Abstract
We evaluated the long-term outcome of vagus nerve stimulation (VNS) in 28 children with refractory epilepsy. Of these 28 children, 15 (53.6%) showed a >50% reduction in seizure frequency and 9 (32.1%) had a >75% reduction. When we compared seizure reduction rates according to seizure types (generalized vs. partial) and etiologies (symptomatic vs. cryptogenic), we found no significant differences. In addition, there was no correlation between the length of the stimulation period and treatment effect. The seizure reduction rate, however, tended to be inversely related to the seizure duration before VNS implantation and age at the time of VNS therapy. VNS also improved quality of life in this group of patients, including improved memory in 9 (32.1%), improved mood in 12 (42.9%), improved behavior in 11 (39.3%), improved altertness in 12 (42.9%), improved achievement in 6 (21.4%), and improved verbal skills in 8 (28.6%). Adverse events included hoarseness in 7 patients, dyspnea at sleep in 2 patients, and wound infection in 1 patient, but all were transient and successfully managed by careful follow-up and adjustment of parameters. These results indicate that VNS is a safe and effective alternative therapy for pediatric refractory epilepsy, without significant adverse events.
Keywords: Vagus Nerve Stimulation, Epilepsy, Child
INTRODUCTION
Vagus nerve stimulation (VNS), repeated stimulation of the left vagus nerve through implanted electrodes, is an empirically based treatment method for epilepsy. Although VNS induces changes in brain electrophysiology, metabolism and neurochemistry in animals and humans, its mechanism of action remains unknown. VNS has been used in epilepsy patients who are refractory to standard medical treatments and unsuitable candidates for resective or disconnective surgery. Due to the possible unfavorable effects of multiple anti-epileptic drugs on development, VNS is of particular interest in children and adolescents; over 25% of the approximately 30,000 patients receiving vagus nerve stimulator implants to date have been children and adolescents younger than 18 yr of age (1). Moreover, VNS has shown a higher degree of efficacy and tolerance in children than in adults (2).
Here we report our experience in this relatively new technique, including its long-term efficacy by seizure type, etiology, and seizure duration, as well as its safety, in pediatric patients with intractable epilepsy.
MATERIALS AND METHODS
The subjects consisted of 28 pediatric patients with intractable epilepsy, defined as uncontrolled seizures on two or more anti-epileptic drugs, in whom vagus nerve stimulators had been implanted at the Epilepsy Centers of Sanggye Paik Hospital, Asan Medical Center, and Severance Hospital in Korea, from July 1999 to March 2005. All 28 patients had multifocal or generalized epilepsy, and were therefore not eligible for epileptic surgery. Patients had various seizure etiologies, seizure types, and/or epilepsy syndromes. All subjects were followed up for at least 12 months, and, in 26 of 28 patients, their regimen of antiepileptic drugs was not changed during the first 6 months.
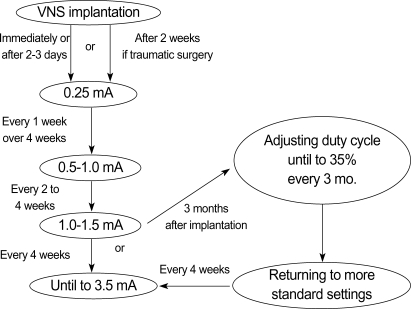
The device used for VNS stimulation was purchased from Cyberonics (Houston, TX). Each stimulation was for 30 sec, initially at 0.25 mA output current, 30 Hz frequency, and 500 µs pulse width, with 5 min between stimulations; parameters for each patient were adjusted in accordance with the manufacturer's guidelines (Fig. 1). We prospectively recorded seizure frequency and severity at 3, 6, and 12 months after implantation, and at last follow-up (up to 6 yr), as well as all adverse events. Quality of life parameters were measured using the Korean version of the Quality of Life in Childhood Epilepsy questionnaire (K-QOLCE) (3).
Fig. 1.
Recommended protocol of VNS parameter settings.
SPSS version 13.0 was used for all statistical analyses. Differences in dependent, categorical and continuous variables were evaluated using two-tailed chi-square and Student's t-tests, and multiple regression analysis was used to evaluate independent variables. A p-value<0.05 was regarded as statistically significant.
RESULTS
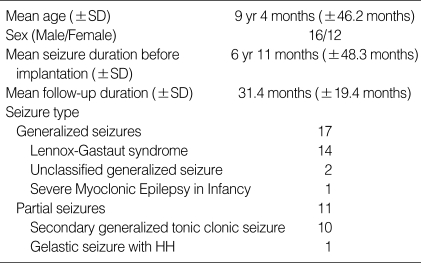
Of the 28 patients, 16 were boys and 12 were girls; their mean (±SD) age at the initiation of VNS was 9 yr 4 months (±3 yr 10.2 months) (range, 2 yr 5 months to 17 yr 10 months). Of these 28 patients, 14 had Lennox-Gastaut syndrome (LGS), 2 had generalized seizures, one had severe myoclonic epilepsy in infancy, and 11 had partial seizures, including one patient with gelastic seizures originating from a hypothalamic hamartoma. Their mean (±SD) seizure duration before VNS implantation was 6 yr, 11 months (±4 yr, 0.3 months) (range, 1 yr, 5 months to 17 yr, 10 months); and the mean (±SD) follow-up after implantation was 31.4 (±19.4) months (range, 12 months to 6 yr, 7 months). Their detailed clinical profiles are summarized in Table 1.
Table 1.
Demographics and clinical characteristics of the subjects
SD, Standard deviation; HH, hypothalamic hamartoma.
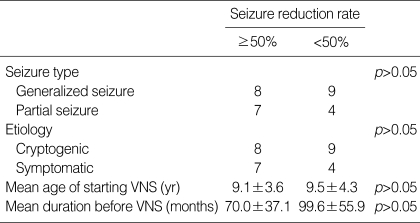
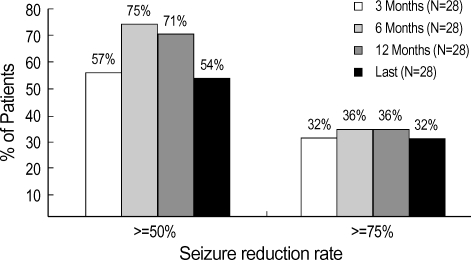
VNS resulted in a >50% reduction in seizure frequency in 15 children (53.6%), with 9 (32.1%) achieving a >75% reduction in seizure frequency at last follow-up. We did not detect any positive correlation between the length of the stimulation period and the treatment effect. There were no significant differences in the seizure type, etiology, mean age at starting VNS therapy, or the mean seizure duration before VNS implantation between children with a >50% and <50% reduction in seizure frequency (Table 2). We found, however, that the seizure reduction rate tended to be inversely related to seizure duration before VNS implantation and to the mean age at the start of VNS therapy.
Table 2.
Relationship of seizure reduction rate to clinical and demographic parameters
VNS, Vagus nerve stimulation.
A >50% reduction in seizure frequency was observed in 7 of the 11 patients (63.6%) with partial seizures and in 8 of the 17 patients (47.1%) with generalized seizures. One patient with complex partial seizures resulting from tuberous sclerosis complex showed a consistently favorable response, with a >90% reduction in seizure frequency during VNS therapy. The one patient who had gelastic seizures from hypothalamic hamartoma did not have a favorable response to VNS therapy.
Although the duty cycle was adjusted from 10% to 35% in 5 patients who did not attain a satisfactory response, this adjustment did not result in the anticipated reduction in seizures, necessitating a return to the previous duty cycle. When these 5 patients were subjected to rapid cycling (longer on-time and shorter off-time), 1 showed a >90% reduction in seizure frequency.
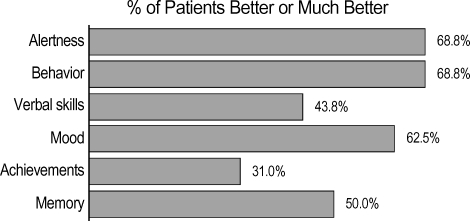
We compared quality of life (K-QOLCE) variables at baseline and at follow-up, (minimum, 12 months). Of the 28 patients, 9 (32.1%) showed improved memory, 12 (42.9%) each showed improved mood and alertness, 11 (39.3%) showed improved behavior, 6 (21.4%) showed improved achievement, and 8 (28.6%) showed improved verbal skills (Fig. 3).
Fig. 3.
Quality of life improvements.
Adverse events included hoarseness in 7 patients, dyspnea at sleep in 2 patients, wound infection in 1 patient, and drooling in 1 patient. All these adverse events were well tolerated or could be controlled by adjustment of output currents. In one patient, wound revision was required. No adverse side-effects such as bradycardia or arrhythmia were observed during implantation and testing of the device inside the operating room.
DISCUSSION
In our evaluation of the long-term outcome and tolerability of VNS in a group of Korean children with intractable epilepsy, we found that VNS resulted in a >50% reduction in seizure frequency in 53.6% of these children and a >75% reduction in 32%. These results were similar to those of earlier studies, in which 30% of patients had a >75% decrease in seizure frequency 6 months after VNS implantation (1) and 45% achieved a >50% reduction (2, 4-6). Although not statistically significant, we found that this reduction in seizure rate tended to be inversely related to seizure duration before VNS implantation and to the mean age at starting VNS therapy (2, 7). VNS also tended to be tolerated better and to be more effective in younger patients. This may be due to the degree of neuronal plasticity in the early years of life, before long-standing epilepsy can cause permanent brain damage.
In agreement with previous findings, we also observed that the efficacy of VNS therapy was not dependent on the seizure type or seizure etiology (1, 8). One patient in our series with tuberous sclerosis complex experienced a >90% reduction in seizure frequency, in agreement with findings that 5 of 10 patients with tuberous sclerosis complex experienced a >90% seizure reduction (9). Contrary to a previous report (10), we found that our single patient with gelastic seizure due to hypothalamic hamartoma did not respond favorably to VNS therapy. Due to the limited number of patients in our study as well as their heterogeneous epileptic syndromes and underlying etiologies, we could not stratify our patients relative to the seizure type or etiology. Our findings suggest, however, that VNS therapy is effective regardless of the seizure type or etiology. Although the effectiveness of VNS has been reported to correlate positively with the length of the treatment period (11, 12), we observed no correlation between the duration of stimulation and treatment effect.
In addition to reducing seizures and seizure intensity, VNS was associated with improvements in quality-of-life measurements, including mood, alertness, verbal skills, memory, and school/professional achievements, in many patients. In contrast to previous findings (11, 13-16), these effects were not related to the anti-epileptic effects of VNS. For example, we found that 2 of the 16 patients who experienced improvements in quality of life measures showed no reduction in seizure frequency. The nonpharmacologic aspects of VNS therapy make it particularly attractive for use, particularly in pediatric patients, due to the side effects and cognitive impairments associated with anticonvulsants, accompanied in many cases by mental retardation and delayed development (17).
The adverse events reported by VNS patients, including voice alterations, coughing during stimulation, and drooling, also occurred in our pediatric population (1). Several patients reported increases in hyperactivity, a side effect unique to this age group (14, 18). We also noted complications such as hoarseness and dyspnea at sleep. Most of these side effects, however, were transient or could be controlled by adjusting the current output. However, most of these children were mentally retarded and could not actively describe their discomforts.
In summary, our results indicate that VNS is a nonpharmacologic option in treating children with intractable epilepsy. It possesses several advantages, such as a lack of adverse effects on cognitive functions, which are a major drawback of antiepileptic drugs in pediatric patients undergoing critical stages of neural development. Our findings indicate that VNS can be used as adjunctive treatment in children and young adults with medically refractory seizures not amenable to resective surgery.
Fig. 2.
Changes in seizure reduction after 3, 6, and 12 months and at last follow-up.
References
- 1.Helmers SL, Wheless JW, Frost M, Gates J, Levisohn P, Tardo C, Conry JA, Yalnizoglu D, Madsen JR. Vagus nerve stimulation therapy in pediatric patients with refractory epilepsy: retrospective study. J Child Neurol. 2001;16:843–848. doi: 10.1177/08830738010160111101. [DOI] [PubMed] [Google Scholar]
- 2.Murphy JV, Torkelson R, Dowler I, Simon S, Hudson S. Vagal nerve stimulation in refractory epilepsy: the first 100 patients receiving vagal nerve stimulation at a pediatric epilepsy center. Arch Pediatr Adolesc Med. 2003;157:560–564. doi: 10.1001/archpedi.157.6.560. [DOI] [PubMed] [Google Scholar]
- 3.Lim K, Kang HC, Kim HD. Validation of a Korean version of the Quality of Life in Childhood Epilepsy Questionnaire (K-QOLCE) J Korean Epilepsy Soc. 2002;6:32–44. [Google Scholar]
- 4.Kang HC, Kim HD, Hwang YS, Park SK. Therapeutic outcomes of vagus nerve stimulation in intractable childhood epilepsy. J Korean Epilepsy Soc. 2003;7:118–124. [Google Scholar]
- 5.Labar D. Vagus nerve stimulation for 1 year in 269 patients on unchanged antiepileptic drugs. Seizure. 2004;13:392–398. doi: 10.1016/j.seizure.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Renfroe JB, Wheless JW. Earlier use of adjunctive vagus nerve stimulation therapy for refractory epilepsy. Neurology. 2002;59:26–30. doi: 10.1212/wnl.59.6_suppl_4.s26. [DOI] [PubMed] [Google Scholar]
- 7.You SJ, Kim DS, Lee JK, Ko TS. Vagus nerve stimulation in intractable pediatric epilepsy patients. J Korean Child Neurol Soc. 2005;13:8–14. [Google Scholar]
- 8.Tanganelli P, Ferrero S, Colotto P, Regesta G. Vagus nerve stimulation for treatment of medically intractable seizures. Evaluation of long-term outcome. Clin Neurol Neurosurg. 2002;105:9–13. doi: 10.1016/s0303-8467(02)00018-5. [DOI] [PubMed] [Google Scholar]
- 9.Parain D, Penniello MJ, Berquen P, Delangre T, Billard C, Murphy JV. Vagal nerve stimulation in tuberous sclerosis complex patients. Pediatr Neurol. 2001;25:213–216. doi: 10.1016/s0887-8994(01)00312-5. [DOI] [PubMed] [Google Scholar]
- 10.Murphy JV, Wheless JW, Schmoll CM. Left vagal nerve stimulation in six patients with hypothalamic hamartomas. Pediatr Neurol. 2000;23:167–168. doi: 10.1016/s0887-8994(00)00170-3. [DOI] [PubMed] [Google Scholar]
- 11.DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, Reed R, Collins S, Tecoma E, Morris GL, Vaughn B, Naritoku DK, Henry T, Laber D, Gilmartin R, Labiner D, Osorio I, Ristanovic R, Jones J, Murphy J, Ney G, Wheless J, Lewis P, Heck C. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 12.Morris GL, 3rd, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01-E05. Neurology. 1999;53:1731–1735. doi: 10.1212/wnl.53.8.1731. [DOI] [PubMed] [Google Scholar]
- 13.Patwardhan RV, Stong B, Bebin EM, Mathisen J, Grabb PA. Efficacy of vagal nerve stimulation in children with medically refractory epilepsy. Neurosurgery. 2000;47:1353–1357. [PubMed] [Google Scholar]
- 14.Valencia I, Holder DL, Helmers SL, Madsen JR, Riviello JJ., Jr Vagus nerve stimulation in pediatric epilepsy: a review. Pediatr Neurol. 2001;25:368–376. doi: 10.1016/s0887-8994(01)00319-8. [DOI] [PubMed] [Google Scholar]
- 15.Buoni S, Mariottini A, Pieri S, Zalaffi A, Farnetani MA, Strambi M, Palma L, Fois A. Vagus nerve stimulation for drug-resistant epilepsy in children and young adults. Brain Dev. 2004;26:158–163. doi: 10.1016/S0387-7604(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 16.Hallbook T, Lundgren J, Stjernqvist K, Blennow G, Strombald LG, Rosen I. Vagus nerve stimulation in 15 children with therapy resistant epilepsy; its impact on cognition, quality of life, behaviour and mood. Seizure. 2005;14:504–513. doi: 10.1016/j.seizure.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Wheless JW, Maggio V. Vagus nerve stimulation therapy in patients younger than 18 years. Neurology. 2002;59:S21–S25. doi: 10.1212/wnl.59.6_suppl_4.s21. [DOI] [PubMed] [Google Scholar]
- 18.Majoie HJ, Berfelo MW, Aldenkamp AP, Renier WO, Kessels AG. Vagus nerve stimulation in patients with catastrophic childhood epilepsy, a 2-year follow-up study. Seizure. 2005;14:10–18. doi: 10.1016/j.seizure.2004.02.003. [DOI] [PubMed] [Google Scholar]