Abstract
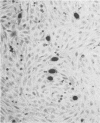
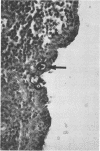
An immunological technique for detecting Chlamydia trachomatis and Chlamydia psittaci inclusions in infected McCoy cell cultures was developed by using a genus-specific monoclonal antibody to Chlamydia spp., rabbit anti-mouse immunoglobulin G bridging antibody, alkaline phosphatase-anti-alkaline phosphatase (APAAP) monoclonal antibody conjugate, and naphthol AS-phosphate/fast red substrate. Chlamydial inclusions stained red and were easily detected against a background of blue hematoxylin-stained nuclei. After 18 h, inclusions of C. trachomatis serovar L2 LGV434/Bu and C. psittaci strain 6BC were stained by APAAP but not by iodine or Giemsa. At 48 h inclusion counts were significantly higher in the APAAP cultures. Both the APAAP procedure and conventional staining detected 35 of 239 (15%) cultures 48 h after inoculation with urethral or endocervical specimens. However, at 24 h after inoculation 22 of 35 (63%) were positive by APAAP staining while negative by iodine. This immunostain also allowed identification of chlamydial inclusions in endometrial biopsies from patients with tubal factor infertility or pelvic inflammatory disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amortegui A. J., Meyer M. P. Enzyme immunoassay for detection of Chlamydia trachomatis from the cervix. Obstet Gynecol. 1985 Apr;65(4):523–526. [PubMed] [Google Scholar]
- Barnes R. C., Wang S. P., Kuo C. C., Stamm W. E. Rapid immunotyping of Chlamydia trachomatis with monoclonal antibodies in a solid-phase enzyme immunoassay. J Clin Microbiol. 1985 Oct;22(4):609–613. doi: 10.1128/jcm.22.4.609-613.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caul E. O., Paul I. D. Monoclonal antibody based ELISA for detecting Chlamydia trachomatis. Lancet. 1985 Feb 2;1(8423):279–279. doi: 10.1016/s0140-6736(85)91056-6. [DOI] [PubMed] [Google Scholar]
- Chernesky M. A., Mahony J. B., Castriciano S., Mores M., Stewart I. O., Landis S. J., Seidelman W., Sargeant E. J., Leman C. Detection of Chlamydia trachomatis antigens by enzyme immunoassay and immunofluorescence in genital specimens from symptomatic and asymptomatic men and women. J Infect Dis. 1986 Jul;154(1):141–148. doi: 10.1093/infdis/154.1.141. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Kuo C. C., Wang S. P., Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986 Jul 17;315(3):161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- Jones M. F., Smith T. F., Houglum A. J., Herrmann J. E. Detection of Chlamydia trachomatis in genital specimens by the Chlamydiazyme test. J Clin Microbiol. 1984 Sep;20(3):465–467. doi: 10.1128/jcm.20.3.465-467.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviat N. B., Wølner-Hanssen P., Peterson M., Wasserheit J., Stamm W. E., Eschenbach D. A., Paavonen J., Lingenfelter J., Bell T., Zabriskie V. Localization of Chlamydia trachomatis infection by direct immunofluorescence and culture in pelvic inflammatory disease. Am J Obstet Gynecol. 1986 Apr;154(4):865–873. doi: 10.1016/0002-9378(86)90473-4. [DOI] [PubMed] [Google Scholar]
- Mahony J. B., Chernesky M. A. Effect of swab type and storage temperature on the isolation of Chlamydia trachomatis from clinical specimens. J Clin Microbiol. 1985 Nov;22(5):865–867. doi: 10.1128/jcm.22.5.865-867.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm W. E., Tam M., Koester M., Cles L. Detection of Chlamydia trachomatis inclusions in Mccoy cell cultures with fluorescein-conjugated monoclonal antibodies. J Clin Microbiol. 1983 Apr;17(4):666–668. doi: 10.1128/jcm.17.4.666-668.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Kuo C. C., Tam M. R. Sensitivity of immunofluorescence with monoclonal antibodies for detection of Chlamydia trachomatis inclusions in cell culture. J Clin Microbiol. 1982 Jul;16(1):4–7. doi: 10.1128/jcm.16.1.4-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Stamm W. E., Handsfield H. H., Stephens R., Kuo C. C., Holmes K. K., Ditzenberger K., Krieger M., Nowinski R. C. Culture-independent diagnosis of Chlamydia trachomatis using monoclonal antibodies. N Engl J Med. 1984 May 3;310(18):1146–1150. doi: 10.1056/NEJM198405033101803. [DOI] [PubMed] [Google Scholar]
- Zapata M., Chernesky M., Mahony J. Indirect immunofluorescence staining of Chlamydia trachomatis inclusions in microculture plates with monoclonal antibodies. J Clin Microbiol. 1984 Jun;19(6):937–939. doi: 10.1128/jcm.19.6.937-939.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]