Abstract
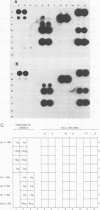
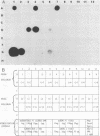
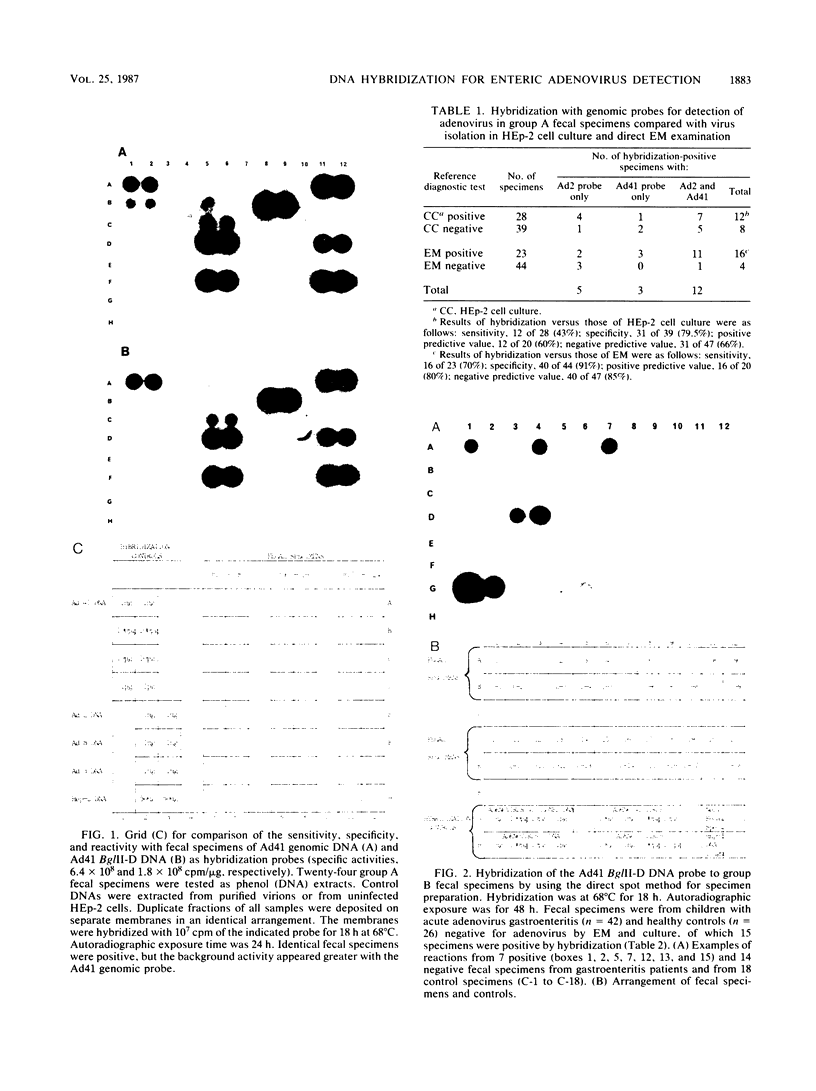
By using a genomic probe, DNA hybridization for adenovirus type 41 (Ad41) showed equivalent sensitivity with a direct spot method from clinical specimens compared with a more laborious DNA phenol extraction procedure. By using this direct spot preparation method, fecal specimens of 67 patients were examined under code for blind testing for the presence of adenovirus by DNA hybridization by using two Ad41 probes (genomic and cloned BglII-D) and an adenovirus type 2 genomic probe. Identical results were obtained with both of the Ad41 probes. Of the fecal specimens from 42 children with adenovirus gastroenteritis studied prospectively (16 of whom had enteric adenoviruses), 13 specimens (81%) were detected by DNA hybridization with a cloned Ad41 BglII-D probe. There were 14 fecal specimens that were positive by electron microscopy (EM) and culture for nonenteric adenovirus, and 2 specimens were positive by DNA hybridization (87% specificity); these 2 specimens may have been from a mixed enteric adenovirus and nonenteric adenovirus infection. None of 26 specimens from age-matched healthy control patients was positive for adenovirus by EM or DNA hybridization. Our data indicated that DNA hybridization gives highly reproducible results. The direct spot technique is the method of choice for specimen preparation in the diagnostic laboratory, since it requires only the simplest manipulations in specimen preparation. By using DNA hybridization with the BglII D fragment of a cloned enteric Ad41, both adenovirus type 40 and Ad41 were detected directly from fecal specimens, but it was less sensitive than EM following direct ultracentrifugation of specimens. The Bg1II-D Ad41 DNA probe was highly specific for enteric adenoviruses, and DNA hybridization with this probe could be a useful diagnostic test for these fastidious adenoviruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard A. K., Wadell G., Evander K. M., Lindman G. K. Specific properties of two enteric adenovirus 41 clones mapped within early region 1A. J Virol. 1985 Apr;54(1):145–150. doi: 10.1128/jvi.54.1.145-150.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. J., Jones S. E., Minson A. C. Diagnosis of human parvovirus infection by dot-blot hybridization using cloned viral DNA. J Med Virol. 1985 Feb;15(2):163–172. doi: 10.1002/jmv.1890150209. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Yolken R. H., Kapikian A. Z., Arrobio J. O., Rodriguez W. J., Wyatt R. G., Chanock R. M., Parrott R. H. Comparative epidemiology of two rotavirus serotypes and other viral agents associated with pediatric gastroenteritis. Am J Epidemiol. 1979 Sep;110(3):243–254. doi: 10.1093/oxfordjournals.aje.a112809. [DOI] [PubMed] [Google Scholar]
- Brown M., Petric M., Middleton P. J. Diagnosis of fastidious enteric adenoviruses 40 and 41 in stool specimens. J Clin Microbiol. 1984 Sep;20(3):334–338. doi: 10.1128/jcm.20.3.334-338.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Nakata S., Nakamura I., Taniguchi K., Urasawa S., Fujinaga K., Nakao T. Outbreak of infantile gastroenteritis due to type 40 adenovirus. Lancet. 1983 Oct 22;2(8356):954–957. doi: 10.1016/s0140-6736(83)90463-4. [DOI] [PubMed] [Google Scholar]
- Gary G. W., Jr, Hierholzer J. C., Black R. E. Characteristics of noncultivable adenoviruses associated with diarrhea in infants: a new subgroup of human adenoviruses. J Clin Microbiol. 1979 Jul;10(1):96–103. doi: 10.1128/jcm.10.1.96-103.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green M., Mackey J. K., Wold W. S., Rigden P. Thirty-one human adenovirus serotypes (Ad1-Ad31) form five groups (A-E) based upon DNA genome homologies. Virology. 1979 Mar;93(2):481–492. doi: 10.1016/0042-6822(79)90251-4. [DOI] [PubMed] [Google Scholar]
- Hammond G. W., Hazelton P. R., Chuang I., Klisko B. Improved detection of viruses by electron microscopy after direct ultracentrifuge preparation of specimens. J Clin Microbiol. 1981 Aug;14(2):210–221. doi: 10.1128/jcm.14.2.210-221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Mauthe G., Joshua J., Hannan C. K. Examination of uncommon clinical isolates of human adenoviruses by restriction endonuclease analysis. J Clin Microbiol. 1985 Apr;21(4):611–616. doi: 10.1128/jcm.21.4.611-616.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M. E., Uhnoo I., Kidd A. H., Madeley C. R., Wadell G. Direct identification of enteric adenovirus, a candidate new serotype, associated with infantile gastroenteritis. J Clin Microbiol. 1980 Jul;12(1):95–100. doi: 10.1128/jcm.12.1.95-100.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd A. H., Harley E. H., Erasmus M. J. Specific detection and typing of adenovirus types 40 and 41 in stool specimens by dot-blot hybridization. J Clin Microbiol. 1985 Dec;22(6):934–939. doi: 10.1128/jcm.22.6.934-939.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtomäki K., Julkunen I., Sandelin K., Salonen J., Virtanen M., Ranki M., Hovi T. Rapid diagnosis of respiratory adenovirus infections in young adult men. J Clin Microbiol. 1986 Jul;24(1):108–111. doi: 10.1128/jcm.24.1.108-111.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite J. P., Pereira H. G., Azeredo R. S., Schatzmayr H. G. Adenoviruses in faeces of children with acute gastroenteritis in Rio de Janeiro, Brazil. J Med Virol. 1985 Feb;15(2):203–209. doi: 10.1002/jmv.1890150213. [DOI] [PubMed] [Google Scholar]
- Niel C., Gomes S. A., Leite J. P., Pereira H. G. Direct detection and differentiation of fastidious and nonfastidious adenoviruses in stools by using a specific nonradioactive probe. J Clin Microbiol. 1986 Nov;24(5):785–789. doi: 10.1128/jcm.24.5.785-789.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki M., Virtanen M., Palva A., Laaksonen M., Pettersson R., Käriäinen L., Halonen P., Söderlund H. Nucleic acid sandwich hybridization in adenovirus diagnosis. Curr Top Microbiol Immunol. 1983;104:307–318. doi: 10.1007/978-3-642-68949-9_20. [DOI] [PubMed] [Google Scholar]
- Singh-Naz N., Naz R. K. Development and application of monoclonal antibodies for specific detection of human enteric adenoviruses. J Clin Microbiol. 1986 May;23(5):840–842. doi: 10.1128/jcm.23.5.840-842.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stålhandske P., Hyypiä T., Allard A., Halonen P., Pettersson U. Detection of adenoviruses in stool specimens by nucleic acid spot hybridization. J Med Virol. 1985 Jul;16(3):213–218. doi: 10.1002/jmv.1890160302. [DOI] [PubMed] [Google Scholar]
- Stålhandske P., Hyypiä T., Gadler H., Halonen P., Pettersson U. The use of molecular hybridization for demonstration of adenoviruses in human stools. Curr Top Microbiol Immunol. 1983;104:299–306. doi: 10.1007/978-3-642-68949-9_19. [DOI] [PubMed] [Google Scholar]
- Takiff H. E., Seidlin M., Krause P., Rooney J., Brandt C., Rodriguez W., Yolken R., Straus S. E. Detection of enteric adenoviruses by dot-blot hybridization using a molecularly cloned viral DNA probe. J Med Virol. 1985 Jun;16(2):107–118. doi: 10.1002/jmv.1890160203. [DOI] [PubMed] [Google Scholar]
- Takiff H. E., Straus S. E., Garon C. F. Propagation and in vitro studies of previously non-cultivable enteral adenoviruses in 293 cells. Lancet. 1981 Oct 17;2(8251):832–834. doi: 10.1016/s0140-6736(81)91104-1. [DOI] [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Johansson M. E. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984 Sep;20(3):365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw A., Davies H., Parry J. Eelectron microscopy of fatal adenovirus gastroenteritis. Lancet. 1977 Feb 12;1(8007):361–361. doi: 10.1016/s0140-6736(77)91158-8. [DOI] [PubMed] [Google Scholar]
- Wigand R., Baumeister H. G., Maass G., Kühn J., Hammer H. J. Isolation and identification of enteric adenoviruses. J Med Virol. 1983;11(3):233–240. doi: 10.1002/jmv.1890110306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J. C., Wigand R., Kidd A. H., Wadell G., Kapsenberg J. G., Muzerie C. J., Wermenbol A. G., Firtzlaff R. G. Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stool. J Med Virol. 1983;11(3):215–231. doi: 10.1002/jmv.1890110305. [DOI] [PubMed] [Google Scholar]