Abstract
Cervical cancer is almost invariably associated with infection by human papillomavirus. It is believed that the host genetic factors such as inflammation-induced cytokines may play a role in cervical carcinogenesis. The IL1B gene, encoding IL-1β cytokine, contains several single nucleotide polymorphisms. One of them which is in the positions -511 (C-T) related with promoter region has been associated with increased IL-1β production and with increased risk of developing a number of inflammatory diseases and gastric carcinoma. We assessed the association between the IL1B -511 polymorphism and cervical cancer risk in a hospital-based case-control study among 546 Korean women (182 cases; 364 age-matched controls). The allele frequencies of the case subjects (C, 0.42; T, 0.58) were not significantly different from those of control subjects (C, 0.43; T, 0.57). Control subjects were in Hardy-Weinberg equilibrium. The carriers with -511 C/T or T/T genotypes were at higher risk of cervical cancer with odds ratio of 2.42 (95% CI 1.31-4.46, p<0.005). However, there was no difference of cervical cancer risk between C/T heterologous genotypes and T/T homologous genotypes. In conclusion, in Korean population, IL1B -511 C/C genotypes were significantly associated with a decreased risk of cervical cancer.
Keywords: Uterine Cervical Neoplasms; Polymorphism, Genetic; Interleukin-1beta; Disease Susceptibility; Case-Control Studies
INTRODUCTION
Cervical cancer is the second major cause of cancer-related mortality in women worldwide and accounts for 250,000 deaths each year (1). It is well established that the infection with high-risk types of human papillomavirus (HPV) plays a central role in the pathogenesis of invasive cervical cancer (2). Although many women are infected with high-risk types of HPV, only a subset of infected women develops cervical cancer, suggesting that other cofactors including host genetic factors must be present for the development of malignancy.
Chronic inflammation has been shown to be an important risk factor for a variety of epithelial cancers (3). Cytokines, as the products of host response to inflammation, play an important role in the defense against viral infections. In cervical cancers, a number of previous reports suggested that chronic inflammation is associated with the precancerous intraepithelial lesion and cancer of uterine cervix (4-6).
The interleukin-1 family of cytokines consists of several members including ineterleukin-1 alpha, interleukin-1 beta and interleukin-1 receptor antagonist. The genes for these cytokines are clustered within a 430-kb segment on human chromosome 2. These cytokines are produced by several cell types and have multiple biological effects. Interleukin-1 beta is a pro-inflammatory cytokine mainly produced by blood monocytes and tissue macrophages and has been implicated in mediating both acute and chronic inflammation (7). Recently, a common polymorphic allele of the regulatory region of the IL1B gene was found to be associated with increased IL-1 production (8). Also, the polymorphism in IL1B was associated with various human cancers (8-11). Since there were several reports supporting the positive association with increased IL1B secretion and cervical cancer risk (12, 13), we hypothesized that an individual with a IL1B genotype producing more IL1B might have an increased risk of cervical cancer. The C>T polymorphism in IL1B -511 site has been correlated with increased intracellular IL1B levels in the previous reports (14). Here we report results from a hospital based case-control study examining the association of IL1B -511 C>T polymorphisms with the risk of cervical cancer.
MATERIALS AND METHODS
Subjects
Case subjects were selected from among cervical cancer patients treated between April 1996 and July 2002 at the Seoul National University Hospital. A total of 182 patients with confirmed cervical squamous cell carcinoma consented to participate in the study and provided a blood specimen. Age-matched (1:2) control subjects were comprised of 364 healthy, unrelated, cancer-free subjects recruited from visitors who attended a comprehensive screening clinic at the same institution and agreed to participate in this study. Informed consent was received from all the cases and controls. All case and control subjects were Korean, and the Institutional Review Board of Seoul National University Hospital approved the protocol used in this study.
Genotyping
All genotyping of 182 cases and 364 control samples was performed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). In brief, IL1B -511 C>T polymorphism was distinguished by PCR-RFLP, using the known primer pairs (forward primers 5'-GCCTGAACCCTGCATACCGT; reverse primers 5'GCCAATAGCCCTCCCTGTCT-3') and restriction enzyme AvaI. Amplification was performed in a volume of 25 µL, containing 2.5 µL of 10×PCR buffer (100 mM Tris-HCI, 15 mM MgCl2, and 500 mM KCl, pH 8.3), 200 nM each dNTP (Roche Diagnostics Korea, Seoul, Korea), 1 µM each primer (Bioneer, Daejeon, Korea), 1 U Taq DNA polymerase (Roche Diagnostics Korea, or Takara Shuzo, Otsu, Japan), and 100 ng of genomic DNA. The thermocycling conditions were as follows: 95℃ for 5 min; then 35 cycles of 95℃ for 30 sec, 58-60℃ for 30 sec, and 72℃ for 1 min; then 72℃ for 10 min. Fifteen microliters of the reaction mixture was treated with 5 U of AvaI (NE Biolabs, Beverly, MA, U.S.A.) at 37℃ for 12 hr and subsequently analyzed on 3% agarose (2% Nusieve [Bio-Whittaker Molecular Applications, Rockland, ME, U.S.A.] and 1% agarose) gel.
Statistical analysis
Hardy-Weinberg equilibrium analyses were performed to compare observed and expected genotype frequencies using the chi-square test (D.F.=1). Allele frequency differences between cases and controls were analyzed using the Pearson chi-square test. Cervical cancer risk was estimated by odds ratios (ORs) and 95% confidence intervals (CIs) using conditional logistic regression model after adjustment for age.
RESULTS
In Korean population, we observed that the allelic frequencies of the 182 case subjects (C, 0.42; T, 0.58) were not significantly different from those of the 364 control subjects (C, 0.43; T, 0.57). The allelic frequencies of control subjects were not statistically different from those reported for Korean populations by other investigators (9). The alleles were in Hardy-Weinberg equilibrium only in control subjects.
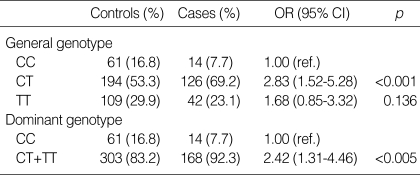
We found that carriers of the T allele had a significantly increased risk of cervical cancer. The frequency distribution of the different genotypes for the IL1B -511 C>T polymorphism is shown in Table 1. The CC genotype was less frequent among the case subjects than among the control subjects (7.7% and 16.8%, respectively). With the use of the chi-square test, we found the significant difference in genotype frequencies between case and control subjects (p<0.001).
Table 1.
IL1B -511 polymorphism and the risk of cervical cancer in Korean women
OR, odds ratio; CI, confidence interval. OR was calculated by logistic regression analysis adjusted for the age.
Using the CC genotype as the reference genotype, we performed logistic regression analysis with adjustment for age variable. The CT genotype was associated with significantly elevated OR of 2.83 (95% CI=1.52 to 5.28, p<0.001). Also, the TT genotype was associated with elevated OR of 1.68 (95% CI=0.85 to 3.32, p=0.136). When we combined CT and TT genotypes together, we found that CT and TT genotypes were associated with OR of 2.42 (95% CI=1.31 to 4.46, p<0.005).
DISCUSSION
Here we reported that -511 polymorphism in IL1B gene was associated with the risk of cervical cancer. Our findings in this hospital-based case-control study suggest that the carriers of -511 T allele may be at increased risk of developing cervical cancer. Our results support the previous hypothesis that -511 T allele is associated with increased production of IL-1β, and that IL-1β may play a role as host factors promoting cervical carcinogenesis.
Since it is assumed that the host immune system is important in the surveillance of HPV-related cervical neoplasia, cytokines, including IL-1β, have been frequently correlated to a risk of cervical cancer. The level of the IL-1β was increased in the cervicovaginal washings of patients with cervical cancer (13). Individuals with high and intermediate IL-1β secretor phenotypes may be more susceptible to lower grade lesions rather than high grade lesion or cervical carcinoma (12). Elevated vaginal lavage IL-1β was associated with a high odds ratio of cervical dysplasia (15). These evidences, including the results of this study, suggest that IL-1β may be involved in early step of cervical carcinogenesis and that individual difference of IL-1β secretion may affect individual susceptibility to cervical cancer progression.
The polymorphism of IL1B gene was reported to be associated with various diseases including cancer, but it is most intensively studied in gastric cancer. El-Omar et al. have recently reported that proinflammatory genotypes of the interleukin-1 gene cluster (IL1B -511/-31 and IL-1RN*2/*2) were associated with increased risk of gastric cancer and its presumptive precursors, gastric atrophy and hypochlorhydria, in white populations from Poland and Scotland (8). Their results contained data that IL1B -511 C>T polymorphism was associated with risk of gastric cancer. While explaining this, they addressed that there were no differences in binding activity between IL1B -511 genotypes, indicating that the effect of IL1B -511 polymorphism may be mediated by linkage disequilibrium with the TATA box polymorphism. This may be possible explanation of our finding, no genedosage relationship between -511 genotypes. In consistence with our data, another recent study reported that IL1B -511 CT heterozygous genotype was the main risk of intestinal type gastric cancer in Korean population (9).
Since our results is the first report about the association between IL1B -511 C>T polymorphism and the risk of cervical cancer, we could not compare our results with other data. However, there were several previous reports suggesting the possible association between cervical cancer risk and the polymorphism of other cytokines or cytokine receptors, such as TNF-alpha, interleukin 10, and interleukin 1 receptor antagonist (16-21). Therefore, it can be speculated that functional variation of inflammatory cytokine may influence on the individual susceptibility of cervical cancer.
Degradation of the p53 gene by oncogenic HPV E6 protein is the most well known carcinogenic mechanism in human cervical cancer. Recent findings indicate an increased p53 mutation load or altered p53 protein function in a number of inflammatory diseases (22). It has been shown that, in rats, intratracheal instillation of IL-1 caused hydrogen peroxide production in lung tissue, initiated neutrophil influx and stimulated their release of reactive oxygen species (23). Therefore, it can be speculated that reactive free radicals produced by inflammatory cells may cause DNA damage in epithelial cells. Inflammatory cytokines have also been shown to induce DNA damage and inhibit DNA repair in vitro (24). In addition, IL-1β has been shown to reduce apoptosis by changing the ratio of BCL-2/BAX proteins (25). Therefore, a higher production of IL-1β may lead to increased p53 mutation load, and the increased level of IL-1β may play a role not only in HPV-related cervical carcinogenesis but also in HPV-non-related cervical carcinogenesis.
The limitations of the present study are as follows. First, the present study is hospital-based and retrospective in nature. Therefore, it cannot be free from any selection bias. Second, our sample size is so limited that it has not enough statistical power to exclude the existence of gene-dosage relationship. Third, the various epidemiologic risk factors of cervical cancer, such as smoking, alcohol intake, diet, or sexual behavior, were not included for analysis. Fourth, since our study did not include all clustered polymorphic site of IL1B and associated genes, the haplotype analysis could not be done. So, it would be worthwhile to perform further large-scale population-based study including the analysis of various clustered polymorphisms. It also should be noted that the risk of cancer caused by foreign pathogen such as virus or bacteria was repeatedly reported by numerous study (8, 26). Therefore, it can be speculated that increased IL-1β production may be associated with host genetic factor defending from foreign carcinogenic pathogen.
In conclusion, in Korean population, IL1B -511 CC genotype was significantly associated with decreased risk of cervical cancer. This relationship supports the idea that polymorphism of inflammatory response genes may be host genetic susceptibility to cervical cancer. IL1B polymorphism should be considered as candidate genetic factor in future study elucidating the genetic risk of cervical cancer.
ACKNOWLEDGEMENT
The authors wish to thank Dr. Yookyung Lee for manuscript preparation.
Footnotes
This study was supported partly by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (0412-CR01-0704-0001) and partly by grant from the CRI Research Fund, Seoul National University (CRI-06-03).
References
- 1.Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 2.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castle PE, Hillier SL, Rabe LK, Hildesheim A, Herrero R, Bratti MC, Sherman ME, Burk RD, Rodriguez AC, Alfaro M, Hutchinson ML, Morales J, Schiffman M. An association of cervical inflammation with high-grade cervical neoplasia in women infected with oncogenic human papillomavirus (HPV) Cancer Epidemiol Biomarkers Prev. 2001;10:1021–1027. [PubMed] [Google Scholar]
- 5.Smith JS, Herrero R, Bosetti C, Munoz N, Bosch FX, Eluf-Neto J, Castellsague X, Meijer CJ, Van den Brule AJ, Franceschi S, Ashley R. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J Natl Cancer Inst. 2002;94:1604–1613. doi: 10.1093/jnci/94.21.1604. [DOI] [PubMed] [Google Scholar]
- 6.Schwebke JR, Zajackowski ME. Effect of concurrent lower genital tract infections on cervical cancer screening. Genitourin Med. 1997;73:383–386. doi: 10.1136/sti.73.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bird S, Zou J, Wang T, Munday B, Cunningham C, Secombes CJ. Evolution of interleukin-1beta. Cytokine Growth Factor Rev. 2002;13:483–502. doi: 10.1016/s1359-6101(02)00028-x. [DOI] [PubMed] [Google Scholar]
- 8.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF, Jr, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 9.Lee KA, Ki CS, Kim HJ, Sohn KM, Kim JW, Kang WK, Rhee JC, Song SY, Sohn TS. Novel interleukin 1 beta polymorphism increased the risk of gastric cancer in a Korean population. J Gastroenterol. 2004;39:429–433. doi: 10.1007/s00535-003-1315-4. [DOI] [PubMed] [Google Scholar]
- 10.Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ, Carneiro F, Sobrinho-Simoes M. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680–1687. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 11.Zienolddiny S, Ryberg D, Maggini V, Skaug V, Canzian F, Haugen A. Polymorphisms of the interleukin-1 beta gene are associated with increased risk of non-small cell lung cancer. Int J Cancer. 2004;109:353–356. doi: 10.1002/ijc.11695. [DOI] [PubMed] [Google Scholar]
- 12.Majeed GS, Glew S, Bidwell J. An association between LSIL and the high secretor phenotype of IL-1beta. Gynecol Oncol. 1999;73:359–361. doi: 10.1006/gyno.1999.5366. [DOI] [PubMed] [Google Scholar]
- 13.Tjiong MY, van der Vange N, ter Schegget JS, Burger MP, ten Kate FW, Out TA. Cytokines in cervicovaginal washing fluid from patients with cervical neoplasia. Cytokine. 2001;14:357–360. doi: 10.1006/cyto.2001.0909. [DOI] [PubMed] [Google Scholar]
- 14.Hall SK, Perregaux DG, Gabel CA, Woodworth T, Durham LK, Huizinga TW, Breedveld FC, Seymour AB. Correlation of polymorphic variation in the promoter region of the interleukin-1 beta gene with secretion of interleukin-1 beta protein. Arthritis Rheum. 2004;50:1976–1983. doi: 10.1002/art.20310. [DOI] [PubMed] [Google Scholar]
- 15.Behbakht K, Friedman J, Heimler I, Aroutcheva A, Simoes J, Faro S. Role of the vaginal microbiological ecosystem and cytokine profile in the promotion of cervical dysplasia: a case-control study. Infect Dis Obstet Gynecol. 2002;10:181–186. doi: 10.1155/S1064744902000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roh JW, Kim MH, Seo SS, Kim SH, Kim JW, Park NH, Song YS, Park SY, Kang SB, Lee HP. Interleukin-10 promoter polymorphisms and cervical cancer risk in Korean women. Cancer Lett. 2002;184:57–63. doi: 10.1016/s0304-3835(02)00193-3. [DOI] [PubMed] [Google Scholar]
- 17.Stanczuk GA, Sibanda EN, Perrey C, Chirara M, Pravica V, Hutchinson IV, Tswana SA. Cancer of the uterine cervix may be significantly associated with a gene polymorphism coding for increased IL-10 production. Int J Cancer. 2001;94:792–794. doi: 10.1002/ijc.1543. [DOI] [PubMed] [Google Scholar]
- 18.Mustea A, Sehouli J, Konsgen D, Stengel D, Sofroni D, Lichtenegger W. Interleukin 1 receptor antagonist (IL-1RA) polymorphism in women with cervical cancer. Anticancer Res. 2003;23:1099–1102. [PubMed] [Google Scholar]
- 19.Stanczuk GA, Sibanda EN, Tswana SA, Bergstrom S. Polymorphism at the -308-promoter position of the tumor necrosis factor-alpha (TNF-alpha) gene and cervical cancer. Int J Gynecol Cancer. 2003;13:148–153. doi: 10.1046/j.1525-1438.2003.13046.x. [DOI] [PubMed] [Google Scholar]
- 20.Kirkpatrick A, Bidwell J, van den Brule AJ, Meijer CJ, Pawade J, Glew S. TNFalpha polymorphism frequencies in HPV-associated cervical dysplasia. Gynecol Oncol. 2004;92:675–679. doi: 10.1016/j.ygyno.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Deshpande A, Nolan JP, White PS, Valdez YE, Hunt WC, Peyton CL, Wheeler CM. TNF-alpha promoter polymorphisms and susceptibility to human papillomavirus 16-associated cervical cancer. J Infect Dis. 2005;191:969–976. doi: 10.1086/427826. [DOI] [PubMed] [Google Scholar]
- 22.Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, Shields PG, Ham AJ, Swenberg JA, Marrogi AJ, Harris CC. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- 23.Hybertson BM, Lee YM, Cho HG, Cho OJ, Repine JE. Alveolar type II cell abnormalities and peroxide formation in lungs of rats given IL-1 intratracheally. Inflammation. 2000;24:289–303. doi: 10.1023/a:1007092529261. [DOI] [PubMed] [Google Scholar]
- 24.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- 25.Simonart T, Van Vooren JP. Interleukin-1 beta increases the BCL-2/BAX ratio in Kaposi's sarcoma cells. Cytokine. 2002;19:259–266. doi: 10.1006/cyto.2002.1964. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Kato N, Hoshida Y, Yoshida H, Taniguchi H, Goto T, Moriyama M, Otsuka M, Shiina S, Shiratori Y, Ito Y, Omata M. Interleukin-1beta gene polymorphisms associated with hepatocellular carcinoma in hepatitis C virus infection. Hepatology. 2003;37:65–71. doi: 10.1053/jhep.2003.50017. [DOI] [PubMed] [Google Scholar]