tsai JW. The cellular roles of the lissencephaly gene LIS1, and what they tell us about brain development. Genes Dev. 2006;20:1384–1393. doi: 10.1101/gad.1417206. [DOI] [PubMed] [Google Scholar]
- Weissman T, Noctor SC, Clinton BK, Honig LS, Kriegstein AR. Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb Cortex. 2003;13:550–559. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- Yingling J, Youn YH, Darling D, Toyo-Oka K, Pramparo T, Hirotsune S, Wynshaw-Boris A. Neuroepithelial stem cell proliferation requires LIS1 for precise spindle orientation and symmetric division. Cell. 2008;132:474–486. doi: 10.1016/j.cell.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- Yu F, Morin X, Cai Y, Yang X, Chia W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell. 2000;100:399–409. doi: 10.1016/s0092-8674(00)80676-5. [DOI] [PubMed] [Google Scholar]
- Yu F, Morin X, Kaushik R, Bahri S, Yang X, Chia W. A mouse homologue of Drosophila pins can asymmetrically localize and substitute for pins function in Drosophila neuroblasts. J Cell Sci. 2003;116:887–896. doi: 10.1242/jcs.00297. [DOI] [PubMed] [Google Scholar]
- Zhong W, Feder JN, Jiang MM, Jan LY, Jan YN. Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron. 1996;17:43–53. doi: 10.1016/s0896-6273(00)80279-2. [DOI] [PubMed] [Google Scholar]
- Zigman M, Cayouette M, Charalambous C, Schleiffer A, Hoeller O, Dunican D, McCudden CR, Firnberg N, Barres BA, Siderovski DP, et al. Mammalian inscuteable regulates spindle orientation and cell fate in the developing retina. Neuron. 2005;48:539–545. doi: 10.1016/j.neuron.2005.09.030. [DOI] [PubMed] [Google Scholar]