Abstract
To evaluate the role of vascular endothelial growth factor (VEGF) in the pathogenesis of preeclampsia, we measured total VEGF, free VEGF and soluble Flt-1 (sFlt-1) concentrations and determined their relationships. Maternal serum samples were collected from 20 patients with preeclampsia and 20 normotensive women with uncomplicated pregnancies matched with the patients with preeclampsia for gestational age and parity. The serum concentrations of total VEGF (2.39±0.75 vs. 0.28±0.14) and sFlt-1 (934.5±235.5 vs. 298.0±161.2) were significantly increased in the patients with preeclampsia compared to the women with uncomplicated pregnancies. However the serum concentration of free VEGF (21.5±6.3 vs. 134.0±16.3) was lower in patients with preeclampsia. There was a positive correlation between the serum concentrations of total VEGF and sFlt-1 with systolic and diastolic blood pressure, respectively. There was a negative correlation between the serum concentration of free VEGF and systolic and diastolic blood pressure. There was a strong negative correlation between free VEGF and sFlt-1 concentrations. In conclusion, we found VEGF and sFlt-1 were related to the pathogenesis of preeclampsia. Although reduced concentrations of free VEGF might interfere with endothelial cell function and survival, further studies are required to clarify its specific role in the pathogenesis of preeclampsia.
Keywords: Pre-eclampsia, Vascular Endothelial Growth Factor, sFlt-1, Vasular Endothelial Growth Factor Receptor-1
INTRODUCTION
Preeclampsia affects about 5-8 percent of pregnancies, resulting in substantial maternal and neonatal morbidity and mortality. Although the etiology remains unclear, the clinical features associated preeclampsia may be initiated by placental factors that enter the maternal circulation and may cause endothelial dysfunction resulting in hypertension and proteinuria (1-3). For a fetus to develop normally, it must receive sufficient oxygen and nutrients from the maternal circulation (4). These are supplied via the maternal spiral arteries in the uterus. In preeclampsia, pseudo-vasculogenesis is defective, and the resultant placental ischemia has been proposed to trigger the release of unknown placenta-derived factors. The latter are thought to induce systemic endothelial dysfunction and thereby contribute to the renal, cardiovascular and neurological problems associated with preeclampsia.
Vascular endothelial growth factor (VEGF) binds with high affinity to two tyrosine kinase receptors, the fms-like tyrosine kinase (Flt-1 or VEGFR-1) and the kinase domain receptor (KDR or VEGFR-2), which are produced predominantly by endothelial cells (5-7). Soluble Flt-1 (sFlt-1) is a splice variant of the Flt-1 lacking the transmembrane and cytoplasmic domains. Thus, this isoform binds VEGF and inhibits its biologic activities as a potent VEGF antagonist (7, 8).
Several studies have demonstrated that circulating total VEGF concentration is significantly elevated in women with preeclampsia (9-11). However, recent studies have demonstrated that biologically active free VEGF concentrations were decreased in women with preeclampsia and that sFlt-1 concentrations were elevated in women with preeclampsia (1, 2, 12, 13). The investigators suggest that causes of endothelial dysfunction in preeclampsia are not the increased total VEGF level, but the decreased free VEGF (2). Nevertheless, VEGF has many characteristics required for a candidate for a circulating factor important to the development of preeclampsia. It induces vascular permeability and also promotes coagulation, two characteristic features of preeclampsia (2, 5). Moreover, in myographic studies, incubation of myometrial resistance arteries with VEGF resulted in a reduction of endothelium-dependent relaxation that mimicked the reduction induced by plasma from women with preeclampsia (14).
In order to clarify the role of VEGF in pathogenesis of preeclampsia, we measured total VEGF, free VEGF and sFlt-1 concentrations at the same time and determined their relationships.
MATERIALS AND METHODS
From May 2002 to December 2003 maternal serum samples were collected from 20 preeclamptic patients either before the onset of labor or prior to medical intervention, at Korea University Medical Center, Guro Hospital, Seoul, Korea. Serum samples were also obtained from 20 normotensive women with uncomplicated pregnancies, who were matched with the preeclamptic patients for gestational age and parity. The diagnosis of preeclampsia was based on a blood pressure of at least 140/90 mmHg on two or more separate occasions and the development of proteinuria of at least 300 mg in a 24 hr urine collection, or the presence of greater than 2+ protein on a catheterized specimen (15). Any woman with a history of chronic hypertension, renal disease, diabetes mellitus, or vascular disease was excluded from the study.
Subjects were recruited with a protocol approved by the institutional review board and written informed consent was obtained from all of the participants. Venipuncture was performed and blood was collected into tubes containing clot activator and separator gel. The samples were centrifuged at 3,000 rpm for 20 min and the serum was then stored in aliquots at -70℃. The total VEGF was measured in duplicates using a competitive enzyme immunoassay (CEIA; Accucyte® Human VEGF EIA, CytImmune Science, Inc., Rockville, MD, U.S.A.). The detection range was from 0.195 to 200.0 ng/mL and intraassay variation and interassay variation were 8.9% and 11.1%, respectively. Free VEGF and sFlt-1 were measured by a sandwich-type enzyme-linked immunosorbent assay (ELISA; Quantikine® human VEGF, Quantikine® human sVEGF R1, R&D Systems Inc., Minneapolis, MN, U.S.A.). The minimum detectable dose of the free VEGF was less than 9 pg/mL and the intraassay variation and interassay variation were 5.4% and 7.3%, respectively. The minimum detectable level of the sFlt-1 was from 1.5-13.3 pg/mL and intraassay variation and interassay variation were 3.2% and 7.6%, respectively.
Demographic and clinical data were compared using Mann-Whitney U test. Mann-Whitney U test was used to determine the difference, in levels of total and free VEGF and sFlt-1, between the two groups studied. ANOVA and linear regression analyses were used to investigate relationships. Statistical significance was assumed at a p value of <0.05. Data were analyzed using SPSS program (Statistical Package for Social Science, release 12.0; SPSS, Inc., Chicago, IL, U.S.A.).
RESULTS
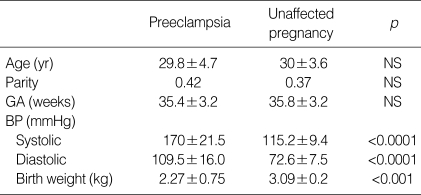
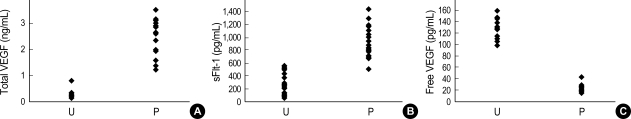
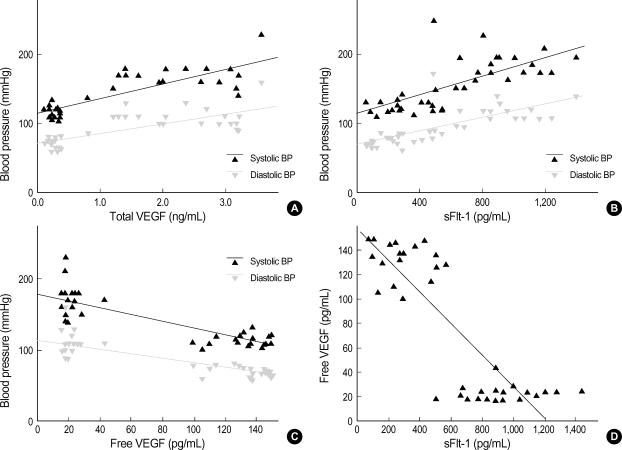
There were no significant differences observed in maternal age, parity and gestational age at blood sampling, between the two groups studied (Table 1). As expected, systolic and diastolic blood pressure were significantly higher in preeclamptic patients (systolic 170±21.5 vs. 115.2±9.4 mmHg, p<0.0001; diastolic 109.5±16.0 vs. 72.6±7.5 mmHg, p<0.0001). Neonatal birth weight was significantly higher in unaffected pregnant women (3.09±0.2 kg vs. 2.27±0.75 kg, p<0.001). Preeclamptic patients had a higher serum concentration of total VEGF and sFlt-1 compared to women with unaffected normotensive pregnancies (total VEGF 2.39±0.75 ng/mL vs. 0.28±0.14 ng/mL, p<0.001; sFlt-1 934.5±235.5 pg/mL vs. 298.0±161.2 pg/mL, p<0.0001) (Fig. 1A, B). However, the serum concentration of free VEGF was found to be lower in preeclamptic patients (21.5±6.3 pg/mL vs. 134.0±16.3 pg/mL, p<0.001) (Fig. 1C). There was a positive correlation between the serum concentration of total VEGF and systolic and diastolic blood pressure (systolic pressure r=0.818, p<0.0001; diastolic pressure r=0.749, p<0.0001); and a positive correlation was also observed for sFlt-1 (systolic pressure r=0.712, p<0.001; diastolic pressure r=0.725, p<0.001) (Fig. 2A, B). There was a negative correlation between the serum concentration of free VEGF and systolic and diastolic pressure (systolic pressure r=-0.826, p<0.0001; diastolic pressure r=-0.814, p<0.0001) (Fig. 2C). There was also a negative correlation between the serum concentration of free VEGF and sFlt-1 (r=-0.841, p<0.0001) (Fig. 2D).
Table 1.
Clinical characteristics of the study population
Values are given as mean±SD.
Statistical significance was assessed using the Mann-Whitney U test.
NS, non-significance; GA, gestational age; BP, blood pressure.
Fig. 1.
Distribution of Total VEGF levels (A), sFlt-1 levels (B), and Free VEGF levels in serum (C). P, preeclampsia; U, unaffected pregnancy.
Difference were significant: (A) p<0.001, (B) p<0.0001, (C) p<0.001. Values are given as mean±SD.
Statistical significance was assessed using Mann-Whitney U test.
Fig. 2.
(A) There was a positive correlation between the serum concentration of total VEGF and systolic pressure and diastolic pressure. (B) Positive correlation between the serum concentration of sFlt-1 and systolic pressure and diastolic pressure. (C) Negative correlation between serum concentration of free VEGF and systolic pressure and diastolic pressure. (D) Negative correlation between serum concentration of free VEGF and serum concentration of sFlt-1.
Difference were siginificant: (A) p<0.0001, (B) p<0.001, (C) p<0.0001, (D) p<0.0001. Values are given as mean±SD.
Statistical significance was assessed using the ANOVA and linear regression analyses.
In addition, we found a positive correlation between the serum concentration of total VEGF and proteinuria (r=0.187, p<0.458), and a negative correlation observed between the serum concentration of free VEGF and proteinuria (r=-0.254, p<0.326). However, there was no significant negative correlation with sFlt-1, and none of these correlations were statistically significant (p>0.05).
DISCUSSION
VEGF is an endothelial cell-specific growth factor that induces endothelial proliferation and chemotaxis, and stimulates new blood vessel formation. In addition to angiogenesis, VEGF is believed to increase microvascular permeability and also promote coagulation, two characteristic features of preeclampsia (16). Although several studies have demonstrated that circulating VEGF concentrations are significantly elevated in women with preeclampsia, recent studies have shown that the levels of serum free VEGF decrease in patients with preeclampsia. This discrepancy could be explained by the fact that VEGF-protein complexes are undetectable by the sandwich-type ELISA because there is a substantial increase in circulating VEGF binding proteins during pregnancy (16, 17). All prior studies reporting on decreased VEGF have used an ELISA kit, which measures free (unbound) VEGF (17, 18), whereas all studies reporting on an increased VEGF in preeclampsia used either a radioimmunoassay or an ELISA system measuring total (bound and unbound) VEGF (19-21).
It is known that there are two main stores for circulating VEGF. One is the platelet which takes up VEGF and releases it on activation in vivo or in vitro; the other is the plasma protein, namely α2-macroglobulin (α2-M) and sFlt-1, which are known to bind VEGF. The sFlt-1 protein is a splice variant of the VEGF receptor; Flt1 lacks the transmembrane and cytoplasmic domains, and acts as a potent VEGF and placental growth factor (PlGF) antagonist (22). It has been recently reported that placental sFlt1 mRNA is up-regulated in preeclampsia, leading to increased systemic levels of sFlt1 that fall after delivery (2). The study also showed that increased circulating sFlt1, in patients with preeclampsia, was associated with decreased circulating levels of free VEGF and PlGF; this has been shown to cause endothelial dysfunction in vitro that can be rescued by exogenous VEGF and PlGF (2). Indeed, a role for blockade of VEGF action, in the pathophysiology of preeclampsia, has been suggested recently by several studies in both animals and humans. Administration of anti-VEGF compounds can induce hypertension and proteinuria in nonpregnant animals and humans enrolled in antiangiogenic trials. Moreover administration of sFlt-1 to pregnant animals has been shown to induce the clinical features of preeclampsia, including hypertension, proteinuria, and glomerular endotheliosis (8).
Experimental evidence suggests that the serum from normal pregnant women is capable of inducing endothelial cells to form tube-like structures, a biologic effect inhibited by adding sFlt-1. By contrast, serum from preeclamptic patients has been shown to inhibit endothelial tube formation. However this process can be restored by adding VEGF and PlGF. The inhibition effect from the serum of women with preeclampsia disappeared after delivery. This suggests that a factor may be released by the placenta. These observations support the concept that the angiogenic properties, from the serum of preeclamptic patients may result from the blockade of VEGF by sFlt-1 (2, 8).
Our study indicates that women with preeclampsia have a higher serum concentration of sFlt-1 compared to unaffected pregnant women. In addition, we observed that serum concentration of total VEGF was higher in women with preeclampsia than in unaffected pregnancies, while serum concentration of free VEGF was lower in women with preeclampsia than in unaffected pregnant women.
Although reduced concentrations of free VEGF could interfere with endothelial cell function and survival (2, 8), there is indirect evidence to support that VEGF itself mediates endothelial cell activation in preeclampsia. In an in vitro study, VEGF induced a significant concentration-dependent increase in prostacyclin production; analogous to the acute effects of plasma from patients with preeclampsia; the increase in prostacyclin production, induced by plasma from women with preeclampsia, could be inhibited by anti-VEGF antibody (23, 24). In addition, VEGF has been reported to increase the nitric oxide production by human endothelial cells (25). There are other important findings in ex vivo models using the technique of wire myography. Ashworth et al. demonstrated that plasma from women with preeclampsia alters the endothelial function of myometrial resistance arteries, compared to women with uncomplicated pregnancies, by demonstrating a reduction in the relaxation of pre-constricted vessels (26). Similarly, incubation of myometrial resistance vessels from normal pregnant women with VEGF resulted in a reduction of endothelium-dependent relaxation, and this mimicked the reduction induced by plasma from women with preeclampsia (14).
It remains to be proven whether changes in the concentration of free VEGF truly reflect functional VEGF in vivo, relative to degradation rates and altered binding by proteins. Circulating VEGF exists in a free and bound form which binds to binding proteins. sFlt-1 and α2-M are known to be binding proteins of VEGF. Out of the total VEGF, only a small portion of VEGF is bound to sFlt-1 in vivo. Another major potential VEGF-binding protein is α2-M. Whereas sFlt-1 has been known as a potent VEGF antagonist, it is not clear how α2-M affects VEGF function (5, 27). Recently, Bhattacharjee et al. demonstrated that under physiological conditions, α2-M did not affect the ability of VEGF to induce cell proliferation or up-regulate Ca2+ (28). We also demonstrated VEGF-induced ET-1 up-regulation was inhibited by sFlt-1, but not by α2-M in human umbilical vein endothelial cells (29). Therefore, VEGF bound with α2-M might preserve biologic action. Considering α2-M is present in human plasma at a higher concentration (2-4 mg/mL) than sFlt-1, if VEGF bound with α2-M preserves its biologic action, increased total VEGF may contribute to the pathogenesis of preeclampsia. However, further studies are required to support this hypothesis. The limitations of this study include its small size. Despite the small sample size, the results were statistically significant. A larger study enrolling additional subjects would likely provide the additional power needed to discern significant difference of the two groups studied.
In summary, we have demonstrated a marked increase in circulating sFlt-1 and total VEGF concentration in patients with preeclampsia, accompanied by a decrease in circulating free VEGF levels. These observations suggest an important role for VEGF and its soluble receptor in the pathogenesis of preeclampsia. However, α2-M, another VEGF-binding protein, has been shown to be elevated in pregnancy; it is not clear how α2-M affects VEGF function to date. Therefore, further studies are needed to evaluate the interaction between α2-M and VEGF
References
- 1.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 2.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee GS, Lee JK, Han WS, Lee Y, Kim SJ, Rha JG, Kim SP, Namkoong SE. The expression of vascular endothelial growth factor, platelet-derived growth factor and intercellular adhesion molecule in severe preeclamptic placenta. Korean J Obstet Gynecol. 2003;46:606–611. [Google Scholar]
- 4.Luttun A, Carmeliet P. Soluble VEGF receptor Flt-1: the elusive preeclampsia factor discovered? J Clin Invest. 2003;111:600–602. doi: 10.1172/JCI18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jelkmann W. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem. 2001;47:617–623. [PubMed] [Google Scholar]
- 6.Sugimoto H, Hamano Y, Charytan D, Cosrove D, Kieran M, Sudhakar A, Kalluri R. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem. 2003;278:12605–12608. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- 8.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Gonclaves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophsiology of preeclampsia. Am J Obstet Gynecol. 2004;190:1541–1550. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 9.Kupferminc MJ, Daniel Y, Englender T, Baram A, Many A, Jaffa AJ, Gull I, Lessing JB. Vascular endothelial growth factor is increased in patients with preeclampsia. Am J Reprod Immunol. 1997;38:302–306. doi: 10.1111/j.1600-0897.1997.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 10.Cho YK, Min HJ, Park KH, Choi H, Kim BR, Lee HK. Serum concentration of vascular endothelial growth factor in preeclamptic women. Korean J Obstet Gynecol. 2000;43:1967–1971. [Google Scholar]
- 11.Lee KJ, Oh MJ, Kim HJ, Kim SH, Lee JK, Hur JY, Saw HS, Park YK. Correlation of serum vascular endothelial growth factor and matrix metalloproteinase-2 in patients complicated with preeclampsia and normotensive pregnant women. Korean J Obstet Gynecol. 2005;48:29–35. [Google Scholar]
- 12.Polliotti BM, Fry AG, Saller DN, Mooney RA, Cox C, Miller RK. Second-trimester maternal serum placental growth factor and vascular endothelial growth factor for predicting severe, early onset preeclampsia. Obstet Gynecol. 2003;101:1266–1274. doi: 10.1016/s0029-7844(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 13.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 14.Brockelsby JC, Hayman R, Ahmed A, Warren A, Johnson I, Baker P. VEGF via VEGF receptor-1 (Flt-1) mimics preeclamptic plasma in inhibiting uterine blood vessel relaxation in pregnancy: implications in the pathogenesis of preeclampsia. Lab Invest. 1999;79:1101–1111. [PubMed] [Google Scholar]
- 15.Levine RJ, Ewell MG, Hauth JC, Curet LB, Catalano PM, Morris CD, Choudhary G, Sibai BM. Should the definition of preeclampsia include a rise in diastolic blood pressure of >/=15 mm Hg to a level <90 mm Hg in association with proteinuria? Am J Obstet Gynecol. 2000;183:787–792. doi: 10.1067/mob.2000.108865. [DOI] [PubMed] [Google Scholar]
- 16.Oh MJ, Shin JC, Popek EJ. Vascular endothelial growth factor expression in patients complicated preeclampsia. Korean J Obstet Gynecol. 2001;44:1877–1882. [Google Scholar]
- 17.Lyall F, Greer IA, Boswell F, Fleming R. Suppression of serum vascular endothelial growth factor immunoreactivity in normal pregnancy and in preeclampsia. Br J Obstet Gynecol. 1997;104:223–228. doi: 10.1111/j.1471-0528.1997.tb11050.x. [DOI] [PubMed] [Google Scholar]
- 18.Reuvekamp A, Velsing-Aarts FV, Poulina IEJ, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factor characterizes pregnancies complicated by preeclampsia. Br J Obstet Gynecol. 1999;106:1019–1022. doi: 10.1111/j.1471-0528.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 19.Baker PN, Krasnow J, Roberts JM, Yeo KT. Elevated serum levels of vascular endothelial growth factor in patients with preeclampsia. Obstet Gynecol. 1995;86:815–821. doi: 10.1016/0029-7844(95)00259-T. [DOI] [PubMed] [Google Scholar]
- 20.Sharkey AM, Cooper JC, Balmforth JR, Mclaren J, Clark DE, Charnock-Jones DS, Morris NH, Smith SK. Maternal plasma levels of vascular endothelial growth factor in normotensive pregnancies and pregnancies complicated by preeclampsia. Eur J Clin Invest. 1996;26:1182–1185. doi: 10.1046/j.1365-2362.1996.830605.x. [DOI] [PubMed] [Google Scholar]
- 21.Brockelsby JC, Anthony FW, Johnson IR, Wheeler T, Baker PN. Increased serum vascular endothelial growth factor concentrations in pre-eclampsia. Hypertens Pregnancy. 1998;17:283–290. [Google Scholar]
- 22.Shibuya M. Role of VEGF-flt receptor system in normal and tumor angiogenesis. Adv Cancer Res. 1995;67:281–316. doi: 10.1016/s0065-230x(08)60716-2. [DOI] [PubMed] [Google Scholar]
- 23.Brockelsby JC, Anthony FW, Johnson IR, Baker PN. The effects of vascular endothelial growth factor on endothelial cells: A potential role in preeclampsia. Am J Obstet Gynecol. 2000;182:176–183. doi: 10.1016/s0002-9378(00)70510-2. [DOI] [PubMed] [Google Scholar]
- 24.Cho YK, Lee HK. The effect of VEGF on endothelial cells in pathogenesis of preeclampsia. Korean J Obstet Gynecol. 2002;45:1354–1359. [Google Scholar]
- 25.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274:H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 26.Ashworth JR, Warren AY, Johnson IR, Baker PN. Plasma from preeclamptic women and functional change in myometrial resistance arteries. Br J Obstet Gynecol. 1998;105:459–461. doi: 10.1111/j.1471-0528.1998.tb10134.x. [DOI] [PubMed] [Google Scholar]
- 27.Anthony FW, Evans PW, Wheeler T, Wood PJ. Variation in detection of VEGF in maternal serum by immunoassay and the possible influence of binding proteins. Ann Clin Biochem. 1997;34:276–280. doi: 10.1177/000456329703400309. [DOI] [PubMed] [Google Scholar]
- 28.Bhattacharjee G, Asplin IR, Wu SM, Gawdi G, Pizzo SV. The conformation-dependent interaction of α2-macroglobulin with vascular endothelial growth factor. J Biol Chem. 2000;275:26806–26811. doi: 10.1074/jbc.M000156200. [DOI] [PubMed] [Google Scholar]
- 29.Jung SH, Oh MJ, Suk YS, Lim JE, Seol HJ, Kim HJ. The effect of α2-macroglobulin on VEGF-induced endothelin-1 upregulation in human umbilical vein endothelial cells. Placenta. 2006;27:A50. [Google Scholar]