Abstract
Claudin-7 has recently been suggested to be a distal nephron marker. We tested the possibility that expression of claudin-7 could be used as a marker of renal tumors originating from the distal nephron. We examined the immunohistochemical expression of claudin-7 and parvalbumin in 239 renal tumors, including 179 clear cell renal cell carcinoma (RCC)s, 29 papillary RCCs, 20 chromophobe RCCs, and 11 renal oncocytomas. In addition, the methylation specific-PCR (MSP) of claudin-7 was performed. Claudin-7 and parvalbumin immunostains were positive in 3.4%, 7.8% of clear cell RCCs, 34.5%, 31.0% of papillary RCCs, 95.0%, 80.0% of chromophobe RCCs, and 72.7%, 81.8% of renal oncocytomas, respectively. The sensitivity and specificity of claudin-7 in diagnosing chromophobe RCC among subtypes of RCC were 95.0% and 92.3%. Those of parvalbumin were 80.0% and 88.9%. The expression pattern of claudin-7 was mostly diffuse in chromophobe RCC and was either focal or diffuse in oncocytoma. All of the cases examined in the MSP revealed the presence of unmethylated promoter of claudin-7 without regard to claudin-7 immunoreactivity. Hypermethylation of the promoter might not be the underlying mechanism for loss of its expression in RCC. Claudin-7 can be used as a useful diagnostic marker in diagnosing chromophobe RCC and oncocytoma.
Keywords: Chromophobe Renal Cell Carcinoma, Oncocytoma, Claudin-7
INTRODUCTION
Renal cell carcinoma (RCC) is the most common malignancy of the adult kidney, comprising 3% of all human cancers (1). The current renal tumor classification system is based on histology, as well as genetic difference (2, 3). More than 90% of clinically notable lesions canve diagnosed as one of the common subtypes of renal epithelial tumors: clear cell RCC, papillary RCC, chromophobe RCC, and renal oncocytomas. Recent study suggested that clear cell RCC has the highest rate of metastasis and poorest survival among common renal malignancies. Chromophobe RCC have relatively indolent biologic behavior (4).
Chromophobe RCC accounts for 5% of the epithelial tumors of the kidney (5). It has been suggested that the cells of chromophobe RCC are related to the normal intercalated cells of the collecting ducts, as it is ultra-structurally characterized by the presence of numerous cytoplasmic vesicles resembling those observed in intercalated cells (6). The diagnosis of chromophobe RCC is sometimes difficult because histological features of chromophobe RCC are similar to those of oncocytoma and the granular variant of clear cell RCC. Ancillary immunohistochemical or molecular markers are often needed to make an accurate subtyping of renal tumors.
A number of immunohistochemical and histochemical markers have been reported to be useful in making a diagnosis of chromophobe RCC. Markers for keratin 7, keratin 20, CD10, epithelial membrane antigen, vimentin, anti-mitochondrial antibody, and Hale's colloidal iron stain have been proposed as having some degree of specificity in diagnosing chromophobe RCC (7-12). Recently, it has been reported that parvalbumin is also specific for chromophobe RCC, which may make it useful as a marker in the differential diagnosis of chromophobe RCC and other subtypes of RCC (13). Parvalbumin, a calcium-binding protein, is selectively expressed in the collecting ducts of the fetal kidney and in the distal nephron of the adult kidney.
Claudin was discovered as a family of tight junction proteins (14), and there are 20 known members of the claudin family (15). Recently, it was demonstrated that claudin-7 is a distal nephron marker (16). Claudin-7 has not been extensively studied as an immunohistochemical marker in diagnosing renal tumors of distal nephron origin, such as chromophobe RCC or oncocytoma.
In the present study, we tested whether the presence of claudin-7 could be used as a marker of renal tumors originating from the distal nephron. We examined 239 cases of renal tumors using immunohistochemical stains for claudin-7 and parvalbumin. Furthermore, methylation specific-PCR (MSP) was performed to see whether the expression of claudin-7 was regulated by hyper-methylation of the promoter.
MATERIALS AND METHODS
Study materials
The study materials consisted of 239 paraffin-embedded tissues of primary renal tumors and one fetal kidney. Tissue samples were obtained from the surgical pathology archives of the Department of Pathology at Chonnam National University Hospital, Seoul National University Hospital, Yonsei University Severans Hospital, Dong-A University Hospital, and Asan Medical Center from the years 1996 to 2004. Of 239 tumors, 179 were clear cell RCC, 29 were papillary RCC, 20 were chromophobe RCC, and 11 were renal oncocytoma. Of the 239 cases, 42 were obtained on standard glass slides and 197 cases on a tissue microarray (TMA), which was constructed by the Korean Genitourinary Pathology Study Group. The diameter of the core was 1 mm, and 3 cores had been obtained in each case. The TMA contained 166 clear cell RCC, 20 papillary RCC, and 11 chromophobe RCC. Chromophobe RCC included 14 cases of typical variant and 6 cases of eosinophilic variant carcinoma (17). All tumors were classified according to standard international criteria (18, 19). For the 228 RCC cases, staging was performed according to the TNM system, and grading according to the system of Fuhrman et al. (20). For the 179 clear cell RCC cases, 98 tumors were stage T1 (54.7%), 42 stage T2 (23.5%), 38 stage T3 (21.2%), and 1 stage T4 (0.6%). Eighteen tumors were grade 1 (10.1%), 100 grade 2 (55.9%), 50 grade 3 (27.9%), and 11 grade 4 (6.1%). For the 29 papillary RCC cases, 15 tumors were stage T1 (51.7%), 7 stage T2 (24.1%), and 7 stage T3 (24.1%). One tumor was grade 1 (3.4%), 12 grade 2 (41.4%), 14 grade 3 (48.3%) and 2 grade 4 (6.9%). For the 20 chromophobe RCC cases, 15 tumors were stage T1 (75.0%), 4 stage T2 (20.0%), and 1 stage T3 (5.0%). Four tumors were grade 1 (20.0%), 13 grade 2 (65.0%), and 3 grade 3 (15.0%).
Immunohistochemical stain
Tissue sections in glass slides were de-paraffinized with xylene, hydrated in serially diluted alcohol, and then immersed in 3% H2O2 in order to quench endogenous peroxidase activity. Antigen retrieval was performed using citrate buffer (Antigen Retrieval Citra; Biogenex, San Ramon, CA, U.S.A.) with a pressure cooker. The sections were then incubated with anti-claudin-7 antibody (rabbit polyclonal; 1:200 dilution; Zymed, CA, U.S.A.) and anti-parvalbumin antibody (mouse polyclonal; 1:200 dilution; Chemicon International, CA, U.S.A.). The LSAB+ (Dakocytomation, Glostrup, Denmark) detection system was applied. The tissue sections were incubated with horseradish peroxidase (HRP)-conjugated streptavidin and the color reaction was developed with liquid 3,3'-diaminobenzidine as a chromogen, and counterstained with hematoxylin.
Methylation-specific PCR (MSP)
We investigated the promoter region of the claudin-7 gene by MSP on the DNA obtained from five cases of clear cell RCC and three cases of chromophobe RCC. Four of the five clear cell RCCs were negative for claudin-7 immunostain, and one case was positive for claudin-7. All of the chromophobe RCCs were positive for claudin-7. MSP of claudin-7 was performed as described in a previous study (21). The primers specific for un-methylated DNA were 5'-TGGGGAAAGGGTGGTGTTG-3'(sense) and 5'-TTACCCAATTTTAACCACCAC-3'(antisense), yielding a 182 bp product. Primers specific for methylated DNA were 5'-GACGTTAGGTTATTTTCGGTC-3'(sense) and 5'-AAACGCGTTTCTAAACGCCG-3'(antisense), yielding a 220 bp product. The genomic DNA (10 µg) treated with 40 U of CpG methylase (New England Biolabs, MA, U.S.A.) at 37℃ for two hours. It was used as a positive control of methylated DNA. The CCRF-CEM cell (Korean Cell Line Bank, Seoul, Korea), an acute lymphoblastic leukemia cell line, was seeded in a 100 mm plate at a density of 1×106 cells. After 24 hr, cells were treated with 10 µM of 5-azacytidine (Sigma, St. Louis, MO, U.S.A.) for 7 days, and the genomic DNA was isolated. It was used as a control of unmethylated DNA. All of the genomic DNA (1 µg) were treated with sodium bisulfite as previously des- cribed before PCR amplification (22).
Interpretation of immunohistochemical stain
All slides were evaluated by light microscopy with a semiquantitative estimation. Immunoreactivity was assessed without previous knowledge of the clinico-pathologic features. Membranous staining for claudin-7, and cytoplasmic staining for parvalbumin were analyzed. The extent of immunoexpression was sub-classified as: focal, <25% of tumor cells; and diffuse, 25-100% of tumor cells. Each tumor was separately examined by three pathologists, and cases with discrepant scores were discussed until consensus unity was achieved.
Statistical analysis
Statistical tests for significant differences in the intensity and extent of immunostains between standard slides and TMA were performed using Wilcoxon two sample test. Statistical analysis for significant differences in expression of claudin-7 and parvalbumin between chromophobe RCC and oncocytoma were performed using a two-tailed Fisher's exact test, with a significant p-value set as <0.05. Statistical comparisons were performed using SPSS, version 12.0 software (SPSS, Chicago, IL, U.S.A.).
RESULTS
Immunohistochemical stain
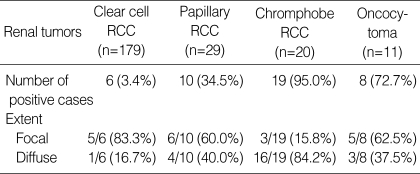
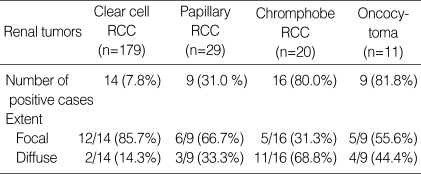
In the normal fetal and adult kidney, claudin-7 immunoreactivity was limited to the distal tubules and collecting ducts, displaying a distinctive membranous pattern, and parvalbumin was expressed in the cytoplasm at the same site (Fig. 1). The results describing the immunoreactivity of claudin-7 and parvalbumin are presented in Table 1 and 2. Positive immunoreactivity for claudin-7 was shown in 3.4% of clear cell RCCs (6 of 179 cases), 34.5% of papillary RCCs (10 of 29 cases), 95.0% of chromophobe RCCs (19 of 20 cases), and 72.7% of renal oncocytomas (8 of 11 cases). Positive immunoreactivity for parvalbumin was shown in 7.8% of clear cell RCCs (14 of 179 cases), 31.0% of papillary RCCs (9 of 29 cases), 80.0% of chromophobe RCCs (16 of 20 cases), and 81.8% of renal oncocytomas (9 of 11 cases). The intensity and extent of claudin-7 or parvalbumin were not different between the standard glass slide and TMA (p>0.5). And, for each subtype of RCC, the expression of claudin-7 or parvalbumin was not correlated with TNM stage and nuclear grade. The expression of claudin-7 and parvalbumin was similar in all of the tumors (Fig. 1). Claudin-7 and parvalbumin were highly expressed in both chromophobe RCC and oncocytoma. Although the expression rate of the two proteins was low in clear cell RCCs and papillary RCCs, immunoreactivity was quite obvious in some cases. The positive immunoreactivity of both claudin-7 and parvalbumin was shown in two cases of clear cell RCC and five cases of papillary RCC. None of these seven cases revealed any histologic features resembling chromophobe RCC. Claudin-7 and parvalbumin were not expressed in one, and in four cases of chromophobe RCC, respectively. At least one of two immunostains was positive in all cases of chromophobe RCC. Although both claudin-7 and parvalbumin were highly expressed in chromophobe RCC and oncocytoma, the expression pattern of claudin-7 was significantly different (p=0.03). The majority of chromophobe RCC revealed a diffuse staining pattern, whereas oncocytoma revealed either a focal or a diffuse pattern. The expression pattern of parvalbumin was not significantly different between chromophobe RCC and oncocytoma (p=0.22).
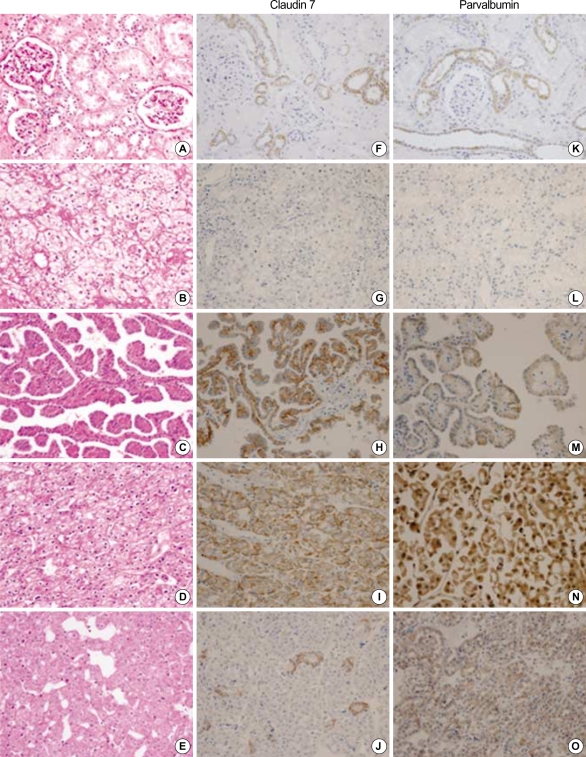
Fig. 1.
Immunohistochemical stains of claudin-7 and parvalbumin. Hematoxylin and eosin stains of normal renal cortex (A), clear cell RCC (B), papillary RCC (C), chromophobe RCC (D), and renal oncocytoma (E) are shown. Claudin-7 is expressed in the distal nephron of normal renal cortex with a distinct membranous pattern (F). It is diffusely expressed in papillary RCC (H) and chromophobe RCC (I), and is focally expressed in oncocytoma (J). It is not expressed in clear cell RCC (G). Parvalbumin is expressed in the cytoplasm of the distal nephron of normal renal cortex (K). It is diffusely expressed in papillary RCC (M), chromophobe RCC (N), and oncocytoma (O). It is not expressed in conventional RCC (L) (A-O, ×200).
Table 1.
Immunoreactivity of claudin-7 in renal epithelial tumors
Table 2.
Immunoreactivity of parvalbumin in renal epithelial tumors
The sensitivity and specificity of claudin-7 and parvalbumin immunostaining in making a diagnosis of chromophobe RCC was evaluated. Claudin-7 had a sensitivity of 95.0% and a specificity of 92.3%. These same measures for parvalbumin were lower than those of claudin-7, with a sensitivity of 80.0% and a specificity of 88.9%.
Methylation-specific PCR
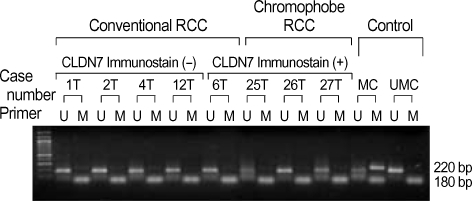
To determine if claudin-7 expression is controlled by hypermethylation-mediated silencing of the promoter, we performed MSP analysis on the extracted DNAs of claudin-7 positive and claudin-7 negative RCCs. All of the cases revealed the presence of unmethylated promoter sequences of claudin-7 (Fig. 2). The evidence provided by MSP supports the idea that hyper-methylation of the promoter might not be the underlying mechanism for the loss of claudin-7 expression in RCC; thus alternative mechanisms should be investigated in evaluating primary RCC.
Fig. 2.
Methylation-specific PCR of claudin-7. Primer sets used for amplification are designated as un-methylated (U), and methylated (M). The methylated control DNA (MC), and un-methylated control DNA (UMC) are designated. The smaller molecular weight fragments seen in the U and M lanes are primer dimers.
DISCUSSION
Shortly after the Heidelberg classification of kidney tumors was published, pathologists and clinicians noted that chromophobe RCC had a distinctly better prognosis than that of other RCC types (7). In this sudy, the nuclear grade and stage in chromophobe RCC cases was lower than those of other RCCs. It is imperative to make a diagnosis of chromophobe RCC correctly, due to the obvious prognostic implications. In this study, we demonstrated that claudin-7 and parvalbumin are specifically expressed in chromophobe RCC and oncocytoma, and the sensitivity and specificity of claudin-7 were higher than those of parvalbumin in the diagnosis of chromophobe RCC from other RCC types.
In this study, claudin-7 was expressed in the cell membrane of distal tubules and collecting ducts. Claudins are components of the tight junctions between epithelial cells. The tight junction structure is important for restricting lateral effusion of lipids and membrane proteins, thereby physically defining the border between the apical and basolateral components of the cell. To date, more than 20 claudins have been identified, and some show tissue specificity. A high-level of claudin-4 was detected in cells of the colon, an intermediate level was detected in prostate, placenta, lung, and pancreastic tissue, and low-levels were found in samples of the small bowel, kidney, and uterus (23). The tissue specificity of claudins strongly suggests that they may have other functions, in addition to being structural components of tight junctions (23). In renal tumors, claudin-7 was expressed in chromophobe RCC and oncocytoma, which supports the suggestion that these tumors originate from cells of the distal tubule and collecting duct.
Parvalbumin, a high affinity calcium-ion binding protein, is expressed in high levels in fast-contracting muscles, and is found at lower levels in brain and several endocrine tissues. It is related in structure and function to calmodulin and troponin C (24). The distribution of parvalbumin in the kidney matches fairly well with that of calcium receptors in the distal tubule and the proximal collecting duct, where the fine regulation of calcium re-adsorption takes place (13). The presence of parvalbumin was reported in most of the chromophobe RCCs with intense cytoplasmic staining (7, 13). In this study, the sensitivity and specificity were 80.0% and 88.9%, respectively.
The expression pattern of claudin-7 was significantly different between chromophobe RCC and oncocytoma. Chromophobe RCC revealed diffuse pattern in 84.2% and focal pattern in 15.8%. Oncocytoma revealed diffuse pattern in 37.5% and focal pattern in 62.5%. Hale's colloidal iron stain has been considered to be the most useful marker for making a differential diagnosis between two tumors (10). But, staining is technically demanding, and interpretation without experience is rather difficult. Therefore, the claudin-7 immunohistochemical stain could be used as a supplementary method of Hale's colloidal iron stain for making a differential diagnosis between two tumors.
Young et al. demonstrated that proximal nephron markers, such as megalin, α-methylacyl CoA racemase were overexpressed in clear cell and papillary RCC, whereas distal nephron markers, such as beta-defensin 1 and claudin-7 were overexpressed in chromophobe RCC and oncocytoma (25). Claudin-7 and parvalbumin were distinctly expressed in a few cases of clear cell RCC. And they were expressed in 34.5% and 31.0% in papillary RCC, respectively. In concordance with our results, the distal nephron markers, parvalbumin and beta-defensin, and a proximal nephron marker, vimentin, can be expressed in some cases of papillary RCCs (25). These findings may reflect the histogenesis of papillary renal cell carcinoma, which has been related to either proximal or distal nephron epithelium (25, 26). Claudin-7 and parvalbumin were not expressed in one case, nor in four cases of chromophobe RCCs, respectively. They were not expressed in three cases, nor two cases of renal oncocytomas, respectively. It is also possible that some tumors in these categories do not show differentiation towards the distal nephron in spite of characteristic histologic features.
Our result suggested that claudin-7 expression might not be controlled by unmethylation/hyper-methylation of its promoter. Similar results on hyper-methylation of claudin-7 were reported in infiltrating ductal carcinoma of breast. Although silencing of claudin-7 was correlated with promoter hyper-methylation in breast cancer cell lines, it did not do so in infiltrating ductal carcinoma of the breast (21). Further evaluation is needed to clarify the epigenetic regulation of claudin-7 expression.
In conclusion, we found that claudin-7 could be used as a valuable diagnostic marker in the diagnosis of chromophobe RCC and oncocytoma.
Footnotes
This work was supported by the Korea Research Foundation Grant (KRF-2001-F20004).
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Reuter VE, Presti JC., Jr Contemporary approach to the classification of renal epithelial tumors. Semin Oncol. 2000;27:124–137. [PubMed] [Google Scholar]
- 3.Zambrano NR, Lubensky IA, Merino MJ, Linehan WM, Walther MM. Histopathology and molecular genetics of renal tumors toward unification of a classification system. J Urol. 1999;162:1246–1258. [PubMed] [Google Scholar]
- 4.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Crotty TB, Farrow GM, Lieber MM. Chromophobe cell renal carcinoma: clinicopathological features of 50 cases. J Urol. 1995;154:964–967. doi: 10.1016/s0022-5347(01)66944-1. [DOI] [PubMed] [Google Scholar]
- 6.Storkel S, Steart PV, Drenckhahn D, Thoenes W. The human chromophobe cell renal carcinoma: its probable relation to intercalated cells of the collecting duct. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;56:237–245. doi: 10.1007/BF02890022. [DOI] [PubMed] [Google Scholar]
- 7.Abrahams NA, MacLennan GT, Khoury JD, Ormsby AH, Tamboli P, Doglioni C, Schumacher B, Tickoo SK. Chromophobe renal cell carcinoma: a comparative study of histological, immunohistochemical and ultrastructural features using high throughput tissue microarray. Histopathology. 2004;45:593–602. doi: 10.1111/j.1365-2559.2004.02003.x. [DOI] [PubMed] [Google Scholar]
- 8.Avery AK, Beckstead J, Renshaw AA, Corless CL. Use of antibodies to RCC and CD10 in the differential diagnosis of renal neoplasm. Am J Surg Pathol. 2000;24:203–210. doi: 10.1097/00000478-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Khoury JD, Abrahams NA, Levin HS, MacLennan GT. The utility of epithelial membrane antigen and vimentin in the diagnosis of chromophobe renal cell carcinoma. Ann Diagn Pathol. 2002;6:154–158. doi: 10.1053/adpa.2002.33901. [DOI] [PubMed] [Google Scholar]
- 10.Tickoo SK, Amin MB, Linden MD, Lee MW, Zarbo RJ. Antimitochondrial antibody (113-1) in the differential diagnosis of granular renal cell tumors. Am J Surg Pathol. 1997;21:922–930. doi: 10.1097/00000478-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Tickoo SK, Amin MB, Zarbo RJ. Colloidal iron staining in renal epithelial neoplasms, including chromophobe renal cell carcinoma: emphasis on technique and patterns of staining. Am J Surg Pathol. 1998;22:419–424. doi: 10.1097/00000478-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Wu SL, Kothari P, Wheeler TM, Reese T, Connelly JH. Cytokeratins 7 and 20 immunoreactivity in chromophobe renal cell carcinomas and renal oncocytomas. Mod Pathol. 2002;15:712–717. doi: 10.1097/01.MP.0000017566.29755.8A. [DOI] [PubMed] [Google Scholar]
- 13.Martignoni G, Pea M, Chilosi M, Brunelli M, Scarpa A, Colato C, Tardanico R, Zamboni G, Bonetti F. Parvalbumin is constantly expressed in chromophobe renal carcinoma. Mod Pathol. 2001;14:760–767. doi: 10.1038/modpathol.3880386. [DOI] [PubMed] [Google Scholar]
- 14.Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- 15.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 16.Schuetz AN, Yin-Goen Q, Amin MB, Moreno CS, Cohen C, Hornsby CD, Yang WL, Petros JA, Issa MM, Pattaras JG, Ogan K, Marshall FF, Young AN. Molecular classification of renal tumors by gene expression profiling. J Mol Diagn. 2005;7:206–218. doi: 10.1016/S1525-1578(10)60547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taki A, Nakatani Y, Misugi K, Yao M, Nagashima Y. Chromophobe renal cell carcinoma: an immunohistochemical study of 21 Japanese cases. Mod Pathol. 1999;12:310–317. [PubMed] [Google Scholar]
- 18.Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, Eble JN, Fleming S, Ljungberg B, Medeiros LJ, Moch H, Reuter VE, Rotz E, Roos G, Schmidt D, Srigley JR, Storkel S, van den Berg E, Zbar B. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Guinan P, Sobin LH, Algaba F, Badellino F, Kameyama S, MacLennan G, Novick A. TNM staging of renal cell carcinoma: Workgroup No. 3. Union International Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80:992–993. doi: 10.1002/(sici)1097-0142(19970901)80:5<992::aid-cncr26>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22:2021–2033. doi: 10.1038/sj.onc.1206199. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Park SJ, Abraham SC, Seo JS, Nam JH, Choi C, Juhng SW, Rashid A, Hamilton SR, Wu TT. Frequent CpG island methylation in precursor lesions and early gastric adenocarcinomas. Oncogene. 2004;23:4646–4654. doi: 10.1038/sj.onc.1207588. [DOI] [PubMed] [Google Scholar]
- 23.Zheng JY, Yu D, Foroohar M, Ko E, Chan J, Kim N, Chin R, Pang S. Regulation of the expression of the prostate-specific antigen by claudin-7. J Membr Biol. 2003;194:187–197. doi: 10.1007/s00232-003-2038-4. [DOI] [PubMed] [Google Scholar]
- 24.Berchtold MW, Epstein P, Beaudet AL, Payne ME, Heizmann CW, Means AR. Structural organization and chromosomal assignment of the parvalbumin gene. J Biol Chem. 1987;262:8696–8701. [PubMed] [Google Scholar]
- 25.Young AN, de Oliveira Salles PG, Lim SD, Cohen C, Petros JA, Marshall FF, Neish AS, Amin MB. Beta defensin-1, parvalbumin, and vimentin: a panel of diagnostic immunohistochemical markers for renal tumors derived from gene expression profiling studies using cDNA microarrays. Am J Surg Pathol. 2003;27:199–205. doi: 10.1097/00000478-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Hughson MD, Johnson LD, Silva FG, Kovacs G. Nonpapillary and papillary renal cell carcinoma: a cytogenetic and phenotypic study. Mod Pathol. 1993;6:449–456. [PubMed] [Google Scholar]