Abstract
Previous neuroimaging studies have implicated the prefrontal cortex (PFC) and nearby brain regions in deception. This is consistent with the hypothesis that lying involves the executive control system. To date, the nature of the contribution of different aspects of executive control to deception, however, remains unclear. In the present study, we utilized an activation likelihood estimate (ALE) method of meta-analysis to quantitatively identify brain regions that are consistently more active for deceptive responses relative to truthful responses across past studies. We then contrasted the results with additional ALE maps generated for 3 different aspects of executive control: working memory, inhibitory control, and task switching. Deception-related regions in dorsolateral PFC and posterior parietal cortex were selectively associated with working memory. Additional deception regions in ventrolateral PFC, anterior insula, and anterior cingulate cortex were associated with multiple aspects of executive control. In contrast, deception-related regions in bilateral inferior parietal lobule were not associated with any of the 3 executive control constructs. Our findings support the notion that executive control processes, particularly working memory, and their associated neural substrates play an integral role in deception. This work provides a foundation for future research on the neurocognitive basis of deception.
Keywords: anterior cingulate, fMRI, lie detection, lying, neuroimaging, prefrontal cortex
Deception can be broadly defined as the attempt to mislead. Although researchers have long been interested in deception and the ability to detect deception (Langfeld 1920; Adler and Larson 1928), until recently such efforts have been limited to the study of indirect physiological and behavioral data such as electrodermal conductance (Guertin and Wilhelm 1954), pupil dilation (Berrien and Huntington 1943), heart rate (Cutrow et al. 1972), and errors in responding (Kintz 1975). Others have gained insight from the study of deception in brain-injured patients and psychologically disturbed individuals (Wiley 1998).
Technological advances have provided new tools for studying deception. For example, scalp-recorded event-related potentials (ERPs) have provided insights into deception (Rosenfeld 2001; Johnson et al. 2004), but the low spatial resolution inherent in ERP methodology has hindered the ability to localize the underlying brain regions involved (Fender 1987; Gonzalez Andino et al. 2001). A growing number of researchers have used functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) to study deception (Spence et al. 2001, 2004; Langleben et al. 2002, 2005; Lee et al. 2002, 2005; Ganis et al. 2003; Kozel, Padgett, and George 2004; Kozel, Revell, et al. 2004; Davatzikos et al. 2005; Kozel et al. 2005; Nuñez et al. 2005; Phan et al. 2005; Abe et al. 2006; Mohamed et al. 2006).
As with prior research, recent neuroimaging studies may be conceptualized as arising from 1 of 2 primary motivations: 1) to detect deception or 2) to differentiate of the neurocognitive processes underlying deception (“differentiation of deception,” Furedy et al. 1988). Although these aims are not necessarily mutually exclusive, it is often the case that studies designed for one purpose are not optimal for the other (for additional discussion, see Furedy et al. 1988).
For example, in one of the first neuroimaging studies on deception, Langleben et al. (2002) employed a variation of the Guilty Knowledge Test (GKT) (Lykken 1959, 1960; Furedy and Ben-Shakhar 1991), a questioning technique that has been used extensively in the forensic field. Importantly, the traditional GKT relies not only on the detection of deception per se but also on the detection of recognition memory for details of the crime scene (Ben-Shakhar and Elaad 2003). In the study of Langleben et al., participants were given a playing card (e.g., a 5 of clubs) and instructed to deny their possession of the card when later queried. Once in the scanner, participants were shown a series of playing cards and were then asked whether or not they possessed each card. With the exception of the aforementioned target card (e.g., 5 of clubs), participants responded truthfully to all cards. Whereas this paradigm may be effective in evaluating the usefulness of neuroimaging techniques in detecting deception, it provides minimal insight into deception as a psychological process insomuch as deceptive responding is confounded with recognition memory for the relevant playing card.
In contrast, several neuroimaging studies have taken a differentiation of deception approach (e.g., Furedy et al. 1988) in hopes of identifying those cognitive processes and associated neural substrates inherent to deception. These studies typically have studied deceptive responding as it relates to 1) personal possession of an item (within the context of a modified version of the aforementioned GKT paradigm), 2) past autobiographical information and/or specific personal experiences, 3) recently completed action events, or 4) knowledge recently acquired through passive experience. In each case, the researchers compared brain activation during a truth condition, in which participants responded correctly to presented stimuli, to brain activation during a deception condition, in which participants intentionally responded incorrectly to stimuli.
Building upon the previously described study by Langleben et al. (2002), subsequent researchers have further adapted the GKT paradigm so as to account for the contribution of recognition memory and therefore provide greater insight into the neurocognitive basis of deception. In these later studies (Davatzikos et al. 2005; Langleben et al. 2005; Phan et al. 2005), researchers modified the methodology by giving participants 2 playing cards (instead of one) and instructing them to deny their possession of one of the cards (i.e., lie) but admit possession of the other card.
Another paradigm involves asking participants about past autobiographical information and/or salient events from their past and prompting them to respond incorrectly (i.e., lie) on a subset of the test items. Nuñez et al. (2005) gave participants a yes/no memory test for autobiographical knowledge (e.g., “Can you ride a bicycle?”) as well as nonautobiographical knowledge (e.g., “Is New York City in Ohio?”). Ganis et al. (2003) gathered information about specific past personal experiences from participants. Later, participants were prompted to generate lies based on rehearsed and unrehearsed false information relating to these past experiences, thus allowing the researchers to make a further distinction between memorized and spontaneous lies. Spence et al. (2008) had participants recount past personal events that someone would typically want to conceal (because the incident was awkward, embarrassing, etc.). Participants were then asked questions about these events and were directed to lie on a subset of the questions (participants were free to choose which items to lie vs. tell the truth on). Studies by Lee et al. (2002, 2005) focused on another particular instance of deception: feigned memory impairments. In these studies, participants were administered a simple visual matching task or a short-answer autobiographical memory test (e.g., “Where were you born?”). In separate conditions, participants were prompted to respond correctly, incorrectly, randomly, or so as to feign memory impairment.
Other studies have focused on deception as it relates to recently completed action events. Both Spence et al. (2001) and Abe et al. (2006) had participants selectively lie during performance of a yes/no memory test for action events that they may have engaged in earlier in the day. Whereas Spence et al. focused on action events that participants may have completed as part of their typical daily routine (e.g., making the bed), Abe et al. had participants actively engage in a subset of action events that were administered by the experimenters shortly before the scanning session. In a similar vein, Mohamed et al. (2006) had a subset of participants perform a single salient action event (i.e., the firing of a starter pistol) and later deny having done so.
Kozel et al. (2005) instructed participants to “steal” a watch or ring (their choice) and place it among their personal belongings in a locker. Later in the scanner, participants were asked questions about taking the items (which they were to deny) as well as general knowledge questions (e.g., “Is it October?”) and questions about minor wrongful behaviors that they may have engaged in (e.g., “Do you speed?”). Participants were told to respond truthfully to these latter 2 types of questions.
Lastly, studies by Kozel, Padgett, and George (2004) and Kozel, Revell, et al. (2004) focused not on memory for action events per se but on knowledge gained through past personal action. Specifically, participants were prompted to lie about the location of money (i.e., a $50 bill) which they had learned through recent personal experience (i.e., participants were instructed to search under various items in a room to locate the money).
Despite the general commonalities noted above, the specific methodology utilized to generate a deceptive response varied across studies and involved differences in context, motivation, spontaneity, and response modality. Given this variability and the apparent difficulty of replication across studies, it is perhaps not surprising that few consistent findings have emerged.
One potential exception relates to the prefrontal cortex (PFC) and anterior cingulate cortex (ACC). Indeed, a majority of the aforementioned studies have found deception-related activity in aspects of one or both of these brain areas. From a cognitive standpoint, this is consistent with the conceptualization of deception as an executive control intensive task (e.g., Spence et al. 2001; Langleben et al. 2002; Johnson et al. 2004; Iacono 2007; Langleben 2008; Spence 2008).
Generally speaking, executive control refers to a set of higher order cognitive processes that allow for the flexible modification of thought and behavior in response to changing cognitive or environmental contexts (Stuss 1992). Converging evidence from patient, animal, and neuroimaging studies (e.g., Goldman-Rakic 1990; Cummings 1993; D'Esposito et al. 1998) suggest that the PFC and surrounding brain regions play a particularly important role in executive control.
Recent work by Miyake and colleagues (Miyake et al. 2000) using a latent variable approach to analyze data from a large sample of young adults on several complex cognitive tasks suggests that executive control may be best conceptualized as comprising at least 3 different component processes: 1) working memory, 2) task switching, and 3) inhibitory control. Within this context, it has been hypothesized that all 3 aspects of executive control may contribute to deception to the extent that deception involves: keeping the truth in mind while formulating a deceptive response (working memory), suppressing a truthful response (inhibitory control), and switching between truthful and deceptive responses (task switching) (e.g., Johnson et al. 2004; Langleben 2008; Spence et al. 2008).
The PFC and ACC are relatively large cortical areas, each comprising multiple regions that may be functionally and anatomically distinct. For example, the dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal cortex (VLPFC) represent 2 subregions of the PFC and have been implicated in maintenance and manipulation of information, respectively (D'Esposito et al. 1998). The ACC is suggested to include dorsal and rostral–ventral subregions that play a role in cognitive and affective processing, respectively (Bush et al. 2000). It is thus of interest to ascertain whether the aforementioned consistent finding of deception-related activity in the PFC and ACC can be further localized to particular subregions of these brain areas.
Previous attempts to address this question have been limited to narrative (e.g., Iacono 2007) and/or table-based literature reviews (e.g., Spence 2008). Such approaches are inherently qualitative in nature and must be interpreted with caution given that they typically rely on author-supplied anatomical labels that may be unduly broad (e.g., “PFC”) or, in some cases, inaccurate. (For example, in their meta-analysis on the Stroop task, Laird, McMillan, et al. [2005] found that, for more than 25% of the reported foci, the author-provided anatomical labels did not agree with the corresponding atlas-derived labels.) Comparison of reported focus coordinates across studies can also prove challenging in that localization of a given set of coordinates to a particular neuroanatomical location is dependent on the target brain atlas and corresponding stereotaxic space in which the data set was registered.
In the present study, recent advancements in meta-analytic computation and foci transformation are brought to bear on this topic. We utilize an activation likelihood estimate (ALE) method of meta-analysis to more precisely identify brain regions that consistently show deception-related activity across past studies. In addition, we employ a new method for translating disparate foci into a common stereotaxic space, thereby improving the accuracy of the quantitative meta-analysis.
This study has 2 major aims. The first is to identify “core” brain regions consistently involved in deception, regardless of the precise nature of the deceptive act. Based on previous research, we hypothesize that these core brain regions will encompass aspects of the PFC and associated brain regions. The second is to evaluate whether different regions implicated in deception are engaged in different aspects of executive control. To this end, we contrast the derived deception ALE map with additional ALE meta-analysis maps generated based on the previous imaging studies focusing on working memory, inhibitory control, and task switching. Consistent with the hypothesis that all 3 aspects of executive control play a role in deception, we predict that the deception ALE map will overlap significantly with each of the 3 executive control ALE maps.
Materials and Methods
Deception Meta-analysis
Consistent with the notion that deception is a more demanding cognitive task than simply telling the truth, few studies report regions showing greater activation for truthful responding as compared with deceptive responding (but see Langleben et al. 2005). Accordingly, we focused our present efforts on the much more common findings of increased neural activation for deceptive relative to truthful responses. Further, in line with previous ALE meta-analyses (e.g., Turkeltaub et al. 2002; Buchsbaum et al. 2005; Owen et al. 2005), the measure of interest was the location of such activation rather than effect size (see Discussion).
To identify appropriate articles for the deception meta-analysis, several online electronic databases (e.g., PsychInfo, MedLine, PubMed) were searched in April 2008 using various combinations of relevant search terms (e.g., deception, lying, fMRI, PET, MRI, neuroimaging). The following inclusion/exclusion criteria were used to select articles for the present meta-analysis:
Only articles that utilized PET or fMRI methodology were considered. Electrophysiological- (e.g., electroencephalography, magnetoencephalography, skin conductance response [SCR]) and behavioral-only studies were excluded. Both blocked and event-related studies were allowed, in order to obtain sufficient data to conduct the meta-analysis. Activations recorded using a block design may represent both transient item-related activity as well as sustained activity related to task set (Visscher et al. 2003). Activations observed in event-related studies reflect only the transient component. As such, brain regions identified via the meta-analysis as showing consistent activation across these 2 different types of studies likely reflect the common component: item-related activity. Similar limitations prevented us from considering additional dimensions (e.g., commonalities/differences in behavioral paradigms, sample characteristics, etc.) in separate meta-analyses.
Only articles with experiments that yielded a clear contrast representing locations of greater activation for deceptive responding as compared with telling the truth and that did not include an obvious limitation (e.g., confound with recognition memory; Langleben et al. 2002) were included.
Only articles that reported areas of peak activation for the lie versus truth contrast in a standardized coordinate space (e.g., Talairach and Tournoux 1988) were considered. Other articles (e.g., only reported Brodmann areas [BAs] or only showed contrast maps) were excluded.
Only peer-reviewed articles reporting previously unpublished data involving a sample size of at least 7 participants were included.
Twelve studies met these criteria and were included in the present meta-analysis. A total of 173 activation foci representing regions of significantly greater activation for deceptive responses (i.e., lies) as compared with truthful responses were compiled from these studies. These peak activations were then used to generate an ALE map (Turkeltaub et al. 2002) to identify brain regions that are frequently implicated in deception across a variety of experimental situations. Table 1 summarizes the included studies.
Table 1.
Data sources included in the deception meta-analysis
| Publication | Foci | Method | Original stereotaxic space | Response |
| Modified GKT paradigm | ||||
| Langleben et al. (2005) | 19 | fMRI | SPM2 | Manual |
| Phan et al. (2005) | 11 | fMRI | SPM99 | Manual |
| Past personal information/experience | ||||
| Ganis et al. (2003) | 12 | fMRI | AFNI | Manual and verbal |
| Lee et al. (2002)—Experiment 2 | 22 | fMRI | AFNIa | Manual |
| Nuñez et al. (2005) | 8 | fMRI | SPM2 | Manual |
| Spence et al. (2004) | 5 | fMRI | SPM99 | Verbal |
| Spence et al. (2008) | 7 | fMRI | SPM2 | Verbal |
| Recent action events | ||||
| Abe et al. (2006) | 4 | PET | SPM2 | Verbal |
| Kozel et al. (2005) | 32 | fMRI | SPM2 | Manual |
| Spence et al. (2001) | 6 | fMRI | SPM99 | Manual |
| Recent knowledge | ||||
| Kozel, Padgett, and George (2004) | 11 | fMRI | SPM2 | Manual |
| Kozel, Revell, et al. (2004) | 10 | fMRI | SPM96 | Manual |
| Lee et al. (2002)—Experiment 1 | 26 | fMRI | AFNIa | Manual |
The authors appear to have utilized a nonstandard registration algorithm; however, the target space (T88) was identical to that employed in AFNI.
Executive Control Meta-analyses
We also generated ALE meta-analysis maps representing brain regions consistently found to be involved in different aspects of executive control, namely working memory, inhibitory control, and task switching. For the working memory map, we adopted studies (24) and associated foci (668) identified in a previously published meta-analysis of the n-back task, a widely used working memory measure (Owen et al. 2005). The studies (19) and foci (205) utilized for the inhibitory control map were based on a previous ALE meta-analysis of the Stroop color-word task published by Laird, McMillan, et al. (2005). Similarly, the studies (18) and foci (231) for the task switching map were the same as those used by Buchsbaum et al. (2005) in their meta-analysis on this latter construct.
The inclusion/exclusion criteria were generally similar across the aforementioned studies and the present deception ALE meta-analysis (e.g., use of PET or fMRI methodology, 3-dimensional [3D] coordinates reported in stereotaxic space, inclusion of canonical contrast of 2 conditions, data from neurologically uncompromised participants). Detailed descriptions of the inclusion criteria are available in the original publications (Buchsbaum et al. 2005; Laird, McMillan, et al. 2005; Owen et al. 2005) and are not reproduced here. Any modest variations in criteria across studies reflect the relative maturity and current state of research in the respective area; therefore, these differences were maintained for the present comparisons. For example, in the case of the working memory and inhibitory control ALE maps, there was sufficient data available to focus in on a specific experimental paradigm (n-back task and Stroop task, respectively), whereas for task switching and the present deception ALE maps, this was not possible.
Projecting Foci by Study-Specific Stereotaxic Projection to the PALS-B12 Atlas Surface
All the compiled foci had been translated previously into one or another standardized stereotaxic atlas space (e.g., Talairach and Tournoux 1988); however, the precise coordinates of the foci were derived using a variety of atlas targets and volume registration methods (e.g., Statistical Parametric Mapping [SPM], AFNI). These differences in anatomical templates and registration algorithms can result in substantial differences in the stereotaxic coordinates of a given geographic location (Van Essen 2005; see below). To compensate for these differences, we utilized Caret 5.5 software (http://brainvis.wustl.edu/caret/) (Van Essen et al. 2001) along with the population-average landmark- and surface-based atlas (PALS) (Van Essen 2005) to translate stereotaxic coordinates from different studies into a common atlas space (Van Essen and Dierker 2007).
Using structural MRI volumes obtained from a group of 12 neurologically uncompromised young adults, Van Essen (2005) generated the PALS-B12 atlas in the Washington University 711-2C space (Buckner et al. 2004; Head et al. 2005) by a process that involved surface-based registration to a population-average target using a standard set of geographic landmarks. Each of the 12 contributing individual hemispheres was resampled to a “standard mesh” format (Saad et al. 2004), represented by 73 730 surface points (nodes) per hemisphere. The 12 contributing left hemispheres were spatially averaged (node-by-node) to generate an average fiducial left hemisphere surface in 711-2C space; the same was done for the 12 individual right hemispheres. Each node on the left or right 711-2C average fiducial surface (e.g., node #14538) represents a particular location in that space and is associated with a cloud of points at geographically corresponding locations in the 12 contributing hemispheres (Van Essen 2005).
To generate average fiducial surfaces for other stereotaxic spaces, each of the PALS-B12 individual brain volumes (starting in 711-2C space) was registered to 5 other stereotaxic spaces (SPM99, SPM2, AFNI, FLIRT, and MRITOTAL) using the methods provided in these other software packages. (The default volume registration algorithms associated with each analysis method were utilized in generating each average fiducial surface. If a given neuroimaging study used a nonstandard processing stream, this might lead to slight deviations in the relationship of the reported foci to geographic landmarks in the PALS atlas surface. However, any such deviations are likely to be much smaller than the misalignments that would occur if comparisons were made by projecting foci from different studies to a single atlas surface that did not respect the stereotaxic space in which the foci were reported [Van Essen and Dierker 2007].) The resulting deformation (e.g., affine matrix for FLIRT and MRITOTAL, piecewise linear transformation prescribed in the +tlrc.HEAD for AFNI, and *sn.mat for SPM) for each individual volume was then applied to each hemisphere's 711-2C surface coordinates, resulting in surfaces for each target space. PALS-B12 average fiducial surfaces were then generated for the left and right hemispheres in each of these stereotaxic spaces using the same spatial averaging process described above. In addition, SPM96 atlas space was equated to MRITOTAL and SPM95 space to AFNI, based on how these earlier registration methods were carried out.
Each node (e.g., node #14538) in the PALS-B12 standard mesh surface represents a particular geographic location (e.g., the medial tip of the central sulcus) on the left or right hemisphere cortical surface but can have substantially different spatial coordinates, depending on the stereotaxic space in which the average fiducial surface is visualized. The differences are largest when comparing spaces in which the target has the dimensions of the original Talaiarach and Tournoux (1988) brain (e.g., AFNI space) to spaces in which the target is the MNI152 population-average brain (e.g., FLIRT, SPM99) or the MNI305 population-average brain (e.g., MRITOTAL). The differences also depend upon brain region and can exceed 1 cm in regions near the dorsal, ventral, anterior, or posterior extrema of the hemisphere.
Recently added functionality in Caret (version 5.5 and higher) allows the user to project stereotaxic foci from the stereotaxic space in which they were originally reported into any other of the 7 previously mentioned stereotaxic spaces (tutorial document and data at http://sumsdb.wustl.edu/sums/directory.do?id=658520&dir_name=CARET_TUTORIAL_SEPT-06). This is done by preserving the spatial relationship between each focus and the nearest tile of the average fiducial surface for the appropriate stereotaxic space. Using this method, all the foci in the present meta-analysis were translated into a common stereotaxic space (FLIRT space). The choice of FLIRT (http://www.fmrib.ox.ac.uk/fsl/flirt/) was motivated by the desirability of using a target atlas (the MNI152 population average) whose dimensions are representative of a normal population and a registration algorithm that is robust and widely used (Van Essen and Dierker 2007). The stereotaxic coordinates for any of the other spaces can be determined by downloading the data and viewing the foci of interest in relation to the average fiducial surface of the stereotaxic space of interest.
ALE Map Generation
We utilized the methodology and software provided by Turkeltaub et al. (2002) to generate a whole-brain statistical map representing the likelihood of activation on a voxel-by-voxel basis. In brief, a 3D Gaussian distribution, with a standard deviation of 6 mm (Full width at half maximum = 15 mm), was used to model the localization probability distribution for each activation focus. The resulting values were then multiplied by a factor of 8 mm3 to reflect the fact that our target spatial resolution was 2 × 2 × 2 mm, and our interest was in the probability of a focus lying “anywhere” within a given voxel rather than at the center of the voxel. This process was repeated such that 172 probability values (one for each of the activation foci obtained from the deception literature) were generated for each voxel. (For the working memory, inhibitory control, and task switching ALE analyses, 668, 205, and 231 probability values were generated, respectively.) These values were, in turn, used to calculate the likelihood that at least one of the activation foci fell within a given voxel. The result was a whole-brain ALE map.
The above described procedure was repeated for 5000 permutations of randomly distributed foci, and the resulting values were used to calculate the expected probability value for a given voxel under the null hypothesis (i.e., a random distribution of foci) at various levels of statistical significance (for more detailed description, see Turkeltaub et al. 2002). The output of this analysis then was used to threshold our whole-brain ALE map so as to achieve a P value of 0.05 while controlling for false discovery rate (Genovese et al. 2002; Laird, Fox, et al. 2005). Localization of significant regions of interest (ROIs) to particular geographic regions was based on a probabilistic map of sulcal identity generated using the 12 contributing brains of the PALS-B12 atlas (Van Essen 2005). Localization to particular cortical areas was based on maps of cortical areas registered to the PALS atlas from the partitioning schemes of Brodmann (1909) and Öngür et al. (2003).
The present data set, as well as other data sets utilizing the PALS-B12 atlas, are available for further visualization and analysis via the SumsDB database (http://sumsdb.wustl.edu/sums/directory.do?id=6600996). Turkeltaub et al. also have generously made their ALE software available online (http://csl.georgetown.edu/software/).
Results
Deception Meta-analysis
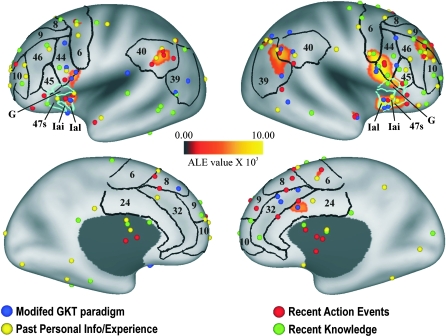
Figure 1 shows the spatial locations of 173 deception-related activation foci displayed in relation to the PALS-B12–inflated left and right hemisphere atlas surfaces. The boundaries of selected Brodmann's (1909) cytoarchitectonic areas (black borders) and the areas of Öngür et al. (2003, light blue borders) as charted on the PALS-B12 atlas surface (Van Essen 2005) are also shown. Foci are scattered across many BAs but with relatively high incidence in areas 24/32, 39/40, 44/45, and 9/10/46 on the right and areas 6, 40, and 44 on the left. The number of foci in the right hemisphere (93) is slightly greater than in the left hemisphere (75), with 5 foci falling on or near the hemispheric midline.
Figure 1.
Previously reported foci demonstrating greater activation for deceptive responses (i.e., lies) as compared with truthful responses overlaid on the results from the ALE meta-analysis. ALE data were thresholded at a value of 0.00502 (which corresponds to p < 0.05 False Discovery Rate corrected). Foci were projected by study-specific stereotaxic projection to the PALS-B12 atlas surface (see Materials and Methods) and are viewed on the inflated PALS atlas surface (Van Essen 2005), color coded based on paradigm type/content. The upper and lower panels show foci in relation to lateral and medial views of the average fiducial surface, respectively. Selected classical Brodmann areas (black borders) as well as orbitofrontal areas (light blue borders) from Öngür et al. (2003) are also illustrated. On all surfaces, foci are shown “pasted” to the surface, irrespective of whether their 3D coordinates lie above or below the surface.
An automated peak search algorithm identified the location (in atlas coordinates) of peak activations within the ALE map on the basis of level of statistical significance with the proviso that they be separated by 12 mm, or else, the peaks were consolidated by coordinate averaging. Regions around the peak activations were identified by choosing contiguous voxels within 10 mm of the peak activation that surpassed the statistical threshold within the z plane of peak activity and in both of the contiguous planes. This method revealed 13 ROIs in the whole-brain volume (Table 2), including 7 ROIs in the right hemisphere, 5 in the left, and 1 (region 12) along the midline. Two ROIs (regions 5 and 8) are centered in subcortical regions in and near the internal capsule and did not intersect the PALS average fiducial surface; these are not considered further. The total volume of right hemisphere ROIs (16.3 cm3) greatly exceeded that of left hemisphere ROIs (4.7 cm3). The greater right hemisphere bias by the ALE analysis presumably reflects tighter clustering of foci on the right.
Table 2.
ROIs identified from ALE analysis (deception > truth) in FLIRT stereotaxic space
| Region | Location | BA | Peak activation |
Volume (cm3) | ALE value × 103* | ||
| x | y | z | |||||
| 1 | Right insula | NA | 37 | 20 | −6 | 3.7 | 10.09 |
| 2 | Right IFG | 6/44/45 | 52 | 14 | 6 | 3.7 | 10.13 |
| 3 | Right middle frontal gyrus | 9/10/46 | 32 | 43 | 26 | 3.4 | 9.29 |
| 4 | Right inferior parietal lobule/supramarginal gyrus | 39/40 | 59 | −50 | 29 | 2.0 | 8.33 |
| 5 | Right internal capsule/thalamus | NA | 13 | −5 | 12 | 1.5 | 8.13 |
| 6 | Left IFG | 44 | −49 | 15 | −4 | 1.4 | 7.23 |
| 7 | Left inferior parietal lobule | 40 | −57 | −49 | 31 | 1.2 | 7.12 |
| 8 | Left internal capsule | NA | −16 | 1 | 13 | 0.8 | 6.41 |
| 9 | Left insula | NA | −35 | 13 | 2 | 0.9 | 6.36 |
| 10 | Left precentral gyrus/middle frontal gyrus | 6 | −42 | 1 | 53 | 0.4 | 6.11 |
| 11 | Right insula | NA | 35 | 30 | −5 | 0.9 | 6.65 |
| 12 | Right anterior cingulate | 24/32 | 5 | 20 | 34 | 0.8 | 5.54 |
| 13 | Right inferior parietal lobule | 7/39 | 47 | −65 | 42 | 0.3 | 5.64 |
Note: NA, not applicable.
P < 0.05 (FDR corrected) in all instances.
Figure 1 also shows the ROIs derived from the ALE analysis after mapping to the PALS atlas surfaces. Comparison with the overlay of the 173 individual foci shows that the significant ROIs generally reflect clusters of foci involving more than one paradigm type; none are associated with just a single study or even paradigm type. About half of the foci are situated far from a significant ROI and thus do not contribute to the remaining analyses.
Regions that were bilaterally symmetric include pars opercularis of the inferior frontal gyrus (IFG, regions 2 and 6), anterior insula (regions 1 and 9), and angular sulcus in the inferior parietal lobule (regions 4 and 7). The unilateral ROIs include left middle frontal gyrus (region 10), the right ACC (region 12), the right intermediate frontal sulcus (region 3), and the right intraparietal sulcus (region 13). The precise location and size of each ROI is detailed in Table 2.
Contributions of Different Paradigms
As noted earlier, sufficient data were not available to conduct a separate meta-analysis for each paradigm type. However, visual inspection of Figure 1 confirms that foci from multiple paradigms appear to contribute to each of the ROIs. For instance, foci from all 4 paradigm types (modified GKT, past personal info/experience, recent action events, and recent knowledge) are associated with the right IFG ROI (region 2). This finding further supports the hypothesis that the identified ROIs are core regions associated with deception across a variety of contexts.
Comparison between Deception and Executive Control Meta-analyses
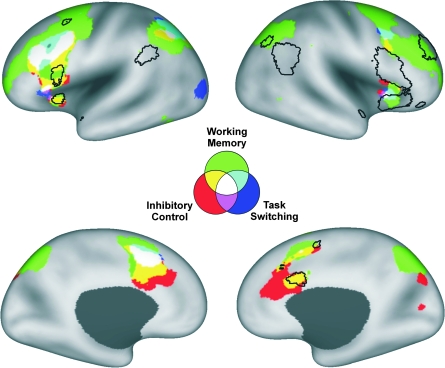
Figure 2 shows the results of the working memory, inhibitory control, and task switching ALE meta-analyses (green, red, and blue, respectively) in relation to the PALS-B12–inflated left and right hemisphere atlas surfaces. There was significant overlap among all 3 executive control maps in portions of the bilateral VLPFC (BA 44/45 and insular regions G and Ial), left DLPFC (BA 6/44/46), left ACC (BA 32), and left posterior parietal cortex (BA 7). Additional overlap between the working memory and inhibitory control ALE maps was observed in the right ACC (BA 24/32).
Figure 2.
Results of the working memory (green), inhibitory control (red), task switching (blue), and deception (black borders) ALE analyses viewed on the inflated PALS atlas surface (Van Essen 2005).
Overall, the working memory ALE map (green) was more extensive than the maps associated with either of the 2 other constructs. Large regions in the right DLPFC (BA 6/10/44/46) and right posterior parietal cortex (BA 7) were associated working memory, but not inhibitory control or task switching. Rostral aspects of the bilateral ACC (right > left) were uniquely associated inhibitory control relative to working memory and task switching. In terms of the task switching ALE map, a region in the left occipital cortex (BA 19) was associated with task switching but not working memory or inhibitory control.
Figure 2 also allows for comparison of the executive control ALE maps and the deception ALE map (regions outlined in black borders). Overlap between the executive control ALE maps and the deception ALE map occurred in the bilateral anterior insula (regions 1 and 9), left IFG (region 6), left middle frontal gyrus (region 10), right intermediate frontal sulcus (region 3), right ACC (region 12), and right intraparietal sulcus (region 13). There was little or no overlap between the executive control and deception ALE maps in bilateral inferior parietal lobule (regions 4 and 7) and right IFG (region 2).
Discussion
The present study supports the hypothesis that prefrontal brain regions play a significant role in deception. Eight of the 13 brain regions identified as consistently showing deception-related activity across studies were located in or near the PFC. This included bilateral aspects of the VLPFC, DLPFC, and anterior insula as well as right ACC.
As noted earlier, a possible explanation for the extensive involvement of the PFC in deception relates to the purported role of executive control processes, particularly working memory (e.g., keeping truth in mind while formulating a deceptive response), inhibitory control (e.g., suppressing a truthful response), and task switching (e.g., switching between truthful and deceptive responses) in deception. To evaluate this possibility, we compared the results from the deception ALE analysis with those from ALE maps generated separately for each of the aforementioned aspects of executive control.
Significant overlap was observed between regions involved in deception and those underlying executive control—suggesting that the aforementioned cognitive processes may indeed contribute to the psychological phenomenon of deception. Specifically, we found that a majority (10 of 13) of the deception-related brain regions were also associated with working memory, inhibitory control, and/or task switching.
“General Use” Executive Control Regions
Deception-related ROIs were identified in bilateral IFG (BA 44), bilateral insular regions, and right ACC (BA 24). The ROIs in the left and right pars opercularis (regions 2 and 6) are centered in the ventral part of area 44 but also extends into part of areas 6 and 45 and gustatory cortex (area G) in the right hemisphere. The ROIs in the anterior insula involves agranular areas Iai and Ial, plus part of area 47s (Öngür et al. 2003). Another ROI is located in the right dorsal ACC (BA 24).
Results from previous studies (e.g., Buchsbaum et al. 2005; Dosenbach et al. 2006) suggest that the frontal regions (i.e., bilateral IFG and insula; ACC) encompassed by the aforementioned ROIs may contribute generally to executive control rather than being associated with one particular aspect of executive control. These regions have been found to be involved in both maintenance of task set as well as moment-by-moment implication of cognitive control (Dosenbach et al. 2006). Consistent with this notion, we found that all the ROIs (with the exception noted below) overlapped with more than one of the ALE maps for working memory, inhibitory control, and task switching. As such, it is difficult to speculate on the specific role that these regions may play in deception above and beyond their general involvement in executive control.
The executive control ALE maps, particularly those associated with working memory and inhibitory control, were more extensive in the left VLPFC than the right. This left hemisphere bias is likely related to the verbal nature of the Stroop task upon which the inhibitory control meta-analysis was based as well as the disproportional contribution of studies utilizing verbal stimuli as compared with those utilizing nonverbal stimuli to the working memory meta-analysis. (Indeed, a previous meta-analysis of the go/no-go paradigm, a nonverbal inhibitory task, found a right-sided bias [Buchsbaum et al. 2005].) Interestingly, although deception is typically conceptualized as a verbal task and the majority of deception studies that contributed to the present meta-analysis involved verbal content, a similar lateralization was not observed in the deception ALE map. Specifically, the deception ALE map encompassed not only a portion of the left IFG (region 6) but also a large region in the right IFG (region 2), suggesting that both verbal and nonverbal aspects of executive control are involved in deception.
In addition to their general involvement in executive control, the insula and parainsular regions are implicated in the control of visceral functions (Augustine 1996) and representation of the body's interoceptive activity (Craig 2003). It is also widely accepted that visceral responses (e.g., blood pressure, heart rate, body temperature) often accompany deception (e.g, Chappell 1929; Cutrow et al. 1972). Hence, it is not surprising that regions involved in visceral function and interoceptive activity also show deception-related responses.
Working Memory–Related Regions
The deception ALE meta-analysis revealed 3 ROIs that overlap with regions implicated in working memory but not inhibitory control or task switching in the ALE maps. These include a region in and near the middle frontal gyrus in the anterior right PFC (region 3), a region in dorsal aspects of the right inferior parietal lobule (region 13), and a small ROI near the junction of the left middle frontal and precentral gyri (region 10).
In contrast, there was no evidence of any cortical area showing isolated overlap between the deception ALE map and either the inhibitory control or task switching maps. This suggests that, whereas these 2 aspects of executive control may play a role in deception, their involvement does not necessitate the recruitment of function-specific regions. Taken together, the current findings support the notion that working memory plays a particularly important role in deception.
Nonexecutive Control Regions
Additional deception-related peak ALE activations were evident in the left and right inferior parietal lobules (regions 4 and 7). These regions did not overlap with any of the 3 executive control ALE maps, suggesting that their involvement in deception may be related to neurocognitive processes other than working memory, inhibitory control, or task switching. Whereas superior parietal areas and more dorsal aspects of the inferior parietal lobule (including region 13) were associated with executive control, the aforementioned 2 parietal ROIs were located ventral to these areas.
These inferior parietal ROIs comprise brain regions that, in concert with more frontal regions, have been implicated previously in selective attention (e.g., Lynch 1980; Petersen et al. 1989) and the detection of salient low-frequency (odd ball) target events (e.g., Linden et al. 1999; Stevens et al. 2000; Kiehl et al. 2001). Within this context, results from Langleben et al. (2005) suggest that activity in the right IPL may be modulated not only by the intent of the participant (truthful responding vs. lying) but also by the relative frequency/saliency of the associated response. The aforementioned study included 3 conditions: a lie condition and 2 truth conditions. In one truth condition, correct responding was associated with the same response (no) as lie trials. In the other truth condition, correct responding was associated with a different, much less frequent response (yes). The results showed greater activation in the right IPL for the lie condition as compared with the same response truth condition. Interestingly, the reverse was true when comparing the lie condition to the latter more salient truth condition. The right IPL showed greater activation for the high saliency truth condition than the lie condition.
Taken together, these findings suggest that the IPL may play a role in maintaining attention to environmental context in order to detect (and respond) appropriately when instances requiring deception arise. Further, its contribution appears to be most evident in situations where the lie condition is equally (if not more) salient as compared with the truth condition. This may help to explain why some deception studies (presumably those with relatively more salient truth conditions) have failed to find lie-related activity in this region (e.g., Ganis et al. 2003; Kozel, Padgett, and George 2004; Kozel, Revell, et al. 2004; Nuñez et al. 2005). Additional research is necessary to more fully understand the role of the IPL in deception.
Interestingly, the link between these parietal association areas and the act of lying is further bolstered by findings that the skin conductance response, an often studied physiological indicator of possible deception, appears to be mediated by these regions (Tranel and Damasio 1994; Critchley et al. 2000).
In summary, the present meta-analyses provide additional insight into the role of different aspects of executive control and their associated neural substrates in deception. Working memory appears to play an important role in deception insomuch as deception-related regions in left DLPFC, right anterior PFC, and right posterior parietal cortex were uniquely associated with working memory (as compared with the other studied aspects of executive control). Regions in VLPFC, insular areas, and ACC may contribute to multiple aspects of executive control (e.g., working memory and inhibitory control) that are involved in deception. In contrast, deception-related regions in bilateral inferior parietal lobule were not associated with any of the 3 executive control constructs. We speculate that the contribution of these regions to deception may be related to attentional control. Lastly, little overlap was observed between the deception and task switching ALE maps thus bringing into question the contribution of this aspect of executive control to deception.
Additional Avenues for Future Research
To the extent that the paradigms utilized in previous neuroimaging studies of deception do not emulate real-life instances of deception, it remains possible that there are additional neurocognitive processes that are involved in deception but that are not revealed by the present meta-analysis. Whereas ethical constraints will likely continue to prevent researchers from fully emulating certain aspects of real-life deceptive situations (e.g., the fear/anxiety associated with being caught, the emotional valence of the subject matter of the lie), recent advancements in fMRI methodology and analysis have allowed researchers to begin to increase the ecological validity of such studies on other fronts. For example, in a recent neuroimaging study by Spence et al. (2008), participants were able to respond vocally (presumably the typical modality for lying) and also were allowed choose when to lie versus tell the truth. Future research hopefully will continue to reduce the gap between laboratory studies and real-world instances of deception.
A major motivation for studying deception is the ultimate goal of being able to reliably detect when a given individual is being truthful versus lying. In pursuit of this goal, there are 2 main questions should be addressed: Are there specific core brain regions involved in deception that should be the focus of study? And can the patterns of brain activation observed in these regions be used to differentiate truth from lying on an instance-by-instance and/or individual-by-individual basis?
The current meta-analysis represents a significant contribution to this line of research by identifying brain regions which appear to be consistently involved in deception across various deceptive situations studied to date. Future efforts may be best directed toward identifying specific “patterns” of activation across these regions that are indicative of deception as compared with other complex cognitive tasks.
Along these lines, several studies have begun to address the second question: Can fMRI signals be used to “detect” deception? By comparing the number of voxels within a particular set of brain regions that were active during deceptive responses (relative to a neutral condition) and the number that were active during truthful responses (minus a neutral condition), Kozel et al. (2005) reported some success detecting deception at the individual participant level. In terms of discriminating lie from truth at the single individual and single-trial level, Davatzikos et al. (2005) suggest that the utilization of machine learning methods to classify spatial patterns of brain activation holds promise for event-by-event identification of deceptive acts (i.e., lying). Interestingly, 2 of the areas identified in the present meta-analysis, right BA 44 and left BA 40, were found to be informative by Davatzikos et al. in terms of classification of lie versus truth on an item-by-item basis. Langleben et al. (2005) took a slightly different approach. Employing logistic regression analysis, Langeben et al. built a predictive model based on the extent of activation in a subset of brain regions. The regions showing the highest predictive ability for classifying lie and truth trials were also identified in the present meta-analysis, namely left BA 40 and left BA 6. These studies represent an initial step in the ongoing pursuit of lie detection ability using fMRI technology.
Limitations
The present paper focused on those brain regions demonstrating greater activation for deceptive as compared with truthful responding. Regions demonstrating the opposite pattern of activation (i.e., greater activation for truthful as compared with deceptive responding) have also been reported (e.g., Langleben et al. 2005). However, there are too few published coordinates for application of ALE methodology to this issue. Future research designed to elucidate the nature of those brain regions showing greater activation for truth than deception will be important. Indeed, shedding further light on these “deactivations” is likely to be instrumental in understanding deception.
In general, the ALE approach to meta-analysis has several apparent advantages over traditional meta-analytic methods. Most importantly, it allows for quantification of both the locations of common activation and the degree of concordance across studies. In addition, the subjective aspects of the meta-analysis process are relatively limited in that most aspects of the ALE computations are automatized.
Importantly, the ALE method does not involve reanalysis of the original raw data and instead must rely on secondary analysis of results previously generated by disparate research groups utilizing different statistical approaches and thresholds. As such, the ALE approach (like other meta-analytic approaches) has limitations. For example, given that each entered focus is given equal weighting in the ALE computations, unequal contribution of foci across studies may, in turn, lead to a bias of the ALE results towards one study (or set of studies) over another. One potential source of such overrepresentation relates to the statistical approach adopted across different studies. Specifically, adoption of a less conservative statistical threshold may result in a relatively greater number of activation foci being reported for a given study as compared with another study utilizing a more conservative threshold. Similarly, ALE results can be biased by overrepresentation of one paradigm over another across the included studies. A focus for future research is the further refinement of the ALE approach thus allowing for the weighting of foci based on secondary factors such as differences in statistical thresholding and behavioral paradigms across studies. Despite such limitations and in light of the alternates, the ALE approach to meta-analysis represents a useful option for integrating findings across different studies in situations where joint reanalysis of the original raw data is not possible.
Conclusions
In conclusion, deception is a very complex phenomenon and may be best conceptualized as a confluence of multiple cognitive processes. Although researchers have long studied deception, it is only recently that technological advancements have allowed for the direct assessment of the neural substrates underlying deception. In the present study, we used newly developed meta-analysis methods to quantitatively identify those brain regions that are consistently active across a variety of situations involving deception. Results support the hypothesis that executive control processes, particularly working memory, and their associated neural substrates play an integral role in deception. This work provides a foundation for future research on the neurocognitive basis of deception.
Funding
National Science Foundation (BCS-0236651 to K.B.M.); National Institute of Mental Health, the National Institute for Biomedical Imaging and Bioengineering, and the National Science Foundation (R01-MH-60974 to D.C.V.).
Acknowledgments
SEC, LEB, and KBM reviewed the selection of relevant studies. All authors were in accord with the major conclusions. We appreciate the assistance of John Harwell and Donna Dierker in developing the Caret software and providing assistance with data analysis and presentation. We also thank Jeffrey Zacks for providing assistance with data analysis. Conflict of Interest: None declared.
References
- Abe N, Suzuki M, Tsukiura T, Mori E, Yamaguchi K, Itoh M, Fujii T. Dissociable roles of prefrontal and anterior cingulate cortices in deception. Cereb Cortex. 2006;16:192–199. doi: 10.1093/cercor/bhi097. [DOI] [PubMed] [Google Scholar]
- Adler HM, Larson JA. Deception and self-deception. J Abnorm Psychol. 1928;22:364–371. [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research: Brain Research Reviews. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Ben-Shakhar G, Elaad E. The validity of psychophysiological detection of information with the guilty knowledge test: a meta-analytic review. J Appl Psychol. 2003;88:131–151. doi: 10.1037/0021-9010.88.1.131. [DOI] [PubMed] [Google Scholar]
- Berrien FK, Huntington GH. An exploratory study of pupillary responses during deception. J Exp Psychol. 1943;32:443–449. [Google Scholar]
- Brodmann K. Liepzig (Germany): J.A. Barth; 1909. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. [Google Scholar]
- Buchsbaum BR, Greer S, Chang W, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting Task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Synder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Elliot R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging. J Neurosci. 2000;20:3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MN. Blood pressure changes in deception. Archives of Psychology. 1929;17:1–39. [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Cutrow RJ, Parks A, Lucas N, Thomas K. The objective use of multiple physiological indices in the detection of deception. Psychophysiology. 1972;9:578–588. doi: 10.1111/j.1469-8986.1972.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Ruparel K, Fan Y, Shen DG, Acharyya M, Loughead JW, Gur RC, Langleben DD. Classifying spatial patterns of brain activity with machine learning methods: application to lie detection. Neuroimage. 2005;28:663–668. doi: 10.1016/j.neuroimage.2005.08.009. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fender DH. Source localization of brain electrical activity. In: Gevins A, Remond A, editors. Handbook of electroencephalography and clinical neurophysiology. Amsterdam (The Netherlands): Elsevier; 1987. pp. 355–399. [Google Scholar]
- Furedy JJ, Ben-Shakhar G. The roles of deception, intention to deceive, and motivation to avoid detection in the psychophysiological detection of guilty knowledge. Psychophysiology. 1991;28:163–171. doi: 10.1111/j.1469-8986.1991.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Furedy JJ, Davis C, Gurevich M. Differentiation of deception as a psychological process: a psychophysiological approach. Psychophysiology. 1988;25:683–688. doi: 10.1111/j.1469-8986.1988.tb01908.x. [DOI] [PubMed] [Google Scholar]
- Ganis G, Kossyln SM, Stose S, Thompson WL, Yurgelun-Todd DA. Neural correlates of different types of deception: an fMRI investigation. Cereb Cortex. 2003;13:830–836. doi: 10.1093/cercor/13.8.830. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols S. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990;85:325–335. doi: 10.1016/s0079-6123(08)62688-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez Andino SL, Blanke O, Lantz G, Thut G, Grave de Peralta Menendez R. The use of functional constraints for the neuroelectromagnetic inverse problem: alternatives and caveats. Int J Bioelectromagnetism. 2001;3:1–17. [Google Scholar]
- Guertin WH, Wilhelm PL. A statistical analysis of the electrodermal response employed in lie detection. J Gen Psychol. 1954;51:153–160. [Google Scholar]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer's disease. Cereb Cortex. 2005;15:732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- Iacono WG. Detection of deception. In: Cacioppo J, Tassinary L, Berntson G, editors. Handbook of psychophysiology. 3rd ed. Cambridge: Cambridge University Press; 2007. pp. 688–703. [Google Scholar]
- Johnson R, Barnhardt J, Zhu J. The contribution of executive processes to deceptive responding. Neuropsychologia. 2004;42:878–901. doi: 10.1016/j.neuropsychologia.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF. An event-related fMRI study of visual and auditory oddball tasks. J Psychophysiol. 2001;15:221–240. [PubMed] [Google Scholar]
- Kintz BL. Lying on a test and in the laboratory. Psychon Bull Rev. 1975;6:207–209. [Google Scholar]
- Kozel FA, Johnson KA, Mu Q, Grenesko EL, Laken SJ, George MS. Detecting deception using functional magnetic resonance imaging. Biol Psychiatry. 2005;58:605–613. doi: 10.1016/j.biopsych.2005.07.040. [DOI] [PubMed] [Google Scholar]
- Kozel FA, Padgett TM, George MS. A replication study of the neural correlates of deception. Behav Neurosci. 2004;118:852–856. doi: 10.1037/0735-7044.118.4.852. [DOI] [PubMed] [Google Scholar]
- Kozel FA, Revell LJ, Lorberbaum JP, Shastri A, Elhai JD, Horner MD, Smith A, Nahas Z, Bohning DE, George MS. A pilot study of functional magnetic resonance imaging brain correlates of deception in healthy young men. J Neuropsychiatry Clin Neurosci. 2004;16:295–305. doi: 10.1176/jnp.16.3.295. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 2005;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfeld HS. Psychophysical symptoms of deception. J Abnorm Psychol. 1920;15:319–328. [Google Scholar]
- Langleben DD. Detection of deception with fMRI: are we there yet? Legal Criminol Psychol. 2008;13:1–9. [Google Scholar]
- Langleben DD, Loughead JW, Bilker WB, Ruparel K, Childress AR, Busch SI, Gur RC. Telling truth from lie in individual subjects with fast event-related fMRI. Hum Brain Mapp. 2005;26:262–272. doi: 10.1002/hbm.20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langleben DD, Schroeder L, Maldjian JA, McDonald GS, Ragland JD, O'Brien CP, Childress AR. Brain activity during simulated deception: an event-related functional magnetic resonance study. Neuroimage. 2002;15:727–732. doi: 10.1006/nimg.2001.1003. [DOI] [PubMed] [Google Scholar]
- Lee TMC, Liu H, Chan CCH, Ng Y, Fox PT, Gao J. Neural correlates of feigned memory impairment. Neuroimage. 2005;28:305–313. doi: 10.1016/j.neuroimage.2005.06.051. [DOI] [PubMed] [Google Scholar]
- Lee TMC, Liu H, Tan L, Chan CCH, Mahankali S, Feng C, Hou J, Fox PT, Gao J. Lie detection by functional magnetic resonance imaging. Hum Brain Mapp. 2002;15:157–164. doi: 10.1002/hbm.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DEJ, Prvulovic D, Formisano E, Völlinger M, Zanella FE, Goebel R, Dierks T. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb Cortex. 1999;9:815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- Lykken DT. The GSR in the detection of guilt. J Appl Psychol. 1959;43:385–388. [Google Scholar]
- Lykken DT. The validity of the guilty knowledge technique: the effects of faking. J Appl Psychol. 1960;44:258–262. [Google Scholar]
- Lynch JC. The functional organization of posterior parietal association cortex. Behav Brain Sci. 1980;3:485–534. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognit Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mohamed FB, Faro SH, Gordon NJ, Platek SM, Ahmad H, Williams JM. Brain mapping of deception and truth telling about an ecologically valid situation: functioning MR imaging and polygraph investigation-initial experience. Radiology. 2006;238:679–688. doi: 10.1148/radiol.2382050237. [DOI] [PubMed] [Google Scholar]
- Nuñez JM, Casey BJ, Egner T, Hare T, Hirsch J. Intentional false responding shares neural substrates with response conflict and cognitive control. Neuroimage. 2005;25:267–277. doi: 10.1016/j.neuroimage.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Öngür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Currie JN. Influences of lesions of parietal cortex on visual spatial attention in humans. Exp Brain Res. 1989;76:267–280. doi: 10.1007/BF00247887. [DOI] [PubMed] [Google Scholar]
- Phan KL, Magalhaes A, Ziemlewicz TJ, Fitzgerald DA, Green C, Smith W. Neural correlates of telling lies: a functional magnetic resonance imaging study at 4 tesla. Acad Radiol. 2005;12:164–172. doi: 10.1016/j.acra.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JP. Event-related potentials in detection of deception. In: Kleiner M, editor. Handbook of polygraphy. New York: Academic Press; 2001. pp. 265–286. [Google Scholar]
- Saad ZS, Reynolds RC, Argall BD, Japee S, Cox RW. Proceedings of the 2004 IEEE International Symposium on Biomedical Imaging Arlington, TX. 2004. SUMA: an interface for surface-based intra- and inter-subject analysis with AFNI; pp. 1510–1513. [Google Scholar]
- Spence SA. Playing devil's advocate: the case against fMRI lie detection. Legal Criminol Psychol. 2008;13:11–25. [Google Scholar]
- Spence SA, Farrow TFD, Herford AE, Wilkinson ID, Zheng Y, Woodruff PW. Behavioral and functional anatomical correlates of deception in humans. Neuroreport. 2001;12:2849–2853. doi: 10.1097/00001756-200109170-00019. [DOI] [PubMed] [Google Scholar]
- Spence SA, Hunter MD, Farrow TFD, Green RD, Leung DH, Hughes CJ, Ganesan V. A cognitive neurobiological account of deception: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2004;359:1755–1762. doi: 10.1098/rstb.2004.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SA, Kaylor-Hughes C, Farrow TFD, Wilkinson ID. Speaking of secrets and lies: the contribution of ventrolateral prefrontal cortex to vocal deception. Neuroimage. 2008;40:1411–1418. doi: 10.1016/j.neuroimage.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Stevens AA, Skudlarski P, Gatenby JC, Gore JC. Event-related fMRI of auditory and visual oddball tasks. Magn Reson Imaging. 2000;18:495–502. doi: 10.1016/s0730-725x(00)00128-4. [DOI] [PubMed] [Google Scholar]
- Stuss DT. Biological and psychological development of executive functions. Brain Cogn. 1992;20:8–23. doi: 10.1016/0278-2626(92)90059-u. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar sterotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tranel D, Damasio AR. Neuroanatomical correlates of electrodermal skin conductance responses. Psychophysiology. 1994;31:427–438. doi: 10.1111/j.1469-8986.1994.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An integrated software system for surface-based analyses of cerebral cortex. J Am Med Informatics Assoc. 2001;41:1359–1378. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Dierker D. On navigating the human cerebral cortex: response to ‘in praise of tedious anatomy’. Neuroimage. 2007;37:1050–1054. doi: 10.1016/j.neuroimage.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Wiley SD. Deception and detection in psychiatric diagnosis. Psychiatr Clin North Am. 1998;21:869–893. doi: 10.1016/s0193-953x(05)70046-0. [DOI] [PubMed] [Google Scholar]