Abstract
Currently finger pricking is the common method of blood glucose measurement in patients with diabetes mellitus. However, diabetes patients have proven to be reluctant to check their glucose profiles regularly because of the discomfort associated with this technique. Recently, a non-invasive and continuous Reverse Iontophoresis based Glucose Monitoring Device (RIGMD) was developed in Korea. The study was conducted during the period November 2003-January 2004 on 19 in-patients. Glucose measurements were performed using RIGMD between 10 a.m. and 4 p.m. Concurrent plasma glucose levels were checked hourly and subsequently compared with RIGMD data. The mean error of RIGMD measurements was -3.45±52.99 mg/dL with a mean absolute relative error of 20±15.16%. Measurements obtained by RIGMD were correlated with plasma glucose levels (correlation coefficient; 0.784 (p<0.05)) and this correlation was independent of time of data collection. However, after excluding confounding variables this correlation coefficient exhibited a tendency to increase. 98.9% of the results were clinically acceptable by Clarke error grid analysis. We concluded that RIGMD does not have the reliability and accuracy required to wholly replace conventional methods. However, further technical advancements that reduce its shortcomings would make this device useful for the management of diabetes.
Keywords: Iontophoresis, Blood Glucose Self-Monitoring, Diabetes Mellitus
INTRODUCTION
It is well known that good metabolic control can delay or prevent diabetes related complications (1-3). In general, basic patient management programs involving diet, exercise, proper patient education, and medication are important for achieving good metabolic control in diabetes mellitus patients. In addition to these basic programs, the frequent and regular monitoring of blood glucose profiles is crucial in terms of predicting outcome and planning treatment strategies (4). However, a single glucose profile obtained during a visit to a clinic provides only superficial information and glucose level testing during a clinic visit is less attractive than daily glucose level checking by patients themselves. For this reason, glucose self-monitoring is important in patients with diabetes mellitus.
Currently self glucose monitoring is mainly performed by obtaining a small amount of blood by finger pricking. However, this method entails apprehension and pain, which results in alarmingly low compliance. According to a study by NHANES, only 47% of diabetes patients with a HbA1c level >8% and 32% of diabetes patients with a HbA1c level <8% check their glucose level more than once a day (5). In Korea, according to the Diabcare study conducted in 2001, only 17% of diabetes patients managed at private clinics check their glucose level on a regular basis (6). These findings all too clearly indicate that a considerable proportion of diabetes patients are receiving inadequate management.
However, even the conventionally recommended frequency of glucose level checking of 4 times a day is rather limited in terms of conveying information on plasma glucose level changes. Some diabetes centers utilize more progressive methods of glucose monitoring, such as, the Continuous glucose monitoring system (CGMS™, Medtronic MiniMed company, Northridge, CA, U.S.A.) to detect daily changes in the interstitial fluid glucose levels, but this system also has the fundamental limitation that it cannot be easily operated by a patient.
Recently, a non-invasive continuous method of measuring glucose concentration in interstitial fluid was introduced, based on the principle of reverse iontophoresis. The GlucoWatch™, automatic glucose biographer (Cygnus, Inc., Redwood, CA, U.S.A.) is the first commercially useful device based on this technology, and many clinical studies conducted to investigate its practical uses have returned favorable results.
A non-invasive glucose monitoring device based on the same principle has also been developed in Korea. This device is tentatively called Reverse Iontophoresis based Glucose Monitoring Device (RIGMD), and it detects and measures interstitial fluid glucose concentrations via an electrochemical enzymatic sensor every 5 min. In this study, the authors investigated the accuracy and clinical acceptability of this new device by comparing the data obtained from it and plasma glucose levels obtained by venous sampling.
MATERIALS AND METHODS
Operating principle of RIGMD
The operating principle of RIGMD was first described in studies that described experiments conducted in vivo in 1995 (7, 8), and is essentially the same as that utilized by the GlucoWatch™, automatic glucose biographer. Briefly, these devices measure fine electrical currents that dependent on glucose concentrations in interstitial fluid by using an electrochemical enzymatic sensor located on forearm skin. The sensor is composed of electrodes and a gelatinous material which contains glucose oxidase (GOx). A microcurrent is produced between the electrodes, which causes reverse iontophoresis that extracts glucose from skin interstitial fluid. The extracted glucose is then converted into gluconic acid and H2O2 by the glucose oxidase contained in sensor gel.
Glucose+O2 GOx → gluconic acid+H2O2
Current is produced by reducing H2O2 to O2 by electrocatalytic oxidation and current differences represent the changing glucose concentrations in interstitial fluid.
H2O2 → O2+2H++2e-
Study subjects
Initial candidates for this study were selected from among type 1 and type 2 diabetes mellitus patients admitted to the Department of Endocrinology and Metabolism in Kyung Hee University Hospital between November 2003 and January 2004. Nine of the 28 candidates were subsequently excluded due to voluntary or non-voluntary discontinuation of the device or noncompliance, leaving a total of 19 study subjects. The exclusion criteria were: 1) continuous hypo- or hyperglycemia (<40 mg/dL or >450 mg/dL); 2) electronic auxiliaries fitted such as cardiac pace maker; 3) an abruption or injury to forearm skin; 4) acetaminophen administration within 72 hr of the initiation of the data-collection; 5) mentally illness; 6) peripheral vascular disease; or 7) anemia.
Study protocol
To evaluate the accuracy and reliability of RIGMD, glucose levels were simultaneously checked hourly in each patient from 10 a.m. to 4 p.m. using RIGMD and by antecubital venous blood sampling; plasma glucose levels were measured using a Biosen analyzer (Biosen 5030; EKF-diagnostic GmbH, Magdeburg, Germany). The first set of data taken at 10 a.m. was used to calibrate the RIGMD vs. plasma glucose levels as determined by vs. sampling. Unwanted side effects at sensor/skin contact site were also noted.
Data analysis
All statistical manipulations were performed using SPSS Version 11.0 (SPSS Inc., Chicago, IL, U.S.A.). Correlation coefficients, slopes and intercepts were calculated using linear regression analysis. Mean absolute relative error (MARE), mean error (ME), and other comparative descriptive statistical values were also calculated for the RIGMD and plasma glucose level testing. The Clarke error grid was utilized to evaluate the clinical applicability of RIGMD.
RESULTS
Patients demographics
Nineteen patients successfully completed the study. There were 9 males and 10 females of mean age 53.52±15.56 yr. Mean disease duration was 7.11±5.70 yr with a mean HbA1c level of 8.36±2.5%.
Mean error and Mean absolute relative error
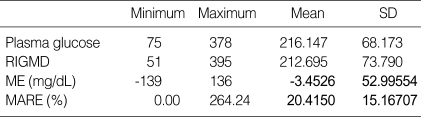
Excluding the 10 a.m. data set, which was used for calibration, all other measurements were subjected to statistical analysis. Differences between measurements are described as ME, and ME divided by the plasma glucose level was defined as the MARE. The mean difference between the paired data was -3.45±52.99 mg/dL with a MARE of 20±15.16% (Table 1).
Table 1.
Mean error (ME) and mean absolute relative error (MARE) of plasma glucose levels and interstitial glucose levels by RIGMD
Linear regression analysis
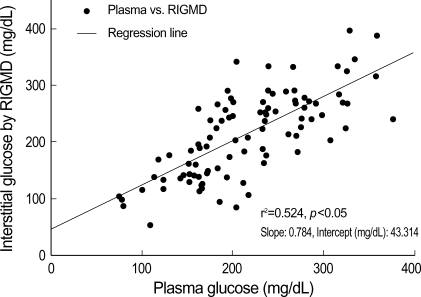
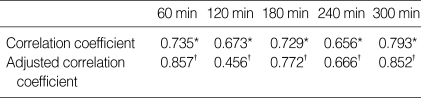
Data from RIGMD was found to be statistically correlated with plasma glucose level by linear regression analysis (r2=0.524; correlation coefficient 0.784, p<0.05) (Fig. 1). Correlation coefficients for hourly measurements were also calculated to determine whether or not time elapsed or a reduction in battery power affected determined glucose oxidase activities. These correlation coefficients were also high, most were within 0.65-0.7, and they remained near constant throughout the study. However, the correlation coefficient between of data taken at 120 min from the start was found to be considerably lower. Adjusted correlation coefficients, i.e., after correcting for confounding factors, such as age and gender, were generally higher; however correlation coefficient of data taken 120 min from start remained low even after correction (Table 2).
Fig. 1.
Linear regression analysis result, showing the relationship between plasma glucose level and interstitial glucose level as determined by RIGMD.
Table 2.
Correlation coefficients and adjusted correlation coefficients between plasma glucose level and interstitial glucose level by RIGMD
*p<0.01 (by Spearman's rho); †Partial correlation coefficient adjusted to age, sex, duration of diseases, other comorbidity, and patient body mass index and HbA1c.
Clarke error grid analysis
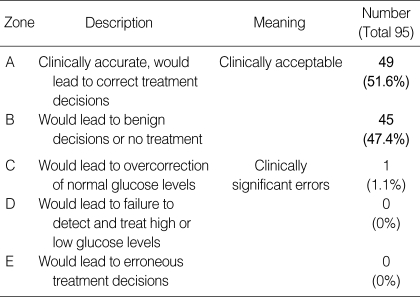
Clarke examined the clinical validity of a glucose monitoring system using an error grid (9). According to this error grid system, the measurements from RIGMD and plasma glucose levels are represented as a scatter plot, which is then divided into 5 zones. Data set falling within zones A and B are defined as clinically acceptable, and data falling within the other zones (C, D, and E) as clinically unacceptable (Table 3). In the present study, only a single set of data fell outside the acceptable zone (into zone C), leaving 98.9% of the data sets within zones A and B, indicating that RIGMD is clinically acceptable (Fig. 2).
Table 3.
Definition of Clarke error grid zones and the number of pairs included in each zone
Fig. 2.
Clarke error grid analysis of plasma glucose level and interstitial glucose level by RIGMD results.
Side effects
Transient itching or erythema was observed at the sensor attachment area in 10.5% (2 of 19) of the subjects. This was thought to be due to the sensor current, and subsided within 90 min after sensor detachment. No specific device complication was discovered during subsequent outpatient follow-up.
DISCUSSION
Several studies have been performed on non-invasive and continuous glucose level monitoring using reverse iontophoresis in vivo (Rao et al. [7, 10] and Tamada et al. [8]). Cygnus Inc. (U.S.A.) is currently marketing a device based on this technology called GlucoWatch™ biographer.
Garg et al. (11) calculated correlation coefficients between finger prick blood glucose levels and GlucoWatch™ determined levels in 28 type 1 diabetes mellitus patients, and obtained correlation coefficient in the r=0.85-0.90 range, and 96% of the measurements were clinically acceptable based on the Clarke error grid analysis. In addition, Tamada et al. (12) performed a study on 92 type 1 and 2 diabetes mellitus patients and also obtained a high correlation coefficient of r=0.88, and found that 96.8% of measurements were clinically acceptable based on the error grid analysis.
The correlation coefficient of the present study (r=0.784) was lower than those of prior GlucoWatch™, biographer studies. Notably, the correlation coefficient calculated from measurements taken at 120 min after start up was markedly lower than those calculated at other times. In addition, the present study was conducted on fewer subjects than the aforementioned studies, which would have resulted in lower reliability.
Although the relatively large measurement error could be attributed to a lack of reliability of RIGMD vs. GlucoWatch™ biographer. It should be noted that in previous studies on GlucoWatch™ biographer, blood glucose levels were determined using the finger prick method and not by using plasma glucose levels measured directly from blood drawn from the forearm, as in the present study. Glucose levels in drawn blood probably better reflect patient glucose profile. This point warrants consideration when tests on GlucoWatch™ biographer are next performed.
Acetaminophen is known to potentially interfere with conventional glucose monitoring devices. For this reason, subjects taking acetaminophen were excluded from the present study. However, according to a study conducted by Tierney et al. (13), subjects being administered acetaminophen daily at 1,000 mg had glucose levels as measured by GlucoWatch™ biographer and by finger prick method that differed by <20% and had clinically acceptable error grid analysis results. Since RIGMD incorporates the same basic principle as GlucoWatch™ biographer a guess can not be effected to acetaminophen administration. However, further clinical studies are required to confirm this relation.
Currently, some limitations restrict the clinical application of RIGMD. For example, the accuracy of RIGMD needs to be investigated to account for the following possible effects, i.e., low or high temperature, in the presence of excessive perspiration at the sensor site, the effects of site location, skin thickeness, etc. Thus, to popularize such methods among diabetes patients, more clinical study of RIGMD is required to provide medical staff and patients with more comprehensive data on daily plasma glucose level shifts and ultimately lead to better management.
The present study shows that as yet RIGMD is not sufficiently reliable or accurate enough to replace conventional methods of monitoring glucose. However, if the device could be made more accurate and convenient we believe that it would offer a highly effective means of glucose level self-testing.
Footnotes
This work was supported from the Korean National Diabetes Program of the Korean Ministry of Health and Welfare (Grant No. A050463).
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. UKPDS 34. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. UKPDS 33. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 4.American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26:S33–S50. doi: 10.2337/diacare.26.2007.s33. [DOI] [PubMed] [Google Scholar]
- 5.Harris MI. Health care and health status and outcomes for patients with type 2 diabetes. Diabetes Care. 2000;23:754–758. doi: 10.2337/diacare.23.6.754. [DOI] [PubMed] [Google Scholar]
- 6.Rhee SY, Kim YS, Oh S, Choi WH, Park JE, Jeong WJ. Diabcare Asia 2001-Korea: Country report on outcome data and analysis. Korean J Intern Med. 2005;20:48–54. doi: 10.3904/kjim.2005.20.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao G, Guy RH, Glikfeld P, LaCourse WR, Leung L, Tamada J, Potts RO, Azimi N. Reverse iontophoresis: Noninvasive glucose monitoring in vivo in humans. Pharm Res. 1995;12:1869–1873. doi: 10.1023/a:1016271301814. [DOI] [PubMed] [Google Scholar]
- 8.Tamada JA, Bohannon NJ, Potts RO. Measurement of glucose in diabetic subjects using noninvasive transdermal extraction. Nat Med. 1995;1:1198–1201. doi: 10.1038/nm1195-1198. [DOI] [PubMed] [Google Scholar]
- 9.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10:622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 10.Rao G, Glikfeld P, Guy RH. Reverse iontophoresis: Development of a noninvasive approach for glucose monitoring. Pharm Res. 1993;10:1751–1755. doi: 10.1023/a:1018926215306. [DOI] [PubMed] [Google Scholar]
- 11.Garg SK, Potts RO, Ackerman NR, Fermi SJ, Tamada JA, Chase HP. Correlation of fingerstick blood glucose measurements with Glucowatch biographer glucose results in young subjects with type 1 diabetes. Diabetes Care. 1999;22:1708–1714. doi: 10.2337/diacare.22.10.1708. [DOI] [PubMed] [Google Scholar]
- 12.Tamada JA, Garg S, Jovanovic L, Pitzer KR, Fermi S, Potts RO. Noninvasive glucose monitoring: Comprehensive clinical results. Cygnus research team. JAMA. 1999;282:1839–1844. doi: 10.1001/jama.282.19.1839. [DOI] [PubMed] [Google Scholar]
- 13.Tierney MJ, Garg S, Ackerman NR, Fermi SJ, Kennedy J, Lopatin M. Effect of acetaminophen on the accuracy of glucose measurements obtained with the Glucowatch biographer. Diabetes Technol Ther. 2000;2:199–207. doi: 10.1089/15209150050025140. [DOI] [PubMed] [Google Scholar]