Abstract
The in vitro antibacterial activities of oral cephem antibiotics and ketolide telithromycin against major respiratory pathogens possessing β-lactam-resistant mutations (within the pbp gene) and/or macrolide-resistant genes (erm and mef) were examined in clinical isolates collected at 66 institutes in all over the Japan between 2002 and 2003. Telithromycin showed the strongest antibacterial activity against methicillin-susceptible Staphylococcus aureus strains with and without macrolide-resistant genes, such as ermA or ermC gene. All the cephem antibiotics showed potent antibacterial activity against Streptococcus pyogenes, with minimum inhibitory concentrations (MICs) of 0.015 mg/L or lower. Cefdinir had a much higher MIC90 against genotypic penicillin-resistant Streptococcus pneumoniae (gPRSP) than cefditoren and cefcapene (8 mg/L cefdinir vs. 1 mg/L cefditoren and cefcapene). The majority of gPRSP harbored either ermB or mefA, and the antibacterial activity of telithromycin against these strains was decreased however some susceptibility was still sustained. Cefditoren exerted the strongest antibacterial activity against β-lactamase-negative ampicillin-resistant Haemophilus influenzae, with an MIC90 of 0.5 mg/L. These results underline the importance of checking the susceptibility and selecting an appropriate antibiotic against target pathogens.
Keywords: cefditoren, telithromycin, Microbial Sensitivity Tests, Minimum Inhibitory Concentration, beta-Lactams
INTRODUCTION
Methicillin-susceptible Staphylococcus aureus (MSSA), Streptococcus pyogenes, Streptococcus pneumoniae and Haemophilus influenzae are major causative pathogens of respiratory infectious disease and established causes of pneumonia, otitis media and paranasal sinusitis. In Japan and other Asian countries, S. pneumoniae has been rapidly developing β-lactam resistance by amino-acid substitutions in penicillin-binding proteins (PBPs). Isolates of these new strains also frequently show resistance to macrolides conferred by macrolide-resistant genes (1-3). Macrolide resistance in S. pneumoniae is conferred by 23S ribosome methylation by ErmB and/or drug efflux systems by MefA (4). Similarly, encounters with ermA-, ermB-, ermC- or mefA-positive MSSA and S. pyogenes have been growing more common in clinical settings. Clinicians in Japan have also been isolating new strains of H. influenzae, β-lactamase-negative ampicillin resistant strains (BLNAR), with increasing frequency (1, 5). Taking all of these developments together, it seems certain that respiratory pathogens are acquiring β-lactam and macrolide resistance. This makes it all the more important to examine the antibacterial activity against multidrug-resistant bacteria isolated from patients with respiratory tract infections (2, 5-9).
In this study we examine the in vitro activities of 7 antibiotics, oral cephems (cefditoren, cefcapene and cefdinir), macrolide (erythromycin), ketolide (telithromycin) and controls (benzylpenicillin and ampicillin) against clinical isolates of four major respiratory pathogens (MSSA, S. pyogenes, S. pneumoniae, and H. influenzae) collected in Japan between 2002 and 2003.
MATERIALS AND METHODS
Clinical isolates
MSSA, S. pyogenes, S. pneumoniae and H. influenzae, which were isolated from the respiratory tract and collected at 66 institutions (20 university hospitals; 34 local central hospitals; 4 clinics; 8 general practitioners) in all over the Japan between 2002 and 2003, were used in this study. Forty-three isolates of MSSA and 49 isolates of S. pyogenes strains were randomly selected. S. pneumoniae was classified in three categories, based on the mutations of the pbp genes associated with β-lactam resistance. As a result, the following isolates were studied: 34 isolates of genotypic penicillin-susceptible S. pneumoniae (gPSSP) with no mutations in the pbp1a, 2x or 2b genes; 47 isolates of genotypic penicillin-intermediate-resistant S. pneumoniae (gPISP) with mutations in two of three pbp genes (1a and 2x, 1a and 2b or 2x and 2b) and 68 isolates of genotypic penicillin-resistant S. pneumoniae (gPRSP) with mutations in all three pbp genes, as described by Ubukata et al. (9). The H. influenzae isolates were classified into two variants. The first, BLNAR, had M377I, S385T, L389F and N526K mutations of the ftsI gene encoding PBP3 without carrying the TEM-1 gene encoding β-lactamase. The second, β-lactamase-negative ampicillin-susceptible H. influenzae (BLNAS) had no mutations of the ftsI gene and did not carry the TEM-1 gene. Twenty-five BLNAS isolates and 24 BLNAR isolates were selected for inclusion in this study.
Detection of drug-resistance genes by PCR
Mutations in the pbp1a, 2x and 2b genes and the presence of the mefA and ermB genes in S. pneumoniae were detected by a penicillin-resistant S. pneumoniae detection kit (Wakunaga Pharmaceutical, Osaka, Japan). Mutations in the ftsI gene and the presence of the TEM-1 gene in H. influenzae were detected using an H. influenzae gene detection kit (Wakunaga Pharmaceutical). The presence of the ermA and ermC genes in MSSA, and the presence of the mefA and ermB genes in S. pyogenes, were detected by conventional PCR methods (6, 10).
Antibiotic susceptibility test
Cefditoren and ampicillin were supplied by Meiji Seika Kaisha, Ltd., Tokyo, Japan. Cefdinir was purchased from Kemprotec Ltd., Middlesborough, U.K. Erythromycin and benzylpenicillin was purchased from Sigma-Aldrich Japan. Cefcapene was synthesized in our laboratory. Telithromycin was purified from the commercial product (Aventis Pharma Japan, Tokyo, Japan). Minimum inhibitory concentrations (MICs) were determined by the agar dilution method with 2-fold serial dilutions of antibiotics. The following Mueller-Hinton agar (MHA, Becton, Dickinson and Company, Sparks, MD, U.S.A.) preparations were used for the drug susceptibility tests: MHA alone for MSSA; MHA supplemented with 5% defibrinated equine blood for S. pneumoniae; MHA supplemented with 5% defibrinated equine blood heated until chocolate for H. influenzae, and MHA supplemented with 5% defibrinated ovine blood for S. pyogenes. Each isolate was inoculated at 104 CFU/spot on each agar plate. The MIC was determined as the minimum drug concentration showing no bacterial growth after 18-20 hr of incubation at 35℃. S. aureus ATCC29213, S. pneumoniae ATCC49619, H. influenzae ATCC49247 and ATCC49766 were used to control the accuracy of the MIC determinations. S. pneumoniae ATCC49619 was used as a control in the measurement of the susceptibility of S. pyogenes isolates. As for the breakpoint values of antibiotics for pneumoniae, we referred to the section of pneumoniae in the journal issued by the Sensitivity Determination Committee for Antibiotics, Japanese Society of Chemotherapy, except for benzylpenicillin that was not provided this journal. Judging from the breakpoint value, we calculated the resistance or susceptibility rate of each antibitotic against pathogens.
RESULTS
Antibacterial activity against MSSA
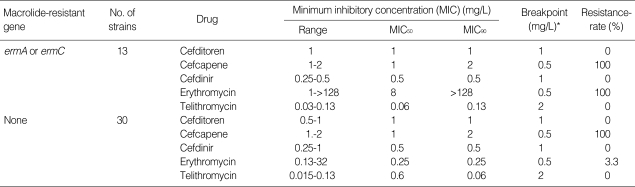
Of the 43 MSSA isolates used in this study, 10 isolates (23.3%) and 3 isolates (7.0%) carried either ermA or ermC, respectively, while none of the strains carried both genes. Telithromycin showed the most potent antibacterial activity against MSSA, followed by cefdinir, cefditoren, and cefcapene in descending order (Table 1). The antibacterial activity of erythromycin against MSSA was markedly attenuated by the presence of the erm genes, whereas the MIC90 of telithromycin was only 0.06 mg/L, even in the presence of the erm genes, with no cross-resistance to erythromycin.
Table 1.
Antibacterial activity of oral cephems, erythromycin and telithromycin against methicillin-susceptible Staphylococcus aureus (MSSA)
*, Breakpoint value of each antibiotic for pneumoniae was cited from the journal issued by that was defined by the Sensitivity Determination Committee for Antibiotics, Japanese Society of Chemotherapy.
Antibacterial activity against S. pyogenes
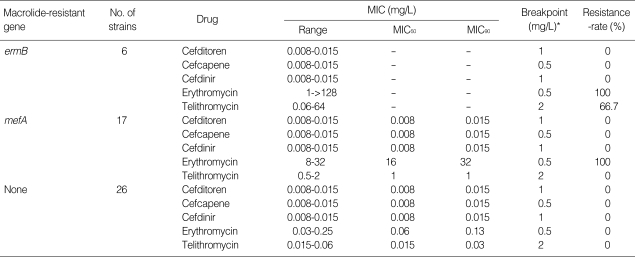
As shown in Table 2, 6 isolates (12.2%) and 17 isolates (34.7%) of the S. pyogenes strains carried the ermB and mefA genes, respectively. The MIC of the cephem antibiotics was no more than 0.015 mg/L against all the isolates of S. pyogenes tested. The MIC90 of telithromycin was increased when the isolates carried either ermB or mefA. Especially high resistance against telithromycin (MIC=64 mg/L) was observed in 4 of 6 isolates carrying the ermB gene.
Table 2.
Antibacterial activity of oral cephems, erythromycin and telithromycin against Streptococcus pyogenes
*, Breakpoint value of each antibiotic for pneumoniae was cited from the journal issued by that was defined by the Sensitivity Determination Committee for Antibiotics, Japanese Society of Chemotherapy.
Antibacterial activity against S. pneumoniae
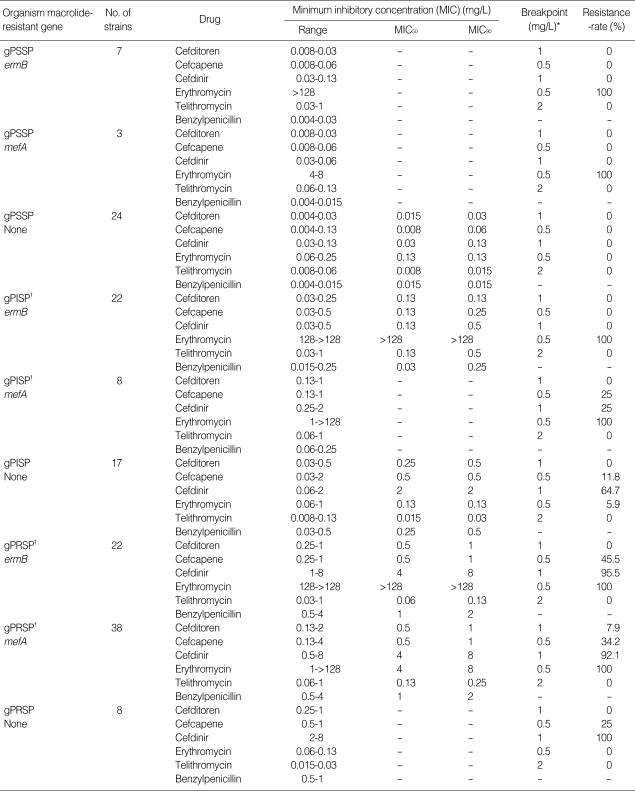
Subdivision of the gPSSP, gPISP, and gPRSP strains based on the presence of the ermB and mefA genes revealed that S. pneumoniae carried either ermB or mefA at a high frequency (Table 3). The β-lactams showed potent antibacterial activity against gPSSP, and cefditoren showed the strongest antibacterial activity against gPSSP, gPISP, and gPRSP of all 3 β-lactam antibiotics. The presence of the ermB and mefA genes within S. pneumoniae strains did not affect the activity the β-lactams. Telithromycin and erythromycin showed no attenuation in antibacterial activity even in the presence of a mutated pbp gene, while telithromycin exerted the strongest antibacterial activities against all three of the S. pneumoniae strains. However, the antibacterial activity of telithromycin was weakened in the presence of either the ermB or mefA gene.
Table 3.
Antibacterial activity of β-lactams, erythromycin and telithromycin against Streptococcus pneumoniae
gPSSP, genotypic susceptible S. pneumoniae; gPISP, genotypic penicillin-intermediate-resistant S. pneumoniae; gPRSP, genotypic penicillin-resistant S. pneumoniae. *, Breakpoint value of each antibiotic for pneumoniae was cited from the journal issued by that was defined by the Sensitivity Determination Committee for Antibiotics, Japanese Society of Chemotherapy; †, Including one strain with both ermB and mefA genes.
Antibacterial activity against H. influenzae
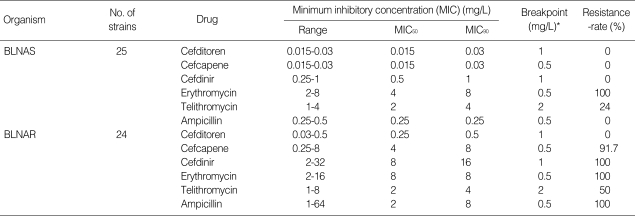
Cefditoren and cefcapene showed stronger antibacterial activity than cefdinir, telithromycin, and erythromycin against BLNAS (Table 4). The antibacterial activities of the oral cephems were weaker against BLNAR than against BLNAS, whereas the MIC90 of cefditoren was 0.5 mg/L, the most potent level measured among all the antibiotics tested in this study. Telithromycin showed an equal MIC90 against BLNAR and BLNAS, and the same result was obtained for erythromycin.
Table 4.
Antibacterial activity of β-lactams, erythromycin and telithromycin against Haemophilus influenzae
BLNAS, β-lactamase-negative ampicillin-susceptible H. influenzae; BLNAR, β-lactamase-negative ampicillin-resistant H. influenzae.
*, Breakpoint value of each antibiotic for pneumoniae was cited from the journal issued by that was defined by the Sensitivity Determination Committee for Antibiotics, Japanese Society of Chemotherapy.
DISCUSSION
The incidence of drug-resistant pathogens differs greatly between countries, presumably in accordance with differences in the dosage and usage of antibiotics. In this study we focused on the antibacterial activities of three different types of antibiotics (cephem, macrolide and ketolide) against recently isolated pathogens from respiratory tract infections carrying various drug-resistance genes in the Japanese population. The different frequencies of drug-resistant pathogens between Japan and neighboring Asian countries have also been considered.
In S. aureus, ribosome methylation and the presence of drug efflux systems have been shown to confer resistance against macrolide antibiotics (4). The isolates that exhibited low susceptibility to erythromycin without the erm gene in this study may have overexpressed multidrug efflux systems such as MsrA (11). Interestingly, all MSSA strains examined in this study were susceptible to telithromycin, even when they were positive for the presence of the erm gene. The expression of the erm gene can be inducible or constitutive, and MSSA is resistant to telithromycin when the expression is constitutive (12). Thus, we know that the isolates used in this study might harbor the inducible type of gene expression. The rate of MSSA resistance to telithromycin in Asia, 20%, is higher than the rates in Europe and the U.S.A. (13).
β-lactam resistance has not yet been reported in S. pyogenes, but macrolide-resistant strains of this pathogen have been frequently isolated (6-8, 13, 14). Some of the S. pyogenes isolates carrying ermB have become highly resistant to telithromycin (MIC >32 mg/L) (6-8). In our study, telithromycin and erythromycin showed reduced antibacterial activity against S. pyogenes isolates carrying either ermB or mefA. The incidence of telithromycin-resistant S. pyogenes (MIC ≥4 mg/L) differs from region to region, presumably due to the different usage of antibiotics. For example, the incidence in Japan stands at less than 1%, while the incidences in Korea and Europe are generally above 10% (13).
As previously reported, we categorized S. pneumoniae into gPRSP or gPISP based on mutations in the pbp gene (9). ermB and mefA have been found to be present at high frequencies in recent Japanese isolates of PISP and PRSP (2, 3). Our results also revealed many gPRSP and gPISP isolates carrying either ermB or mefA. In Korea and Hong Kong, PRSP accounts for ≥60% of clinical isolates of S. pneumoniae, and 50% or more of these isolates carry either ermB or mefA (3). It thus comes as no surprise that the MIC of telithromycin has already risen to 4 mg/L against newly emerging strains of S. pneumoniae in Taiwan (15) and to 2-8 mg/L against strains in other countries (7).
Just as Japan is witnessing a rising prevalence of BLNAR and falling prevalence of β-lactamase-producing strains, the very opposite trends are being observed in Korea (1, 3). It might be reasonable to assume that penicillin is more frequently prescribed in Korea than in Japan. Accordingly, the oral cephems such as cefditoren, an agent stable against β-lactamase in H. influenzae, may be useful and effective in both of these countries. Although macrolide-resistant genes such as erm or mef have not yet been found in H. influenzae, the antibacterial activity of telithromycin and erythromycin was lower than that of cefditoren against the H. influenzae isolates analyzed in the present study. Peric et al. demonstrated that more than 98% of H. influenzae strains have a macrolide efflux mechanism (16). Thus, the H. influenzae isolates investigated in this study might also express efflux pumps, such as AcrAB (17).
In conclusion, the variable antibacterial activity against the clinical pathogens in the presence of drug-resistant genes demonstrated in this study suggests that the inappropriate prescription of antibiotic agents may lead to clinical failure. It will be important to choose suitable antibiotics and to make the most of the ability of the prescribed agents in order to prevent the further emergence of drug-resistant pathogens.
References
- 1.Ubukata K. Problems associated with high prevalence of multidrug-resistant bacteria in patients with community-acquired infections. J Infect Chemother. 2003;9:285–291. doi: 10.1007/s10156-003-0278-y. [DOI] [PubMed] [Google Scholar]
- 2.Ubukata K, Iwata S, Sunakawa K. In vitro activities of new ketolide, telithromycin, and eight other macrolide antibiotics against Streptococcus pneumoniae having mefA and ermB genes that mediate macrolide resistance. J Infect Chemother. 2003;9:221–226. doi: 10.1007/s10156-003-0258-2. [DOI] [PubMed] [Google Scholar]
- 3.Inoue M, Lee NY, Hong SW, Lee K, Felmingham D. PROTEKT 1999-2000: a multicentre study of the antibiotic susceptibility of respiratory tract pathogens in Hong Kong, Japan and South Korea. Int J Antimicrob Agents. 2004;23:44–51. doi: 10.1016/j.ijantimicag.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Roberts MC, Sutcliffe J, Courvalin P, Jensen LB, Rood J, Seppala H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother. 1999;43:2823–2830. doi: 10.1128/aac.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa K, Chiba N, Kobayashi R, Murayama SY, Iwata S, Sunakawa K, Ubukata K. Rapidly increasing prevalence of beta-lactamase-nonproducing, ampicillin-resistant Haemophilus influenzae type b in patients with meningitis. Antimicrob Agents Chemother. 2004;48:1509–1514. doi: 10.1128/AAC.48.5.1509-1514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morosini MI, Canton R, Loza E, del Campo R, Almaraz F, Baquero F. Streptococcus pyogenes isolates with characterized macrolide resistance mechanisms in Spain: in vitro activities of telithromycin and cethromycin. J Antimicrob Chemother. 2003;52:50–55. doi: 10.1093/jac/dkg303. [DOI] [PubMed] [Google Scholar]
- 7.Farrell DJ, Morrissey I, Bakker S, Felmingham D. Molecular characterization of macrolide resistance mechanisms among Streptococcus pneumoniae and Streptococcus pyogenes isolated from the PROTEKT 1999-2000 study. J Antimicrob Chemother. 2002;50(Suppl S1):39–47. doi: 10.1093/jac/dkf806. [DOI] [PubMed] [Google Scholar]
- 8.Sunaoshi K, Nakayama E, Kobayashi R, Suzuki E, Tajima T, Ubukata K. Antibiotic susceptibility and T type identification of Streptococcus pyogenes isolated from pediatric outpatients with pharyngotonsillitis. Jpn J Chemother. 2004;52:401–407. [Google Scholar]
- 9.Ubukata K, Chiba N, Hasegawa K, Kobayashi R, Iwata S, Sunakawa K. Antibiotic susceptibility in relation to penicillin-binding protein genes and serotype distribution of Streptococcus pneumoniae strains responsible for meningitis in Japan, 1999 to 2002. Antimicrob Agents Chemother. 2004;48:1488–1494. doi: 10.1128/AAC.48.5.1488-1494.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lina G, Quaglia A, Reverdy ME, Leclercq R, Vandenesch F, Etienne J. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob Agents Chemother. 1999;43:1062–1066. doi: 10.1128/aac.43.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross JI, Eady EA, Cove JH, Cunliffe WJ, Baumberg S, Wootton JC. Inducible erythromycin resistance in staphylococci is encoded by a member of the ATP-binding transport super-gene family. Mol Microbiol. 1990;4:1207–1214. doi: 10.1111/j.1365-2958.1990.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz FJ, Petridou J, Milatovic D, Verhoef J, Fluit AC, Schwarz S. In vitro activity of new ketolides against macrolide-susceptible and -resistant Staphylococcus aureus isolates with defined resistance gene status. J Antimicrob Chemother. 2002;49:580–582. doi: 10.1093/jac/49.3.580. [DOI] [PubMed] [Google Scholar]
- 13.Canton R, Loza E, Morosini MI, Baquero F. Antimicrobial resistance amongst isolates of Streptococcus pyogenes and Staphylococcus aureus in the PROTEKT antimicrobial surveillance programme during 1999-2000. J Antimicrob Chemother. 2002;50(Suppl S1):9–24. doi: 10.1093/jac/dkf811. [DOI] [PubMed] [Google Scholar]
- 14.Okubo T, Iyobe S, Fujiki Y, Sagai H. Antimicrobial activities of macrolides against recent clinical isolates, and analysis of resistant mechanisms. Jpn J Antibiot. 2003;56:163–170. [PubMed] [Google Scholar]
- 15.Hsueh PR, Teng LJ, Wu TL, Yang D, Huang WK, Shyr JM, Chuang YC, Wan JH, Yan JJ, Lu JJ, Wu JJ, Ko WC, Chang FY, Yang YC, Lau YJ, Liu YC, Lee CM, Leu HS, Liu CY, Luh KT. Telithromycin and fluoroquinolone-resistant Streptococcus pneumoniae in Taiwan with high prevalence of resistance to macrolides and beta-lactams: SMART program 2001 data. Antimicrob Agents Chemother. 2003;47:2145–2151. doi: 10.1128/AAC.47.7.2145-2151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peric M, Bozdogan B, Jacobs MR, Appelbaum PC. Effects of efflux mechanism and ribosomal mutations on macrolide susceptibility of Haemophilus influenzae clinical isolates. Antimicrob Agents Chemother. 2003;47:1017–1022. doi: 10.1128/AAC.47.3.1017-1022.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez L, Pan W, Vinas M, Nikaido H. The acrAB homolog of Haemophilus influenzae codes for a functional multidrug efflux pump. J Bacteriol. 1997;179:6855–6857. doi: 10.1128/jb.179.21.6855-6857.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]