Abstract
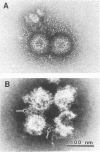
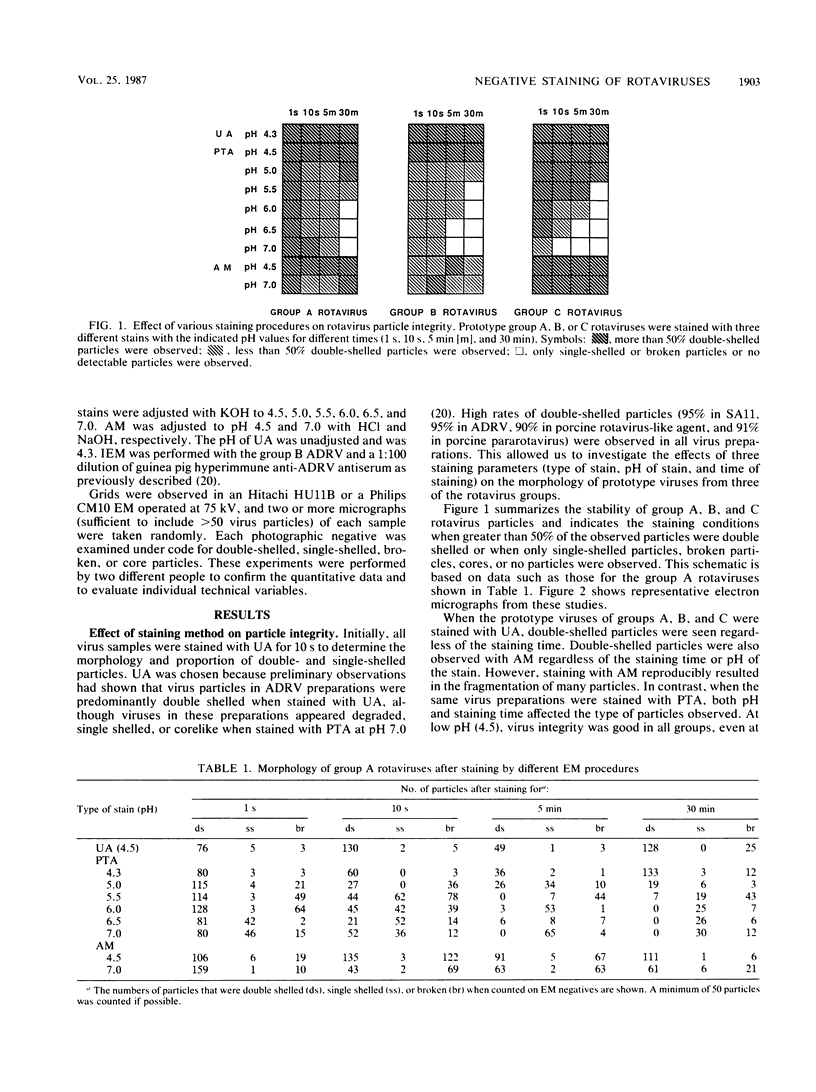
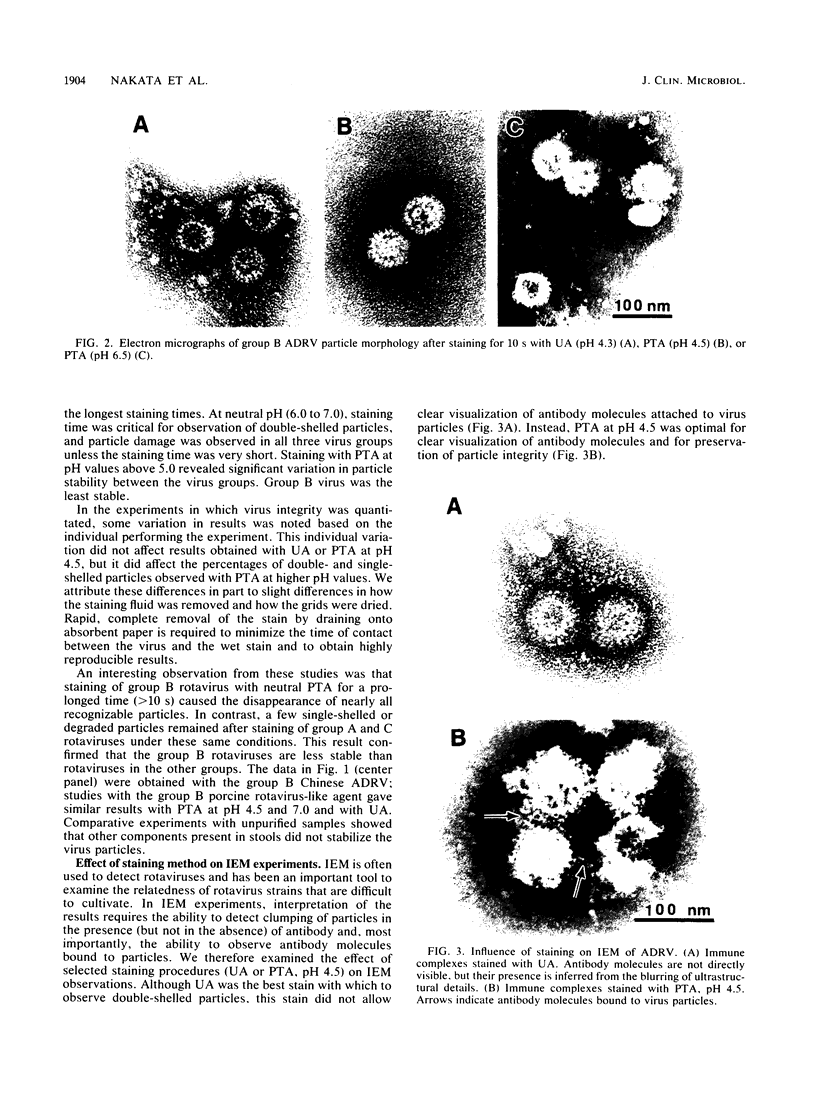
Technical parameters of electron microscope staining procedures (type of stain, pH of stain, and time of staining) influence particle integrity for three groups of rotaviruses. Simian rotavirus SA11 (group A), Chinese adult diarrhea rotavirus and porcine rotavirus-like agent (group B), and porcine pararotavirus (group C) were tested. All rotavirus strains were quite stable in uranyl acetate and phosphotungstic acid at pH 4.5 and relatively stable in ammonium molybdate. However, staining with phosphotungstic acid at higher pH values with increased staining time yielded a reduction in the number of particles and particles that were broken or degraded to single-shelled particles or core particles. The different staining procedures were also tested in immunoelectron microscopy experiments. Antibody molecules bound to rotavirus particles were observed clearly only with phosphotungstic acid staining and not with uranyl acetate. We therefore recommend that uranyl acetate and phosphotungstic acid at pH 4.5 be used for negative staining of rotaviruses; phosphotungstic acid at pH 4.5 is optimal for immunoelectron microscopy. These technical points may be critical for rotavirus detection and are important for studies pertaining to the epidemiology and clinical importance of the non-group A rotaviruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS W. R., KRAFT L. M. Epizootic diarrhea of infant mice: indentification of the etiologic agent. Science. 1963 Jul 26;141(3578):359–360. doi: 10.1126/science.141.3578.359. [DOI] [PubMed] [Google Scholar]
- Almeida J. D. Practical aspects of diagnostic electron microscopy. Yale J Biol Med. 1980 Jan-Feb;53(1):5–18. [PMC free article] [PubMed] [Google Scholar]
- Bishop R. F., Davidson G. P., Holmes I. H., Ruck B. J. Detection of a new virus by electron microscopy of faecal extracts from children with acute gastroenteritis. Lancet. 1974 Feb 2;1(7849):149–151. doi: 10.1016/s0140-6736(74)92440-4. [DOI] [PubMed] [Google Scholar]
- Bohl E. H., Saif L. J., Theil K. W., Agnes A. G., Cross R. F. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982 Feb;15(2):312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Thomas L., Yolken R. H., Arrobio J. O., Kapikian A. Z., Parrott R. H., Chanock R. M. Comparison of direct electron microscopy, immune electron microscopy, and rotavirus enzyme-linked immunosorbent assay for detection of gastroenteritis viruses in children. J Clin Microbiol. 1981 May;13(5):976–981. doi: 10.1128/jcm.13.5.976-981.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C., Pedley S., McCrae M. A. Group C rotaviruses in humans. J Clin Microbiol. 1986 Apr;23(4):760–763. doi: 10.1128/jcm.23.4.760-763.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. M., Hung T., Bridger J. C., McCrae M. A. Chinese adult rotavirus is a group B rotavirus. Lancet. 1985 Nov 16;2(8464):1123–1124. doi: 10.1016/s0140-6736(85)90710-x. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Puerto F., Soler C., González N. Characterization of a human pararotavirus. Infect Immun. 1984 Apr;44(1):112–116. doi: 10.1128/iai.44.1.112-116.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y. Rotavirus antigens. Adv Exp Med Biol. 1985;185:201–214. doi: 10.1007/978-1-4684-7974-4_13. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Davies H., Bryden A. S., Robertson M. J. Diagnostic electron microscopy of faeces. II. Acute gastroenteritis associated with reovirus-like particles. J Clin Pathol. 1974 Aug;27(8):608–614. doi: 10.1136/jcp.27.8.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T., Chen G. M., Wang C. G., Chou Z. Y., Chao T. X., Ye W. W., Yao H. L., Meng K. H. Rotavirus-like agent in adult non-bacterial diarrhoea in China. Lancet. 1983 Nov 5;2(8358):1078–1079. [PubMed] [Google Scholar]
- Hung T., Chen G. M., Wang C. G., Yao H. L., Fang Z. Y., Chao T. X., Chou Z. Y., Ye W., Chang X. J., Den S. S. Waterborne outbreak of rotavirus diarrhoea in adults in China caused by a novel rotavirus. Lancet. 1984 May 26;1(8387):1139–1142. [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Much D. H., Zajac I. Purification and characterization of epizootic diarrhea of infant mice virus. Infect Immun. 1972 Dec;6(6):1019–1024. doi: 10.1128/iai.6.6.1019-1024.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata S., Estes M. K., Graham D. Y., Loosle R., Tao H., Wang S. H., Saif L. J., Melnick J. L. Antigenic characterization and ELISA detection of adult diarrhea rotaviruses. J Infect Dis. 1986 Sep;154(3):448–455. doi: 10.1093/infdis/154.3.448. [DOI] [PubMed] [Google Scholar]
- Nicolas J. C., Cohen J., Fortier B., Lourenco M. H., Bricout F. Isolation of a human pararotavirus. Virology. 1983 Jan 15;124(1):181–184. doi: 10.1016/0042-6822(83)90302-1. [DOI] [PubMed] [Google Scholar]
- Payne C. M., Ray C. G., Yolken R. H. The 30- to 54-nm rotavirus-like particles in gastroenteritis: incidence and antigenic relationship to rotavirus. J Med Virol. 1981;7(4):299–313. doi: 10.1002/jmv.1890070406. [DOI] [PubMed] [Google Scholar]
- Pedley S., Bridger J. C., Chasey D., McCrae M. A. Definition of two new groups of atypical rotaviruses. J Gen Virol. 1986 Jan;67(Pt 1):131–137. doi: 10.1099/0022-1317-67-1-131. [DOI] [PubMed] [Google Scholar]
- Pereira H. G., Leite J. P., Azeredo R. S., de Farias V., Sutmoller F. An atypical rotavirus detected in a child with gastroenteritis in Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 1983 Jul-Sep;78(3):245–250. doi: 10.1590/s0074-02761983000300002. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Bishop R. F., Holmes I. H. Detection of a rotavirus-like agent associated with diarrhea in an infant. J Clin Microbiol. 1982 Oct;16(4):724–726. doi: 10.1128/jcm.16.4.724-726.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Herring A. J., Campbell I., Inglis J. M., Hargreaves F. D. Comparison of atypical rotaviruses from calves, piglets, lambs and man. J Gen Virol. 1984 May;65(Pt 5):909–914. doi: 10.1099/0022-1317-65-5-909. [DOI] [PubMed] [Google Scholar]
- Spratt H. C., Marks M. I., Gomersall M., Gill P., Pai C. H. Nosocomial infantile gastroenteritis associated with minirotavirus and calicivirus. J Pediatr. 1978 Dec;93(6):922–926. doi: 10.1016/S0022-3476(78)81212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil K. W., Saif L. J. In vitro detection of porcine rotavirus-like virus (group B rotavirus) and its antibody. J Clin Microbiol. 1985 May;21(5):844–846. doi: 10.1128/jcm.21.5.844-846.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil K. W., Saif L. J., Moorhead P. D., Whitmoyer R. E. Porcine rotavirus-like virus (group B rotavirus): characterization and pathogenicity for gnotobiotic pigs. J Clin Microbiol. 1985 Mar;21(3):340–345. doi: 10.1128/jcm.21.3.340-345.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]