Abstract
The purpose of this study was to determine the feasibility of identifying the sentinel lymph nodes (SNs) as well as to evaluate factors that might influence the SN detection rate in patients with cervical cancer of the uterus. Eighty nine patients underwent intracervical injection of 1% isosulfan blue dye at the time of planned radical hysterectomy and lymphadenectomy between January 2003 and December 2003. With the visual detection of lymph nodes that stained blue, SNs were identified and removed separately. Then all patients underwent complete pelvic lymph node dissection and/or para-aortic lymph node dissection. SNs were identified in 51 of 89 (57.3%) patients. The most common site for SN detection was the external iliac area. Metastatic nodes were detected in 21 of 89 (23.5%) patients. One false negative SN was obtained. Successful SN detection was more likely in patients younger than 50 yr (p=0.02) and with a history of preoperative conization (p=0.05). However, stage, histological type, surgical procedure and neoadjuvant chemotherapy showed no significant difference for SN detection rate. Therefore, the identification of SNs with isosulfan blue dye is feasible and safe. The SN detection rate was high in patients younger than 50 yr or with a history of preoperative conization.
Keywords: Sentinel Lymph Node Biopsy, Uterine Cervical Neoplasms, iso-sulfan blue, Conization
INTRODUCTION
Radical hysterectomy and pelvic lymphadenectomy has been widely accepted as the primary surgical modality for the treatment of early stage cervical cancer. Pelvic node involvement, which is known as one of the most important prognostic factors, is detected in only 10-35% of patients with early stage cervical cancer (1-3). Most pelvic lymph nodes are dissected unnecessarily as the only method for detecting metastasis. Pelvic lymphadenectomy can cause complications such as vessel injury, nerve injury, lymphocyst and adhesion formation (4). An accurate method, that could reflect the status of the entire lymphatic area, without removing all lymph nodes would spare patients the loss of unaffected lymph nodes and give the chance of radical hysterectomy without lymphadenectomy; whereas patients with affected nodes would be treated with chemoradiation.
The sentinel lymph node (SN) is the first node draining the lymphatic flow from a primary tumor, and represents the status of lymphatic spread (5). SN biopsy is a standard treatment modality in patients with cutaneous melanoma (6). If this concept of SN biopsy could be applied to women with cervical cancer, pelvic lymphadenectomy may not be needed in patients who have SN results with no tumor cells on frozen section at surgery. Previous studies have used different techniques to identify the SN, which must be not only accurate and reliable but also safe and easy to assess (5-17). One of the widely used materials for detection, has been blue dye; this method has been shown to be easy for physicians and safe for patients; however, the SN detection rate is low and anaphylactic reactions have been reported.
We used isosulfan blue dye, in relatively small amounts (1% lymphazurin 2 mL) compared to other studies, to determine the feasibility and SN detection rate in patients with early cervical cancer.
MATERIALS AND METHODS
Between January 2003 and December 2003 eighty nine patients underwent radical hysterectomy and pelvic lymphadenectomy, primarily by laparotomy or laparoscopy, for early stage cervical cancer all of whom were enrolled.
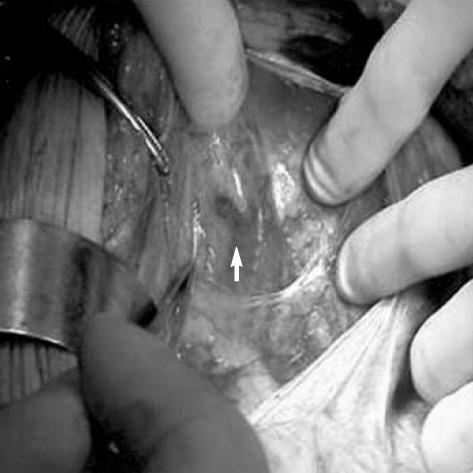
After the induction of general anesthesia, the uterine cervix was exposed with a speculum in the dorsolithotomy position. Using a syringe with a 25-guage spinal needle, 1 mL of isosulfan blue dye was injected into the cervix at the three and nine o'clock orientation. After injection of the dye, disinfection was performed and subsequently the patient was toweled. The abdominal cavity and the retroperitoneum were opened, taking care not to disturb the vessels and lymphatics. Under direct visualization, the blue colored lymphatic channels were identified (Fig. 1). All blue colored nodes were considered SNs. All surgically removed lymph nodes including SNs were sent for frozen section. If the pelvic lymph nodes were negative, radical hysterectomy was performed. When tumor cell was found in the frozen section, hysterectomy was not performed but rather para-aortic lymphadenectomy was done to determine the boundaries for radiation after surgery. Surgical specimens were examined using routine hematoxylin and eosin staining.
Fig. 1.
Identification of a sentinel lymph node (arrow) stained with blue dye after entering the retroperitoneal space.
To determine the important factors influencing the SN detection rate, we dichotomized the variables (patient age, FIGO stage, histopathology of tumor, preoperative conization and neoadjuvant chemotherapy and status of SN) and applied the chi-square test in dBSTAT 4.0.
RESULTS
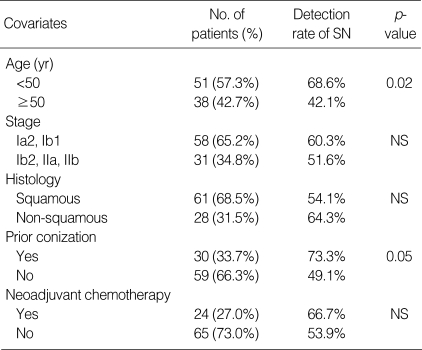
There was no adverse reaction associated with the isosulfan dye injection such as: urticaria, edema, circulatory collapse or respiratory problems. The mean age of patients was 48.3 yr (range 30-78). FIGO stage Ib1 was the most common stage (54/89, 60.6%). The most common histological type of tumor was a squamous cell carcinoma (61/89, 68.5%). Preoperative conization was performed in 30 patients (33.7%) and neoadjuvant chemotherapy in 24 patients (27.0%) (Table 1).
Table 1.
Clinical characteristics
*Adenosquamous and clear cell type.
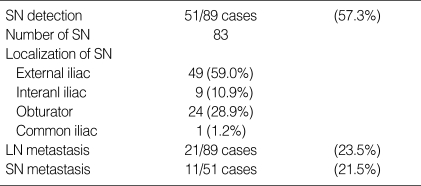
Out of 89 patients, 83 SNs were detected in 51 (57.3%) patients. The most common site for the SN was the external iliac area; no SN was found in the para-aortic area. In 21 (23.5%) patients, surgically removed lymph nodes were pathologically proven to be positive. Among 51 patients in whom SNs were detected, 11 (21.5%) patients were positive for tumor cells (Table 2).
Table 2.
The localization and status of the sentinel lymph node
SN, Sentinel lymph node; LN, Lymph node.
The patients were grouped according to age (<50 yr vs. ≥50), stage (below Ib1 vs. above Ib2), pathology (squamous cell carcinoma vs. others), preoperative conization and neoadjuvant chemotherapy. The detection rate of SNs was compared among the dichotomized variables, and was found to be high in patients younger than 50 yr or with a history of preoperative conization (Table 3).
Table 3.
Factors affecting the detection rate of SNs
SN, Sentinel lymph node; NS, not significant.
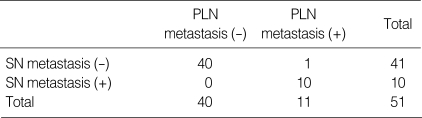
The results of the frozen section were identical to those of the permanent staining. The positive predictive value (PPV, 100%, 10/10), sensitivity (90.9%, 10/11), and specificity (100%, 40/40) were assessed by comparing the status of SNs with the status of all lymph nodes. The negative predictive value (NPV) was 97.6% (40/41); this was because a non-colored lymph node, from only one patient, contained tumor cells with a negative SN (Table 4).
Table 4.
Relationship between the pathology of SNs and other pelvic lymph nodes
SN, Sentinel lymph node; PLN, Pelvic lymph node.
The SN detection rate was 53.3% in 43 patients recruited during the first six months and 60.9% in 46 patients recruited during the last six months of the study period. The difference was not statistically significant (Table 5).
Table 5.
Comparison of sentinel lymph node (SN) detection rate between the first six months and the last six months
NS, Not significant.
DISCUSSION
Since 1977 when Cabanas introduced the SN concept, in the treatment of penile carcinoma, SN detection has been accepted as a standard diagnostic method for breast cancer and melanoma. It has also been applied to gynecologic cancers (5, 7-15).
Dye application to map a SN does not require new equipment and is relatively easy to learn. However, there are several drawbacks including a low detection rate and anaphylactic reaction. Multiple factors such as dosage, depth of dye injection, type of marker used, tumor size and elapsed time between administration of the dye and detection can influence the rate of SN detection (9-13, 21-23). Echt et al., pioneers in SN mapping for cervical cancer, injected 2 mL of lymphazurin and detected the SN in 15.4% of patients (9). Dargent et al. succeeded in detecting the SN in 85.5% (59/69) of patients and confirmed that the detection rate improved with increased amounts of dye (50%:<1.5 mL, 83%:2 mL, 90%:4 mL) (11).
We injected 1% isosulfan blue 2 mL (Lymphazurin) at three and nine o'clock orientations of the cervix (1 mL per each site) and detected SN in 57.3% (51/89) of patients. The low detection rate might be due to the small amount of dye used to prevent the occurrence of an anaphylactic reaction.
Previous studies reported that an anaphylactic reaction developed in 1-2% of breast cancer or melanoma patients and showed a dose-response relationship (16, 17). In patients with cervical cancer, 10% (1/10) of patients injected with high density patent blue dye (2.5%, 0.2 mL) have been reported to develop an anaphylactic reaction (15). With Lymphazurin (1% isosulfan blue) 4 mL, 5% (1/20) of patients had circulatory collapse and 15% (3/20) experienced oxygen desaturation measured by pulse oximetry (18). In our study, only temporary low oxygen saturation was noted in a few patients without clinical consequence.
Our results have confirmed the results of previous studies in which SNs were detected at the external iliac, obturator, internal iliac, and common iliac nodes in descending order (19). Some authors have found SNs in the para-aortic area but only concomitantly with pelvic nodes (10). We did not detect SN in the extra-pelvic area.
SN detection in patients with cervical cancer, even if suspected metastasis occurs through multiple routes to various areas such as the locations reported in our results, can be very useful. However, the false negative (negative SN in the presence of positive non-SN nodes) rate must be very low, and the SN easy to detect, then perhaps SN study can be introduced for cervical cancer patients (11, 12).
We observed that one patient had metastatic nodes out of 41 patients with visible negative SNs. The negative predictive value was therefore 97.5% (40/41) in our study. Previous studies have reported only four false negative cases out of 447 patients involved in SN mapping in patients with cervical cancer. In other words the false negative rate was about 1% (20).
In our study, SN detection rate was high only in patients over 50 yr or in those with a history of preoperative conization. Stage, histologic type of tumor or performance of neoadjuvant chemotherapy was not associated with SN detection. Dargent et al. reported that the SN detection rate, of patients with preoperative conization, was 74%; this was lower than that of patients without preoperative conization (86%); they also reported that detection failure was not influenced by age, stage, BMI or pathology (24).
O'Boyle et al. reported that care must be taken to estimate the remnant volume of the cervix, and that it was easy to cause intraperitoneal or rectal spillage (13). Preoperative conization had no effect on the detection rate in their study. In prior studies, investigators were more concerned about the residual cervix after conization as a volumetric structure rather than as a histologic tissue where dye flow might change (11-13). Our data may stimulate future study on the impact of preoperatvie conization; which may be a more important factor than the dye injection method.
In previous studies on patients with cervical cancer, there has been no correlation noted between the patient's age and the SN detection rate. The cause of a low detection rate in patients over 50 yr may result from decreased lymphatic flow resulting from the aging process. Age and physician's experience are known to be important factors influencing the SN detection rate in breast cancer patients. As women get older, fatty tissue in the mammary gland increases and lymphatic flow lessens. An increase in the age of one year or one unit of BMI decreases the odds of success for SN detection by 5% (21).
The learning curve for laparoscopic retrieval of SN showed that the average time from injection to retrieval of the first node was shorter with time (24). Attending physicians with experience in lymphatic mapping of vulvar cancer, a short learning curve has been observed (12). In our study, laparotomic or laparoscopic SN detection was performed according to the preference of surgeons who had experience with radical surgery. The detection rate was similar between the first 43 patients and the last 46 patients, which suggested the need for a very short learning period. If physicians are accustomed to radical surgery, lymphatic mapping with blue dye and detection of SNs are relatively easy to learn.
Limitations of this study include the following: we did not consider time elapsed, tumor size and depth of injection; which were important factors noted in previous studies. This could have lowered our detection rate.
In conclusion, SN detection, with a small amount of isosulfan blue dye (1% Lymphazurin 2 mL) is feasible and safe; however, our results show a low detection rate. Age and history of preoperative conization are important factors influencing the success rate of SN detection; which was higher in patients younger than 50 yr or with a history of preoperative conization.
References
- 1.Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, Fujimoto T, Oikawa M, Fujino T, Fujimoto S. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer. 1999;85:1547–1554. doi: 10.1002/(sici)1097-0142(19990401)85:7<1547::aid-cncr16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti-Panici P, Maneschi F, D'Andrea G, Cutillo G, Rabitti C, Congiu M, Coronetta F, Capelli A. Early cervical carcinoma: the natural history of lymph node involvement redefined on the basis of thorough parametrectomy and giant section study. Cancer. 2000;88:2267–2274. [PubMed] [Google Scholar]
- 3.Park CT, Seong SJ, Kim TJ, Lim KT, Chung HW, Park IS, Shim JU, Lee KH. Clinico-pathologic profiles and 5-year survival rate of 2209 patients with invasive cancer of the uterine cervix. Korean J Obstet Gynecol. 2003;46:1404–1410. [Google Scholar]
- 4.Beyersdorff D, Bahnsen J, Frischbier HJ. Nodal involvement in cancer of the uterine cervix: value of lymphography and MRI. Eur J Gynaecol Oncol. 1995;16:274–277. [PubMed] [Google Scholar]
- 5.Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 6.Statius Muller MG, van Leeuwen PA, Borgstein PJ, Pijpers R, Meijer S. The sentinel node procedure in cutaneous melanoma. An overviex of 6 years experience. Eur J Nucl Med. 1996;26(Supple 4):S20–S25. doi: 10.1007/pl00014791. [DOI] [PubMed] [Google Scholar]
- 7.Cabanas RM. An approach for the treatment of penile carcinoma. Cancer. 1977;39:456–466. doi: 10.1002/1097-0142(197702)39:2<456::aid-cncr2820390214>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, Feldman S, Kusminsky R, Gadd M, Kuhn J, Harlow S, Beitsch P. The sentinel node in breast cancer. N Engl J Med. 1998;339:941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 9.Echt ML, Finan MA, Hoffman MS, Kline RC, Roberts WS, Fiorica JV. Detection of sentinel lymph nodes with Lymphazurin in cervical, uterine and vulvar malignancies. South Med J. 1999;92:204–208. doi: 10.1097/00007611-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Malur S, Krause N, Kohler C, Schneider A. Sentinel lymph node detection in patients with cervical cancer. Gynecol Oncol. 2001;80:254–257. doi: 10.1006/gyno.2000.6041. [DOI] [PubMed] [Google Scholar]
- 11.Dargent D, Martin X, Mathevet P. Laparoscopic assessment of the sentinel lymph node in early stage cervical cancer. Gynecol Oncol. 2000;79:411–415. doi: 10.1006/gyno.2000.5999. [DOI] [PubMed] [Google Scholar]
- 12.Levenback C, Coleman RL, Burke TW, Lin WM, Erdman W, Deavers M, Delpassand ES. Lymphatic mapping and sentinel node identification in patient with cervix cancer undergoing radical hysterectomy and pelvic lymphadenectomy. J Clin Oncol. 2002;20:688–693. doi: 10.1200/JCO.2002.20.3.688. [DOI] [PubMed] [Google Scholar]
- 13.O'Boyle JD, Coleman RL, Bernstein SG, Lifshitz S, Muller CY, Miller DS. Intraoperative lymphatic mapping in cervix cancer patients undergoing radical hysterectomy: A pilot study. Gynecol Oncol. 2000;79:238–243. doi: 10.1006/gyno.2000.5930. [DOI] [PubMed] [Google Scholar]
- 14.Rhim CC, Park JS, Bae SN, Namkoong SE. Sentinel node biopsy as an indicator for pelvic nodes dissection in early cervical cancer. J Korean Med Sci. 2002;17:507–511. doi: 10.3346/jkms.2002.17.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verheijen RH, Pijpers R, van Diest PJ, Burger CW, Buist MR, Kenemans P. Sentinel node detection in cervical cancer. Obstet Gynecol. 2000;96:135–138. doi: 10.1016/s0029-7844(00)00831-0. [DOI] [PubMed] [Google Scholar]
- 16.Leong SP, Donegan E, Heffernon W, Dean S, Katz JA. Adverse reactions to isosulfan blue during selective sentinel lymph node dissections in melanoma. Ann Surg Oncol. 2000;7:361–366. doi: 10.1007/s10434-000-0361-x. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery LL, Thorne AC, Van Zee KJ, Fey J, Heerdt AS, Gemignani M, Port E, Petrek J, Cody HS, 3rd, Borgen PI. Isosulfan blue dye reactions during sentinel lymph node mapping for breast cancer. Anesth Analg. 2002;95:385–388. doi: 10.1097/00000539-200208000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Rhim CC, Namkoong SE. A case of anaphylactic reactions to isosulfan blue dye in a cervical cancer patient. Korean J Obstet Gynecol. 2003;46:484–487. [Google Scholar]
- 19.Buist MR, Pijpers R, van Lingen A, van Diest PJ, Dijkstra Jan, Kenemans P, Verheijen RH. Laparoscopic detection of sentinel lymph nodes followed by lymph node dissection in patients with early stage cervical cancer. Gynecol Oncol. 2003;90:290–296. doi: 10.1016/s0090-8258(03)00277-4. [DOI] [PubMed] [Google Scholar]
- 20.Barranger E, Darai E. Sentinel lymph node biopsy is not accurate in predicting lymph node status for patients with cervical carcinoma. Cancer. 2004;101:1919–1920. doi: 10.1002/cncr.20589. [DOI] [PubMed] [Google Scholar]
- 21.Cox CE, Dupont E, Whitehead GF, Ebert MD, Nguyen K, Peltz ES, Peckham P, Cantor A, Reintgen DS. Age and body mass index may increase the chance of failure in sentinel lymph node biopsy for women with breast cancer. Breast J. 2002;8:88–91. doi: 10.1046/j.1524-4741.2002.08203.x. [DOI] [PubMed] [Google Scholar]
- 22.Rob L, Strnad P, Robova H, Charvat M, Pluta M, Schlegerova D, Hrehorcak M. Study of lymphatic mapping and sentinel node identification in early stage cervical cancer. Gynecol Oncol. 2005;98:281–288. doi: 10.1016/j.ygyno.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Wydra D, Sawicki S, Emerich J, Romanowicz G. The influence of depth of marker administration on sentinel node detection in cervical cancer. Nucl Med Rev Cent East Eur. 2003;6:131–133. [PubMed] [Google Scholar]
- 24.Dargent D, Enria R. Laparoscopic assessment of the sentinel lymph nodes in early cervical cancer. Technique-preliminary results and future developments. Crit Rev Oncol Hematol. 2003;48:305–310. doi: 10.1016/s1040-8428(03)00129-x. [DOI] [PubMed] [Google Scholar]