Abstract
AIM: To investigate whether the iron stores regulator hepcidin is implicated in colon cancer-associated anaemia and whether it might have a role in colorectal carcinogenesis.
METHODS: Mass spectrometry (MALDI-TOF MS and SELDI-TOF MS) was employed to measure hepcidin in urine collected from 56 patients with colorectal cancer. Quantitative Real Time RT-PCR was utilized to determine hepcidin mRNA expression in colorectal cancer tissue. Hepcidin cellular localization was determined using immunohistochemistry.
RESULTS: We demonstrate that whilst urinary hepcidin expression was not correlated with anaemia it was positively associated with increasing T-stage of colorectal cancer (P < 0.05). Furthermore, we report that hepcidin mRNA is expressed in 34% of colorectal cancer tissue specimens and was correlated with ferroportin repression. This was supported by hepcidin immunoreactivity in colorectal cancer tissue.
CONCLUSION: We demonstrate that systemic hepcidin expression is unlikely to be the cause of the systemic anaemia associated with colorectal cancer. However, we demonstrate for the first time that hepcidin is expressed by colorectal cancer tissue and that this may represent a novel oncogenic signalling mechanism.
Keywords: Iron, Hepcidin, Colon, Cancer, Anaemia, Mass spectrometry
INTRODUCTION
Anaemia is a common presenting symptom of colorectal cancer, a recent study showing that up to 6% of patients presenting with iron deficiency anaemia (IDA) had colorectal cancer[1]. IDA is commoner in patients with right sided than left sided colorectal cancers[2]. The reason for this is assumed to be because of the chronic occult blood loss associated with cancers in the right colon compared to overt bleeding of left sided colonic disease which is detected sooner.
What remains unclear is whether a component of the anaemia might be a consequence of the inflammation associated with the neoplastic process. The major pro-inflammatory cytokine implicated in the pathogenesis of anaemia of chronic inflammation is interleukin 6 (IL-6). Recent work has shown that the induction of hepcidin release stimulated by IL-6 is crucial to this process[3–5]. Precisely how hepcidin mediates anaemia of chronic disease has been comprehensively investigated by several groups and it is now well established that hepcidin acts to cause a block on cellular iron export by internalization and degradation of ferroportin[6,7]. At the level of the macrophage, the major cell type responsible for iron recycling, this results in iron sequestration and interrupts iron delivery to erythroid precursor cells thus causing anaemia[8]. Increased hepcidin levels are likely to also cause an accumulation of iron in other ferroportin expressing cell lineages such as colonocytes. Our recent studies have shown that raising colonocyte iron levels can result in increased Wnt signalling which has been shown to be crucial in colorectal carcinogenesis[9,10].
However, to date there are no studies addressing whether hepcidin expression contributes to the anaemia in colorectal cancer and whether hepcidin itself may be considered a pro-oncogenic factor.
There are several strands of evidence to suggest that this hypothesis may be relevant: firstly, the major inducer of hepcidin, IL-6, shows increased expression in both colorectal cancer tissue and in the serum of colorectal cancer patients and is indicative of a more advanced phenotype[11–13]. Secondly, consistent with a hepcidin-colonocyte axis, we have reported that ferroportin is cytoplasmic in cellular localization and expression levels are repressed with increasing stage of colorectal cancer[14].
This raises the possibility that hepcidin may have a dual effect in colorectal cancer patients; contributing to the systemic anaemia by acting at the level of the macrophage whilst acting locally at the colonocyte level promoting iron accumulation and Wnt signalling.
Thus the aims of this study were: (1) To measure urinary hepcidin levels by Matrix-assisted laser desorption/ionization time of flight mass spectroscopy (MALDI-TOF MS) and Surface-enhanced laser desorption ionization time of flight (SELDI-TOF MS) in colorectal cancer patients and determine if hepcidin expression was associated either with anaemia or with disease state including T-stage, and nodal involvement; (2) To examine if hepcidin mRNA expression could also be detected in colorectal cancer tissue and, if so, determine cellular localization.
MATERIALS AND METHODS
Sample collection
Urine was collected from 56 patients with colorectal cancer prior to surgery, centrifuged at 3000 r/min for 20 min and stored at -80°C. In a separate set of colorectal cancer patients (n = 34) surgical resection specimens of colorectal carcinoma matched with normal colonic mucosa from the same resection specimen were collected and processed for RNA extraction.
Ethics
This work has been carried out in accordance with the declaration of Helsinki (2000) of the World Medical Association. Ethical approval for this study was approved by South Birmingham LREC No. 05/Q2702/17. All patients provided informed written consent.
Urine hepcidin measurements
SELDI-TOF-MS was performed as previously described[15]. Briefly, urine samples were diluted to 10 μg protein/mL in 0.5 mol/L NaCl, 100 mmol/L sodium phosphate (pH 7.0) and applied to Cu2+ loaded IMAC30 ProteinChip arrays. MALDI spectra were obtained either by applying diluted urine (20 μg protein/mL) or urine desalted using ClinProt C8 magnetic beads (BrukerDaltronic) to GoldChips. Spectra were acquired on a PBS IIc ProteinChip Reader (Ciphergen) using sinapinic acid as the matrix. Spectra were normalized to total ion current (as previously described for urinary biomarker profiling[16,17]), baselines subtracted and peaks picked using Ciphergen ProteinChip software. Synthetic hepcidin-25 (Peptides International) was employed for peak/assay validation. Immunocapture was performed as previously described[15,19]. Briefly Protein G sepharose beads loaded with or without rabbit polyclonal anti-hepcicin-25 (Abcam 31877) were incubated with human urine containing hepcidin 20, 22 and 25. The beads were washed extensively with 20 mmol/L ammonium bicarbonate and the captured proteins eluted with 50% acetonitrile/0.1% trifluoroacetic acid and analysed by MALDI.
Real time RT-PCR
Real Time RT-PCR was performed on colorectal cancer tissue specimens (C) and matched uninvolved normal colonic mucosa (N) with all reactions containing 18S ribosomal RNA as an internal standard (PE Biosystems, Roche, USA), and human specific hepcidin probe and primers (Probe 5'-FAM 3' TAMRA AGCTGCAACCCCAGG, Forward primer CCCACAACAGACGGGACAA and Reverse primer TCTGGAACATGGGCATCC) as previously described[18].
Immunohistochemistry
Immunohistochemistry was performed using paraffin sections of human normal colon (n = 10) and colorectal cancer (n = 15). All sections were incubated at 95-100°C in WCAP reagent (Surgipath) for 30 min, washed and then incubated in 10:1 Methanol: H2O2 for 10 min. Sections were then incubated in rabbit polyclonal hepcidin antibody (Abcam 31877; 1:20) for 1hr after which immunoreactivity detected with an Envision assay kit and DAB reagent (DakoCytomation). All sections were counterstained in haematoxylin and mounted prior to visualisation. Normal liver sections were utilized as a positive control. Negative control included omission of primary antibody and liver sections incubated with antibody which had prior incubation with the immunizing peptide (Abcam 31875).
Statistical analysis
Statistical significance was calculated using the Mann-Whitney test to compare continuous data and the Spearman rank test to assess correlations between data sets. Significance was accepted at P ≤ 0.05. All analyses were performed using SPSS version 14.0 (SPSS Inc, USA). Data are presented with 2 standard errors of the mean.
RESULTS
Validation of MALDI-TOF MS for the detection of urinary hepcidin
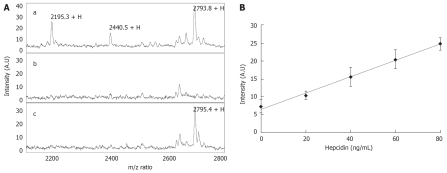
Using a pre determined hepcidin positive urine sample we performed MALDI-TOF MS and were able to detect the two major forms of hepcidin; the mature hepcidin 25 (m/z 2793.8), and the N-terminally truncated hepcidin 20 (m/z 2195.3). In addition we were also able to detect hepcidin 22 (m/z 2440.5) which corresponds to a urinary degradation product of hepcidin 25 (Figure 1Aa). Using a human urine sample devoid of hepcidin (Figure 1Ab) we were able to demonstrate the appearance of a hepcidin 25 peak on spiking with a synthetic human hepcidin peptide (Figure 1Ac). To determine if MALDI-TOF MS could be used in a semi-quantitative manner as has previously been reported for SELDI-TOF MS we spiked a urine sample with low endogenous hepcidin and demonstrated a linear relationship between hepcidin concentration and intensity of the hepcidin 25 peak (Figure 1B).
Figure 1.
Validation of hepcidin expression in urine by MALDI-TOF MS. A: (a) A human urine specimen showing the two dominant forms of hepcidin; hepcidin 20 (m/z 2195.3) and hepcidin 25 (m/z 2793.8). In addition the degradation product of hepcidin 25 hepcidin 22 could also be detected by MALDI-TOF MS (m/z 2440.5); (b) A human urine specimen completely devoid of hepcidin which when spiked with synthetic hepcidin 25 clearly shows a detectable peak at 2795.4 (c); B: Synthetic hepcidin was spiked into a low hepcidin containing urine sample at concentrations between 0-80 ng/mL and analysed by MALDI-TOF MS followed by analysis of the hepcidin 25 peak intensity.
Analysis of urinary hepcidin levels in colorectal cancer patients
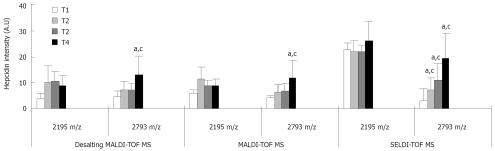
To determine hepcidin levels in urine samples from colorectal cancer patients (n = 56) we performed SELDI-TOF MS and MALDI-TOF MS (Figure 2). In addition, as the presence of salt can interfere with mass spectrometry, we desalted the urine samples and once more performed MALDI-TOF MS. Since hepcidin 22 is a urine specific degradation product of hepcidin[19] we chose to analyse only the expression level of hepcidin 20 and 25. Using a Spearman Rank test we were able to demonstrate a correlation between all three data sets (MALDI-TOF MS vs SELDI-TOF MS, correlation coefficient = 0.59 P < 0.0001, Desalting MALDI-TOF MS vs MALDI-TOF MS, correlation coefficient = 0.56 P < 0.0001, Desalting MALDI-TOF MS vs SELDI-TOF MS, correlation coefficient = 0.71 P < 0.0001).
Figure 2.
Hepcidin expression is associated with T-stage of colorectal cancer. Expression of hepcidin 20 (m/z 2195) and 25 (m/z 2793) was determined in urine samples of 56 colorectal cancer patients by MALDI-TOF MS, before and after desalting with desalting beads (Desalting MALDI-TOF MS), and SELDI-TOF MS and analysed with respect to T-stage. Expression of hepcidin 20 was not altered with T-stage by any of the three techniques. However, all three techniques demonstrated a significant increase in hepcidin 25 expression in T4 cancers compared to T1. Statistical significance compared to T1 (aP < 0.05); Statistical significance compared to preceding T-stage (cP < 0.05). Error bars denote 2 SEM.
Data was analysed with respect to the site of cancer (left or right sided), TNM classification, and the presence or absence of anaemia where anaemia was classified as haemoglobin level of less than 13 g/dL for men and 12 g/dL for women. Of the 56 cancers examined, 63% were left sided cancers of which 40% were anaemic, whereas of the right sided cancers 77% were anaemic[22]. Our data analyses using all data sets individually (Desalting MALDI-TOF MS, MALDI-TOF MS, and SELDI-TOF MS) for both hepcidin 20 and 25 showed that neither forms of hepcidin were significantly altered in expression in respect to the site of cancer, local nodal involvement, metastasis or haemoglobin level.
However, when examining the relationship between hepcidin and T-stage of disease, whilst hepcidin 20 was not correlated with T-stage, hepcidin 25 significantly increased with increasing stage. All three mass spectrometry techniques demonstrated a significant increase in hepcidin 25 in T4 cancers compared to T1. Moreover, SELDI-TOF MS data delineated a significant stepwise increase between T2 and T1 (7.28 vs 2.77 P < 0.05), T3 and T2 (11.07 vs 7.28 P < 0.05), and T4 and T3 (19.17 vs 11.07 P < 0.05).
Determination of hepcidin expression and localization in colorectal cancer tissue
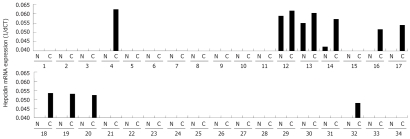
The observation that hepcidin is associated with advanced disease led us to speculate that it may have an effect at the level of the colonocyte and that hepcidin may even be expressed by colorectal cancer tissue. Thus we examined hepcidin mRNA expression in 34 cancer specimens (C) each with adjacent normal uninvolved mucosa (N) (Figure 3). Our data shows that hepcidin mRNA was detected in 34% of colorectal cancer tissue specimens (10 out of 34) and, whilst there was no hepcidin mRNA detectable in the majority of matched adjacent uninvolved mucosa, where there was hepcidin mRNA, it was found at a lower level than in the associated cancer. Furthermore we find that hepcidin mRNA expression is inversely correlated with ferroportin mRNA expression (data not shown).
Figure 3.
Hepcidin mRNA expression in colorectal cancer tissue. Using Real Time RT-PCR on 34 cancer specimens (C) each with adjacent normal uninvolved mucosa (N) we demonstrate that hepcidin mRNA expression could be detected in 34% of colorectal cancer tissue specimens (10 out of 34). mRNA expression is presented as 1/dCT. Positive control included human liver. Negative control included omission of cDNA.
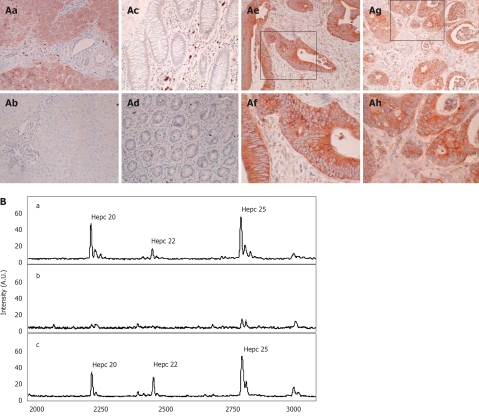
To further verify expression and determine the cellular localization of hepcidin we performed immunohistochemistry. As a positive control for hepcidin expression we utilized human liver. As anticipated we were able to show strong immunoreactivity in all hepatocytes (Figure 4Aa), and this immunoreactivity could be blocked by co-incubation with hepcidin peptide (Figure 4Ab). In normal colon, both on the luminal surface and in the crypts, we were unable to detect any hepcidin immunoreactivity (Figure 4Ac-Ad). However, in approximately 46% (7 out of 15) of colorectal cancer specimens examined there was an abundance of both membranous (Figure 4Ae-Af) and cytoplasmic immunoreactivity (Figure 4Ag-Ah).
Figure 4.
Cellular localization of hepcidin in colorectal cancer tissue. A: To determine hepcidin cellular localization in colorectal tissue immunohistochemistry was performed with a hepcidin specific antibody (Abcam 31877). (Aa) Human Liver; (Ab) Human Liver, incubated with both hepcidin antibody and immunizing hepcidin peptide; (Ac) Normal colon; (Ad) Normal colon with crypts in cross section; (Ae) Colorectal cancer (x 20); (Af) Colorectal cancer (x 40); (Ag) colorectal cancer (x 20); (Ah) Colorectal cancer (x 40). Boxes denote area subsequently magnified; B: Immunocapture of urinary hepcidin. (Ba) A human urine sample containing hepcidin 20 (m/z 2193.6), 22 (m/z 2438.2) and 25 (m/z 2792.0) was subject to immunocapture with either (Bb) protein G sepharose or with (Bc) protein G-sepharose and a polyclonal anti hepcidin antibody. Immunocaptured proteins were then eluted from the protein G sepharose and analysed by MALDI-TOF MS.
To determine what form of hepcidin was being detected by our antibody and thus expressed in colorectal cancer tissue, we performed an immuno-capture experiment on human urine followed by MALDI-TOF MS (Figure 4B). We initially chose a urine sample which had clear and abundant hepcidin 20, 22 and 25 peaks (Figure 4Ba), and incubated with either protein G sepharose or with protein G-sepharose and the polyclonal anti hepcidin antibody. Immunocaptured proteins were then eluted from the protein G sepharose and analysed by MALDI-TOF MS. We clearly demonstrated that incubation with the rabbit hepcidin antibody results in binding of all three forms of hepcidin (Figure 4Bc), whilst incubation with protein G sepharose alone did not bind any form of hepcidin (Figure 4Bb). Thus from this data we infer that the immunoreactivity in colorectal cancer tissue could be due to any of these hepcidin moieties.
DISCUSSION
Anaemia is a common presenting symptom of colorectal cancer, and it is widely assumed to be a consequence of blood loss[1,2]. However, it is possible that a component of this anaemia may be a result of inflammatory processes. In particular the anti-microbial peptide hepcidin a down-stream target of cytokines such as IL-6 has been widely accepted as a mediator of anaemia of inflammation[3–5]. Thus in this study we aimed to address whether hepcidin was involved in the anaemia associated with colorectal carcinogenesis.
We demonstrated using SELDI-TOF MS; a previously validated method for the detection of urinary hepcidin[19–21], and MALDI-TOF MS; a more common proteomic technique, that urinary hepcidin 25 expression was not associated with the presence of anaemia in a small cohort of 56 colorectal cancer patients. However, it is important to point out that the only parameter which was utilized to assess anaemia was a haemoglobin level of less than 13 g/dL for men and 12 g/dL for women. Thus it is quite possible that some of these anaemic individuals may actually have anaemia of chronic disease rather than pure iron deficiency anaemia. To unequivocally discriminate between these two types of anaemia it would be essential to assess both the level serum ferritin and soluble transferrin receptor levels.
However, our result is consistent with previous reports which suggest that the phenotype of the systemic anaemia associated with colorectal cancer is more likely to be an iron deficiency rather than anaemia of chronic disease and that early detection of blood loss due to colon cancer is life saving[23].
Interestingly using both MALDI-TOF MS and SELDI-TOF MS we showed that urinary hepcidin 25 expression was associated with stage of disease. This association was not observed for the N-terminally truncated hepcidin 20 moiety. This observation led us to speculate that it may have an effect at the level of the colonocyte and that hepcidin may even be expressed by colorectal cancer tissue. Such a hypothesis is strengthened by evidence that the major inducer of hepcidin, IL-6, is over expressed in colorectal cancer tissue and its expression is positively associated with stage of disease[11–13]. Moreover, we have recently reported both ferroportin internalization and iron loading in colorectal cancer tissue: both predicted effects of hepcidin[14]. Thus consistent with such a hypothesis we were able to demonstrate for the first time that approximately a third of all colorectal cancer tissues examined expressed hepcidin mRNA.
What the signal for hepcidin induction is in these colorectal cancers is unclear. The two original reports describing murine hepcidin suggested that there was either very low or no hepcidin expressed in the colon consistent with our findings[24,25]. However, more recently it has been suggested that as a consequence of an acute phase response the colon does have the potential to induce hepcidin expression[26] and furthermore several of the upstream regulators of hepcidin including IL-6, STAT3, TfR2 and BMP4 have been shown to be over expressed in colorectal cancer tissue[11–13,27–29]. In addition a recent report suggests that hepcidin expression is dependent on p53 status, a tumour suppressor which is commonly mutated in colorectal cancers[30].
Irrespective of the mechanism of hepcidin expression the downstream consequences are likely to be internalization and degradation of the cellular iron export protein ferroportin. In the background of an elevation in the cellular iron import proteins such as transferrin receptor 1 and Divalent metal transporter 1, this culminates in cellular iron accumulation; a phenotype which we have previously reported in colorectal cancer[14]. Furthermore, we have recently demonstrated that elevating intracellular iron in the presence of mutations in either adenomatous polyposis coli (APC) or β-catenin results in increased Wnt signalling; the major oncogenic signalling pathway in the colon[9,10]. Thus hepcidin expression at the level of the tumour may be a mechanism of ultimately accentuating carcinogenesis and that abrogating hepcidin expression either directly or indirectly through IL-6 may provide a strategy for therapeutic intervention.
COMMENTS
Background
Anaemia is a common presenting symptom of colorectal cancer, though whether the anaemia is a consequence of blood loss is not known. Recent studies have suggested that anaemia in the context of chronic disease is mediated by the antimicrobial peptide hepcidin.
Research frontiers
To date there have been no studies addressing whether hepcidin could be the cause of the anaemia associated with colorectal cancer. Similarly, whether colorectal cancer tissue itself can express hepcidin is unknown.
Innovations and breakthroughs
This is the first study to show that systemic hepcidin levels were positively associated with stage of colorectal cancer. Furthermore, this study suggests that colonic epithelial cancer cells have acquired the ability to express hepcidin; a protein which was previously thought to be largely expressed by the liver.
Applications
What still remains unclear is whether the circulating level of hepcidin is derived from the liver or whether it has come from the colorectal tumour tissue itself. Irrespective of this, this study suggests that circulating hepcidin levels may provide a useful tool for aiding in the diagnosis of colorectal cancer.
Terminology
Hepcidin is an antimicrobial peptide which regulates iron metabolism. In instances of high hepcidin it depresses duodenal iron absorption and sequesters iron in the reticulo-endothelial system culminating in anaemia. This type of anaemia is classified as the anaemia of chronic disease and not iron deficiency anaemia which can result from extensive blood loss, compromised duodenal iron absorption and or low dietary iron intake.
Peer review
This is the first study to associate hepcidin expression with carcinogenesis.
Peer reviewer: Filip Braet, Associate Professor, Australian Key Centre for Microscopy and Microanalysis, Madsen Building (F09), The University of Sydney, Sydney NSW 2006, Australia
S- Editor Li DL L- Editor Roberts SE E- Editor Ma WH
References
- 1.Raje D, Mukhtar H, Oshowo A, Ingham Clark C. What proportion of patients referred to secondary care with iron deficiency anemia have colon cancer? Dis Colon Rectum. 2007;50:1211–1214. doi: 10.1007/s10350-007-0249-y. [DOI] [PubMed] [Google Scholar]
- 2.Beale AL, Penney MD, Allison MC. The prevalence of iron deficiency among patients presenting with colorectal cancer. Colorectal Dis. 2005;7:398–402. doi: 10.1111/j.1463-1318.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- 3.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganz T. Molecular pathogenesis of anemia of chronic disease. Pediatr Blood Cancer. 2006;46:554–557. doi: 10.1002/pbc.20656. [DOI] [PubMed] [Google Scholar]
- 6.De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569–2578. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 8.Ganz T. Hepcidin--a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18:171–182. doi: 10.1016/j.beha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Brookes MJ, Boult J, Roberts K, Cooper BT, Hotchin NA, Matthews G, Iqbal T, Tselepis C. A role for iron in Wnt signalling. Oncogene. 2008;27:966–975. doi: 10.1038/sj.onc.1210711. [DOI] [PubMed] [Google Scholar]
- 10.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 11.Esfandi F, Mohammadzadeh Ghobadloo S, Basati G. Interleukin-6 level in patients with colorectal cancer. Cancer Lett. 2006;244:76–78. doi: 10.1016/j.canlet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Becker C, Fantini MC, Wirtz S, Nikolaev A, Lehr HA, Galle PR, Rose-John S, Neurath MF. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–220. [PubMed] [Google Scholar]
- 13.Chung YC, Chang YF. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol. 2003;83:222–226. doi: 10.1002/jso.10269. [DOI] [PubMed] [Google Scholar]
- 14.Brookes MJ, Hughes S, Turner FE, Reynolds G, Sharma N, Ismail T, Berx G, McKie AT, Hotchin N, Anderson GJ, et al. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut. 2006;55:1449–1460. doi: 10.1136/gut.2006.094060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward DG, Suggett N, Cheng Y, Wei W, Johnson H, Billingham LJ, Ismail T, Wakelam MJ, Johnson PJ, Martin A. Identification of serum biomarkers for colon cancer by proteomic analysis. Br J Cancer. 2006;94:1898–1905. doi: 10.1038/sj.bjc.6603188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munro NP, Cairns DA, Clarke P, Rogers M, Stanley AJ, Barrett JH, Harnden P, Thompson D, Eardley I, Banks RE, et al. Urinary biomarker profiling in transitional cell carcinoma. Int J Cancer. 2006;119:2642–2650. doi: 10.1002/ijc.22238. [DOI] [PubMed] [Google Scholar]
- 17.Rogers MA, Clarke P, Noble J, Munro NP, Paul A, Selby PJ, Banks RE. Proteomic profiling of urinary proteins in renal cancer by surface enhanced laser desorption ionization and neural-network analysis: identification of key issues affecting potential clinical utility. Cancer Res. 2003;63:6971–6983. [PubMed] [Google Scholar]
- 18.Sharma N, Laftah AH, Brookes MJ, Cooper B, Iqbal T, Tselepis C. A role for tumour necrosis factor alpha in human small bowel iron transport. Biochem J. 2005;390:437–446. doi: 10.1042/BJ20050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemna EH, Tjalsma H, Podust VN, Swinkels DW. Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem. 2007;53:620–628. doi: 10.1373/clinchem.2006.079186. [DOI] [PubMed] [Google Scholar]
- 20.Tomosugi N, Kawabata H, Wakatabe R, Higuchi M, Yamaya H, Umehara H, Ishikawa I. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood. 2006;108:1381–1387. doi: 10.1182/blood-2005-10-4043. [DOI] [PubMed] [Google Scholar]
- 21.Kemna E, Tjalsma H, Laarakkers C, Nemeth E, Willems H, Swinkels D. Novel urine hepcidin assay by mass spectrometry. Blood. 2005;106:3268–3270. doi: 10.1182/blood-2005-05-1873. [DOI] [PubMed] [Google Scholar]
- 22.Bloem RM, Zwaveling A, Stijnen T. Adenocarcinoma of the colon and rectum: a report on 624 cases. Neth J Surg. 1988;40:121–126. [PubMed] [Google Scholar]
- 23.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 24.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 25.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 26.Sheikh N, Dudas J, Ramadori G. Changes of gene expression of iron regulatory proteins during turpentine oil-induced acute-phase response in the rat. Lab Invest. 2007;87:713–725. doi: 10.1038/labinvest.3700553. [DOI] [PubMed] [Google Scholar]
- 27.Lassmann S, Schuster I, Walch A, Gobel H, Jutting U, Makowiec F, Hopt U, Werner M. STAT3 mRNA and protein expression in colorectal cancer: effects on STAT3-inducible targets linked to cell survival and proliferation. J Clin Pathol. 2007;60:173–179. doi: 10.1136/jcp.2005.035113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calzolari A, Oliviero I, Deaglio S, Mariani G, Biffoni M, Sposi NM, Malavasi F, Peschle C, Testa U. Transferrin receptor 2 is frequently expressed in human cancer cell lines. Blood Cells Mol Dis. 2007;39:82–91. doi: 10.1016/j.bcmd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Deng H, Makizumi R, Ravikumar TS, Dong H, Yang W, Yang WL. Bone morphogenetic protein-4 is overexpressed in colonic adenocarcinomas and promotes migration and invasion of HCT116 cells. Exp Cell Res. 2007;313:1033–1044. doi: 10.1016/j.yexcr.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Weizer-Stern O, Adamsky K, Margalit O, Ashur-Fabian O, Givol D, Amariglio N, Rechavi G. Hepcidin, a key regulator of iron metabolism, is transcriptionally activated by p53. Br J Haematol. 2007;138:253–262. doi: 10.1111/j.1365-2141.2007.06638.x. [DOI] [PubMed] [Google Scholar]