Abstract
AIM: To assess the intestinal permeability (IP) in patients with Crohn’s disease (CD) and study the association of IP with the patient and disease characteristics.
METHODS: One hundred and twenty five consecutive patients of CD (Males: 66) were diagnosed on the basis of a combination of standard clinical, endoscopic, imaging and histological features. CD activity index (CDAI) was used to calculate the activity of the disease while the behavior of the disease was assessed by the modified Montreal classification. IP was measured by the ratio of the percentage excretion of ingested doses of lactulose and mannitol in urine (LMR). The upper limit of normality of LMR (0.037) was derived from 22 healthy controls.
RESULTS: Thirty six percent of patients with CD had increased IP. There was no significant difference in mannitol excretion (patients vs controls = 12.5% vs 14.2%, P = 0.4652), but lactulose excretion was significantly higher in patients compared to healthy controls (patients vs controls = 0.326% vs 0.293%, P = 0.0391). The mean LMR was also significantly higher in the patients as compared to healthy controls [0.027 (0.0029-0.278) vs 0.0164 (0.0018-0.0548), P = 0.0044]. Male patients had a higher LMR compared to females [0.036 (95% CI 0.029, 0.046) vs 0.022 (95% CI 0.0178, 0.028) (P = 0.0024), though there was no difference in the number of patients with abnormal IP in both the sexes. Patients with an ileo-colonic disease had a higher LMR than those with only colonic disease [0.045 (95% CI 0.033, 0.06) vs 0.021 (95% CI 0.017, 0.025) (P < 0.001)]. Of patients with ileo-colonic disease, 57.8% had an abnormal IP, compared to 26.7% with colonic and 15.6% with small intestinal disease. Patients with a stricturing disease had significantly higher LMR compared to non-fistulising non-stricturing disease [0.043 (95% CI 0.032, 0.058) vs 0.024 (95% CI 0.019, 0.029) (P = 0.0062)]. There was no correlation of IP with age, disease activity, duration of illness, D-xylose absorption, upper GI involvement, perianal disease, and extra-intestinal manifestations. On multiple regression analysis, male gender and ileo-colonic disease were independent factors associated with increased IP. Gender, location, behavior of the disease and upper GI involvement could explain up to 23% of variability in IP (R2 = 0.23).
CONCLUSION: IP was increased in 36% of patients with CD. Male gender and an ileo-colonic disease were the independent factors associated with increased IP.
Keywords: Lactulose mannitol ratio, Crohn’s disease, Inflammatory bowel disease, Intestinal barrier, Crohn’s disease activity index, Intestinal permeability
INTRODUCTION
Intestinal permeability (IP) is the property of the intestinal epithelium which refers to the facility with which it allows molecules to pass through by non-mediated diffusion[1]. IP has been implicated in the pathogenesis and frequent relapses of Crohn’s disease (CD)[2–6]. Seven to 18% higher relapse rate has been reported in patients with increased IP compared to those with normal IP[7–11]. Moreover, 10%-20% of healthy first-degree relatives have been also shown to have increase IP[4,12,13]. IP is measured as a ratio of two non-metabolizable probe molecules that pass across the mucosal barrier and are excreted in the urine. Quantitation of these probes in a timed urine collection provides a measure of the fraction of the ingested dose that penetrated the mucosal barrier[14]. The individual variations due to non-mucosal factors (gastric emptying, intestinal transit, dilution by secretions, renal clearance and incomplete urine recovery) are circumvented when the urinary recovery is expressed as a ratio, since both the sugars are equally affected by these factors except the route of permeation[1,15]. A few studies have reported the association of IP with activity and location of Crohn’s disease; however, there is a lack of literature on the association of IP with the disease characteristics (duration, extent, behavior, extra-intestinal manifestations) and patient characteristics (age and gender). There are no reports on IP in Asian patients with CD. Hence, this study was planned to assess IP in patients with CD and also to explore the relationship of IP with the patient and disease characteristics.
MATERIALS AND METHODS
Patients
One hundred and twenty five consecutive patients with CD from the outpatient and inpatient of Gastroenterology Department, All India Institute of Medical Sciences, New Delhi, were studied between May 2005 and September 2006. The diagnosis of CD was made on the basis of clinical manifestations (chronic diarrhea, hematochezia, abdominal pain and intestinal obstructive symptoms), endoscopic features (skip lesions, asymmetrical involvement, deep ulcers, aphthous ulcers, ileocecal valve and terminal ileal involvement) and histological evidence (acute on chronic colitis, inflammation extending beyond muscularis mucosa, lymphoid follicles and non caseating granuloma). The involvement of small intestine was assessed by barium meal follow through, small bowel enema, magnetic resonance enteroclysis and/or retrograde ileoscopy. Disease activity was assessed using the Crohn’s disease activity index (CDAI)[16]. The location and behavior of the disease were classified using the modified Montreal classification[17]. Patients were treated with maintenance doses of mesalamines and azathioprine, along with hematinics, multivitamins and calcium supplements. Those in the active phase of the disease were treated with steroids. None of the patients in active phase of the disease had any evidence of bacterial or parasitic infection at the time of inclusion into the study.
Healthy controls
Twenty-two healthy controls comprising of hospital staff and family members of patients with a diagnosis other than inflammatory bowel disease were included to decide the upper limit of normality (cut-off) for lactulose mannitol ratio (LMR). None of the controls had signs and symptoms of gastrointestinal disorders; renal diseases, diabetes and none of them had taken NSAIDs for at least four weeks prior to the test. None had a history of alcohol intake and smoking.
Ethical Consideration: The Ethics Committee of our institution approved the study. An informed consent was taken from all the participants.
Assessment of intestinal permeability
IP was measured using the lactulose and mannitol (L:M) excretion test in all the patients and healthy controls. The results were expressed as the ratio of percentage excretion of the ingested dose of lactulose and mannitol in urine [lactulose mannitol ratio (LMR) = % lactulose/% mannitol].
Test procedure
After an overnight fast, the patients evacuated the urinary bladder, collected a pre-test sample and then drank the test solution containing 5 g of lactulose, 2 g of mannitol and 5 g of D-xylose in 100 mL water. No food or drink other than water was allowed until the completion of the test. Water was permitted after one hour of the ingestion of the test solution. All the urine passed in the subsequent five hours was collected into a plastic can containing 20% chlorhexidine as a preservative. Aliquots of the collected urine were stored at -20°C until analysis. Patients were instructed to abstain from NSAIDs for four weeks prior to the test. All the patients and controls tolerated the test solution well.
Estimation of mannitol in urine
This test was based on the principle of oxidation of mannitol to formaldehyde by periodic acid. The formaldehyde so produced was measured by the method of MacFadyen as described by Corcoran and Page[18].
Periodic acid (0.03 mol/L in 0.25 mol/L sulfuric acid; 500 μL) was added to 2 mL of urine specimen in a test tube and kept at room temperature for 10 min. Stannous chloride (0.125 mol/L; 500 μL) was added to the sample and mixed well. Oxidation of stannous chloride to stannic acid produced a milky precipitate, which was dissolved by adding 5 mL of chromotropic acid reagent. The tubes were then placed in a boiling water bath for 30 min. After cooling the tubes the volume of the solution was made up to 25 mL by adding distilled water and the temperature was stabilized at 25°C in a water bath. A reagent blank was prepared containing 2 mL of distilled water instead of sample and was treated in the same manner along with the sample tubes. The optical density was measured at 570 nm using a spectrophotometer[18].
Estimation of lactulose in urine
The estimation of lactulose was based on the enzymatic assay of fructose which was produced after the hydrolysis of lactulose to fructose and galactose. In a series of enzymatic reactions, fructose was converted to NADPH. The amount of NADPH produced was directly proportional to lactulose concentration, which was measured by the change in absorbance at 340 nm on a spectrophotometer[19]. D-xylose was estimated in urine using the standard method of Roe and Rice[20].
Estimation of the cut-off for the LMR
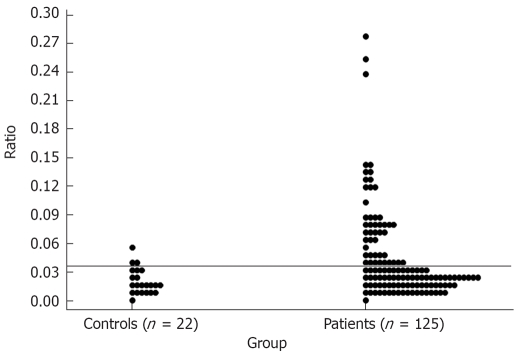
The results of the LMR in the healthy controls were used to fix the upper limit of IP at one tailed 90% tolerance interval. Since the distribution of LMR in controls was non-normal, a square root-transformation was carried out to determine the mean and standard deviation (SD) and subsequently the cut-off value was established as mean + 1.28 SD of the square root of LMR, assuming that in normal population 10% subjects will have abnormal IP. Thus obtained cut off value would correspond to the 90th percentile. A LMR of > 0.0373 was considered abnormal (Figure 1).
Figure 1.
Upper limit of normality for lactulose mannitol ratio (LMR).
Statistical analysis
STATA 9.0 (College station, TX, USA) statistical software was used for data analysis. The data were expressed as number (percentage), median (range) and mean (95% CI). The difference in proportions of abnormal IP between different categories of disease area, behavior, activity and gender were compared using χ2 test. Non-normal continuous variables were compared between the patients and controls using non-parametric test (Wilcoxon rank-sum test). Bivariate analysis was done using t-test, ANOVA or Spearman’s correlation as appropriate followed by multiple regression analysis to find the factors related with increased IP. The P-value < 0.05 was considered statistically significant.
RESULTS
The median (range) age of 125 patients was 36 (14-66) years. The median (range) duration of the disease was 50 (3-432) mo. The duration of the disease was less than 36 mo in 52 (41.6%) and more than that in 73 (58.4%) patients. The distribution of the patients according to activity, duration, location, and behavior of the disease is shown in Table 1.
Table 1.
Characteristics of the patients with CD (n = 125)
| Variables | n (%) |
| Sex | |
| Males | 66 (52.8) |
| Females | 59 (47.2) |
| Age (yr) | |
| Median (range) | 36 (14-66) |
| Patients with age < 40 | 76 (60.8) |
| Patients with age ≥ 40 | 49 (39.2) |
| Duration of the disease (mo) | |
| Median (range) | 50 (3-432) |
| Duration < 36 mo | 52 (41.6) |
| Duration ≥ 36 mo | 73 (58.4) |
| Activity of the disease (CDAI) | |
| Median (range) | 109 (10-343) |
| CDAI < 150 | 80 (64) |
| CDAI 151-220 | 29 (23.2) |
| CDAI 221-400 | 16 (12.8) |
| CDAI > 400 | 0 (0) |
| Area of involvement of the GI tract | |
| Small intestine only (L1) | 16 (12.8) |
| Large intestine only (L2) | 62 (49.6) |
| Ileo-colonic (L3) | 47 (37.6) |
| UGI involvement (L4) | 23 (18.4) |
| Behaviour of the disease | |
| Non-fistulizing non-stricturing (B1) | 74 (59.2) |
| Stricturing disease (B2) | 37 (29.6) |
| Fistulizing disease (B3) | 14 (11.2) |
| Perianal disease | 25 (20) |
| Extra-intestinal manifestations | 49 (39.2) |
CD: Crohn’s disease; CDAI: Crohn’s disease activity index.
Twenty three (18.4%) patients had involvement of the UGI tract, of which 6 (26.08%) had ileal, 6 (26.08%) had colonic and 11 (47.8%) had ileo-colonic involvement.
Lactulose and mannitol excretion and LMR in patients with CD
The percentage excretion of lactulose and mannitol, values of LMR and D-xylose excretion are shown in Table 2. Using the upper limit of normality for LMR at 0.0373, 45 of 125 (36%) patients had an abnormal IP, while 80 (64%) had a normal IP.
Table 2.
Comparison of intestinal permeability and D-xylose absorption between patients and healthy controls
| Parameter | Patients (n = 125) | Controls (n= 22) | P value |
| Lactulose excretion (%) | 0.326 (0.0204-2.76) | 0.293 (0.0089-0.665) | 0.0391a |
| Mannitol excretion (%) | 12.5 (1.43-43.75) | 14.2 (4.95-30.8) | 0.4652 |
| Lactulose mannitol ratio (LMR) | 0.027 (0.0029-0.278) | 0.0164 (0.0018-0.0548) | 0.0044a |
| D-xylose (g/5 g per 5 h) | 1.45 (0.32-4.5) | 1.89 (0.80-4.73) | 0.0277a |
All values expressed as median (range);
P < 0.05.
Lactulose, mannitol and D-xylose excretion in healthy controls and patients with CD
As shown in Table 2 there was no significant difference in percentage excretion of mannitol in patients and healthy controls (P = 0.4652). The percentage excretion of lactulose was significantly higher in patients compared to healthy controls (P = 0.0391). Similarly, the LMR was higher in the patients compared to healthy controls (P = 0.0044). D-xylose excretion was also significantly low in patients compared to healthy controls (P = 0.0277).
Relationship of LMR with patient and disease characteristics
As shown in Table 3, male patients had a significantly higher LMR in comparison to that in females (P = 0.0024). There was no difference in the number of patients with abnormal IP in both the sexes (P = 0.05). There was no significant correlation between IP and age of the patients (Table 4).
Table 3.
Relationship between intestinal permeability (log LMR) and characteristics of the patients and disease (n = 125)
| Variables | n | Mean LMR (95% CI) | P value |
| Gender | |||
| Males | 66 | 0.036 (0.029, 0.046) | 0.0024a |
| Females | 59 | 0.022 (0.0178, 0.028) | |
| Area of involvement of the GI tractb | |||
| Small intestine only (L1) | 16 | 0.028 (0.0175, 0.0447) | 0.0001a |
| Large Intestine only (L2) | 62 | 0.021 (0.0174, 0.0255) | |
| Ileo-colonic (L3) | 47 | 0.0451 (0.0336, 0.0605) | |
| UGI Involvement | |||
| Absent | 102 | 0.0268 (0.0224, 0.0321) | 0.0406a |
| Present | 23 | 0.0416 (0.027, 0.062) | |
| Perianal disease | |||
| Absent | 100 | 0.028 (0.023, 0.033) | 0.3573 |
| Present | 25 | 0.0339 (0.0234, 0.049) | |
| Behavior of the diseased | |||
| Non-fistulizing Non-stricturing (B1) | 74 | 0.024 (0.0194, 0.0298) | 0.0062a |
| Stricturing (B2) | 37 | 0.043 (0.032, 0.058) | |
| Fistulizing (B3) | 14 | 0.028 (0.018, 0.042) | |
| Extra intestinal manifestations | |||
| Absent | 76 | 0.028 (0.0226, 0.034) | 0.5674 |
| Present | 49 | 0.030 (0.0236, 0.040) | |
Values are expressed as geometric mean (95% CI),
P < 0.05; Large intestine only vs ileo-colonic,
P = 0.001 < 0.01; Non-fistulizing non-stricturing vs stricturing,
P = 0.004 < 0.01.
Table 4.
Relationship of LMR with the patient and disease characteristics using Spearman’s correlation coefficient
| Variables | r |
| Age (yr) | -0.1414 |
| Activity of the disease (CDAI) | 0.1414 |
| Duration of the disease (mo) | -0.0271 |
| D-xylose (g/5 g per 5 h) | -0.1210 |
LMR and location of the disease: On comparison of LMR in patients with different location of the disease (L1, L2, L3), a significant (P = 0.0001) difference was found. Patients with ileo-colonic involvement had a higher LMR in comparison to those with only colonic involvement (P = 0.0001). Higher percentage (57.8%) of patients with ileo-colonic disease had abnormal IP compared to the colonic (26.7%) and small intestine (15.6%) disease, which was statistically significant (P < 0.001).
LMR and behavior of the disease: There was a signifi-cant difference (P = 0.0062) in the LMR of patients with different behavior of the disease (B1, B2, B3). Patients with stricturing disease (B2) had significantly higher LMR in comparison to those with a non-fistulizing and non-stricturing disease (P = 0.004). Higher percentage (46.7%) of patients with a stricturing disease had abnormal IP compared to the non-stricturing non-fistulizing (42.2%) and fistulizing (11.1%) disease (P = 0.006).
LMR and UGI involvement, perianal disease and extra-intestinal manifestations: There was no significant difference in the LMR of the patients with and without extra-intestinal manifestations and perianal disease (P > 0.05). However, patients with an UGI involvement had a significantly higher LMR as compared to those without an UGI involvement (P = 0.046).
LMR and activity and duration of the disease: Twenty eight of 83 (33.7%) patients in the remission phase and 17 of 42 (40.47%) in the active phase of the disease had increased IP, however, it was not statistically significant. Spearman’s correlation did not show any association of LMR with activity and duration of the disease (Table 4).
Factors associated with increased IP
Sex, area, behavior and UGI involvement were the significant factors on a bivariate analysis. A multiple regression analysis revealed that all these factors could explain up-to 23% of variability in IP (R2 = 0.23) (Table 5). Out of all these factors gender of the patients and ileo-colonic disease were independent factors associated with increased IP.
Table 5.
Assessment of the effect of disease and patients characteristics on log LMR using multiple regression analysis
| Variable |
Regression co-efficient β (95% CI) |
|
| Unadjusted | Adjusted | |
| Sex | ||
| Females1 | ||
| Males | 0.39 (0.089, 0.69) | 0.429 (0.130, 0.727)a |
| Area | ||
| Small intestine only1 | ||
| Large intestine only | -0.284 (-0.764, 0.196) | 0.002 (-0.539, 0.543) |
| Ileo-colonic | 0.475 (-0.020, 0.971) | 0.579 (0.065, 1.09)a |
| Behaviour | ||
| Non-fistulizing non-stricturing1 | ||
| Stricturing disease | 0.587 (0.230, 0.945) | -0.237 (-0.763, 0.288) |
| Fistulizing disease | 0.152 (-0.366, 0.670) | 0.085 (-0.457, 0.628) |
| Upper GI involvement | ||
| Absent1 | ||
| Present | 0.437 (0.019, 0.856) | 0.233 (-0.163, 0.630) |
R2 = 23%,
P < 0.05, significant sqrt-square-root transformation.
Reference category; β: Regression coefficient; CI: Confidence interval.
On an average, log LMR value among males was 0.429 (95% CI 0.130, 0.727) more as compared to log LMR value in females. Patients with ileo-colonic disease on an average had a 0.58 (95% CI 0.065, 1.09) log units increase in the LMR compared to those with a small intestine disease (Table 5). Thus, male sex and ileo-colonic disease were found to be a risk factor for increased IP in patients with CD.
DISCUSSION
In our study, 36% of patients with CD had increased intestinal permeability. Males, patients with ileo-colonic disease, stricturing disease and those with UGI tract involvement had a significantly higher IP than females, patients with colonic disease, those with non-stricturing non-fistulizing disease and those without the UGI tract involvement, respectively. All these factors together could explain up to 23% of variability in IP. Male sex and ileo-colonic location of the disease were the independent factors associated with increased IP. However, there was no correlation of IP with age, duration, activity of the disease and D-xylose absorption.
Thirty three to 68% patients with CD have been reported to have increased IP using a variety of marker probes such as polyethylene glycol (PEG) 400, PEG 1000, 51CrEDTA, 99mTcDTPA, 51CrEDTA/14C-mannitol, lactulose, mannitol, rhamnose and cellobiose[21–24]. In this study, we used lactulose and mannitol (L/M) excretion to quantify IP. L/M test is a simple, non-invasive, reliable and a frequently used test for estimation of IP in clinical practice[25,26]. Our study showed that IP, as assessed by LMR, was significantly higher in patients compared to the healthy controls. There was no difference in the mannitol excretion amongst patients and controls, but the excretion of lactulose, was significantly higher in patients as compared to controls. Therefore, an abnormal LMR was due to higher excretion of lactulose in the urine than a reduced excretion of mannitol. Pearson et al[15], Murphy et al[3], Wyatt et al[7], and Katz et al[27] have also reported similar findings. The probes used to measure IP are water soluble, which cannot penetrate the lipid cell membrane of enterocytes and thus use the paracellular route through the tight junctions (TJs) for permeation. There is a difference between the TJs of the villous tips and villous crypts. The smaller probes can easily pass through the small, more numerous and more accessible TJs of the villous tips, whereas the larger probes make use of the larger, less accessible, and less numerous pores at the crypt of the villous[28,29]. While mannitol uses predominantly smaller and more numerous TJs at the villous tips, lactulose being a bigger molecule passes through larger pores in the crypts of the villous. As patients with CD do not generally have diffuse villous atrophy, the mannitol excretion remains unaffected in them and this could explain a normal mannitol excretion values observed in this study. Contrary to this, villous atrophy is the hallmark of celiac disease, which results in loss of smaller TJs at the villous tips, thus affecting excretion of mannitol[15,30]. Therefore, an increased IP observed in patients with CD, is predominantly because of increased permeation of lactulose, possibly due to defective TJs at the crypt. Nonetheless, one study has shown a decreased mannitol excretion in patients with CD as compared to the healthy controls[31], where reduced mannitol absorption reflects a reduction in the absorptive area of mucosa rather than change in mucosal leakiness.
We did not find any correlation between IP and age of the patients. Similar findings have been shown by Soderholm et al[23]. While Munkholm[32] and Jogerson et al[21] found a negative correlation of age with the absorption of mannitol and excretion of 51CrEDTA, respectively. Male sex was found to be a risk factor for increased IP on a multiple regression analysis in this study. Jorgensen et al reported an association between the male gender and increased permeation of 14C-mannitol[21]. On the contrary, Soderholm et al[23] did not find any correlation between IP and gender of the patients with CD. The reason of higher IP in male patients is not clear. We presume that it may be attributed to some other environmental factors, like consumption of tobacco and alcohol which was only prevalent among males in our study.
We also observed a significantly higher IP in patients with ileo-colonic disease as compared with those with only a colonic disease. Ukabam et al[31] and Ainsworth et al[33] have also reported a significantly increased IP in patients with ileal CD than those with colonic CD using L/M ratio and 51CrEDTA, respectively. Peters et al[22] reported a significantly higher median permeability of 51Cr EDTA in patients with ileal CD compared to normal controls; no significant difference was seen in patients with colonic CD and normal controls. The possible reason that could be given in support of our findings is the choice of marker probes. Different marker probes with regional selectivity are used to study IP in different locations of GI tract. Sucrose is used for gastric and proximal duodenal permeability; lactulose and mannitol for small intestine permeability; sucralose, poly sucralose and EDTA for both large and small intestine permeability[28]. Nevertheless, most of the probes are much better in detecting the permeability abnormalities of the small intestine than that of the colon[34]. Moreover, mannitol permeability does not differentiate between CD of the small intestine and large intestine. Abnormal IP in patients with ileo-colonic involvement in this study may be a reflection of selection of a marker probe, which assessed the small intestinal permeability only. In a few studies on the other hand, no relation was observed between IP and location of the disease[23,27,35]. No association was found between IP and perianal disease.
It was also seen that patients with a stricturing disease had a significantly higher LMR than a non-fistulizing non-stricturing disease. Similar observations have not been reported in the English literature to the best of our knowledge. We also hypothesize that an abnormal IP might lead to frequent and prolonged relapses, which in turn may have lead to the development of intestinal strictures.
Increased IP, molecular mimickry, translocation of antigens and circulating cytokines are the proposed pathogenic mechanisms of extra-intestinal manifestations in patients with CD[36]. We, however, did not observe an association between increased IP and extra-intestinal manifestations in this study.
A number of studies have reported a relationship between abnormal IP and the activity of the disease[3,5,37,38]. In some reports it has been observed that the magnitude of alterations in L/M ratio increases with the increase in disease activity index. In fact, assessment of IP has been recommended as a more objective way of assessing the activity and severity of the disease[39]. But, we did not find any correlation between L/M ratio and activity of the disease. Though there were a greater number of patients with abnormal IP in the active phase of the disease than in the remission phase, this did not, however, reach a value of statistical significance. Ukabam et al[31] and Soderholm et al[23] also did not observe a relationship between IP and activity of the disease. Similarly, Turck et al[35] reported that the highest excretion values of 51Cr EDTA were not always observed in children with the most active CD.
In conclusion, we observed increased intestinal permeability in 36% of patients with CD and this was mainly because of the increase in the excretion of lactulose. Male gender and ileo-colonic involvement were independent risk factors for increased intestinal permeability in our patients.
ACKNOWLEDGMENTS
The authors are grateful to Dr. A Srinivasan, Associate Professor, Department of Biophysics, All India Institute of Medical Sciences, New Delhi for his continuous intellectual inputs in conducting this study. We are also thankful to all the patients and healthy volunteers who willingly participated in this study.
COMMENTS
Background
Intestinal permeability (IP) is an important aspect of the gut barrier function of the intestinal mucosa. Its role has been emphasized recently in a number of conditions including Crohn’s disease (CD). In case of CD it has been implicated in its etiopathogenesis, assessment of the disease activity, and a predictor of relapse. The incidence of CD has been recently reported to be on a rise in the Asian countries including India. Hence, we decided to study IP and its correlation with the patient and disease characteristics in Crohn’s disease in India
Research frontiers
There are a number of studies from the western world on the study of IP. It has been reported that IP may be abnormal even in those with a macroscopically normal gut. It is believed that IP is maintained by the tight junctions (TJs). It will be interesting to further study that apart from atrophy of the mucosal surface what else makes the TJs ineffective. Probability of some defect or abnormality in TJs proteins has been suggested for the abnormal IP. It will be further interesting to know the characterization of these proteins and then improve upon them by some intervention
Innovation and breakthroughs
There are several studies reporting abnormal IP not only in CD, but also in blood relations of the patients. There is also a case report where a patient with abnormal IP ultimately developed CD. There have been no reports on IP in Asian patients with CD. Hence this study is an initial attempt in this direction to study IP and identify those patients & disease characteristics which might influence IP in patients with CD.
Application
Our study reveals that up to 36% of patients have abnormal IP. This shows that IP in Indian patients is the same as that of the western population. We thought that India, being a tropical country, might have greater prevalence of abnormal IP than the western world. The gender difference shown in IP in our patients signifies that there may be a variation in the presentation and clinical course of the disease in both the sexes, as has been reported in pediatric patients with CD.
Terminology
Intestinal permeability (IP): IP can be defined as the property of the intestinal epithelium or of a membrane which refers to the facility with which it allows molecules to pass through by non-mediated diffusion. Tight Junctions (TJs): The tight junctions encircle epithelial cells at the apical pole, being a narrow belt that both connects adjacent cells and maintains cell polarity. TJs represent the gating mechanism in the paracellular pathway.
Peer review
This research adds to the growing body of literature regarding intestinal permeability in a variety of pathogenic states including inflammatory bowel disease. It suggests that the underlying mucosal defect is at the crypt and not at the villous level and thus it yields a new level of understanding to the underlying mechanism.
Supported by The Indian Council of Medical Research (ICMR)
Peer reviewer: Fabrizio Michelassi, MD, Deparment of Surgery and Lewis Atterbury Stimson, Weill Cornell Medial College, 525 East 68 Street, Box 129, Rm F-739, New York 10021, United States
S- Editor Zhu LH L- Editor Rippe RA E- Editor Ma WH
References
- 1.Travis S, Menzies I. Intestinal permeability: functional assessment and significance. Clin Sci (Lond) 1992;82:471–488. doi: 10.1042/cs0820471. [DOI] [PubMed] [Google Scholar]
- 2.Hollander D. Crohn’s disease--a permeability disorder of the tight junction? Gut. 1988;29:1621–1624. doi: 10.1136/gut.29.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy MS, Eastham EJ, Nelson R, Pearson AD, Laker MF. Intestinal permeability in Crohn’s disease. Arch Dis Child. 1989;64:321–325. doi: 10.1136/adc.64.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 5.Adenis A, Colombel JF, Lecouffe P, Wallaert B, Hecquet B, Marchandise X, Cortot A. Increased pulmonary and intestinal permeability in Crohn’s disease. Gut. 1992;33:678–682. doi: 10.1136/gut.33.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secondulfo M, de Magistris L, Fiandra R, Caserta L, Belletta M, Tartaglione MT, Riegler G, Biagi F, Corazza GR, Carratu R. Intestinal permeability in Crohn’s disease patients and their first degree relatives. Dig Liver Dis. 2001;33:680–685. doi: 10.1016/s1590-8658(01)80045-1. [DOI] [PubMed] [Google Scholar]
- 7.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 8.Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol. 2000;35:1163–1169. doi: 10.1080/003655200750056637. [DOI] [PubMed] [Google Scholar]
- 9.D'Inca R, Di Leo V, Corrao G, Martines D, D'Odorico A, Mestriner C, Venturi C, Longo G, Sturniolo GC. Intestinal permeability test as a predictor of clinical course in Crohn's disease. Am J Gastroenterol. 1999;94:2956–2960. doi: 10.1111/j.1572-0241.1999.01444.x. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt J, Hubl W, Vogelsang H: Intestinal permeability predicts acute phases of Crohn’s disease. Gastroenterology. 1991;100:A848. [Google Scholar]
- 11.Hilsden RJ, Meddings JB, Hardin J, Gall DG, Sutherland LR. Intestinal permeability and postheparin plasma diamine oxidase activity in the prediction of Crohn’s disease relapse. Inflamm Bowel Dis. 1999;5:85–91. doi: 10.1097/00054725-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 12.May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn’s disease? Gastroenterology. 1993;104:1627–1632. doi: 10.1016/0016-5085(93)90638-s. [DOI] [PubMed] [Google Scholar]
- 13.Hilsden RJ, Meddings JB, Sutherland LR. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn’s disease. Gastroenterology. 1996;110:1395–1403. doi: 10.1053/gast.1996.v110.pm8613043. [DOI] [PubMed] [Google Scholar]
- 14.Hollander D. The importance of intestinal permeability in the pathogenesis of Crohn’s disease. In: Rachmilewtz D, editor. Inflammatory bowel disease. Falk Symposium 72. London: Kluwer Academic Publishers; 1994. pp. 41–51. [Google Scholar]
- 15.Pearson AD, Eastham EJ, Laker MF, Craft AW, Nelson R. Intestinal permeability in children with Crohn's disease and coeliac disease. Br Med J (Clin Res Ed) 1982 Jul 3;285(6334):20–21. doi: 10.1136/bmj.285.6334.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 17.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 18.Corcoran A, Page J. A method for determination of mannitol in plasma and urine. J Biol Chem. 1947;170:165–171. [Google Scholar]
- 19.Behrens RH, Docherty H, Elia M, Neale G. A simple enzymatic method for the assay of urinary lactulose. Clin Chim Acta. 1984;137:361–367. doi: 10.1016/0009-8981(84)90125-6. [DOI] [PubMed] [Google Scholar]
- 20.Roe JH, and Rice EW. A photometric method for the determination of free pentoses in animal tissues. J Biol Chem. 1948;173:507–512. [PubMed] [Google Scholar]
- 21.Jorgensen J, Ranlov PJ, Bjerrum PJ, Diemer H, Bisgaard K, Elsborg L. Is an increased intestinal permeability a valid predictor of relapse in Crohn disease? Scand J Gastroenterol. 2001;36:521–527. [PubMed] [Google Scholar]
- 22.Peeters M, Ghoos Y, Maes B, Hiele M, Geboes K, Vantrappen G, Rutgeerts P. Increased permeability of macroscopically normal small bowel in Crohn’s disease. Dig Dis Sci. 1994;39:2170–2176. doi: 10.1007/BF02090367. [DOI] [PubMed] [Google Scholar]
- 23.Soderholm JD, Olaison G, Lindberg E, Hannestad U, Vindels A, Tysk C, Jarnerot G, Sjodahl R. Different intestinal permeability patterns in relatives and spouses of patients with Crohn's disease: an inherited defect in mucosal defence? Gut. 1999;44:96–100. doi: 10.1136/gut.44.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Inca R, Annese V, di Leo V, Latiano A, Quaino V, Abazia C, Vettorato MG, Sturniolo GC. Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1455–1461. doi: 10.1111/j.1365-2036.2006.02916.x. [DOI] [PubMed] [Google Scholar]
- 25.van Elburg RM, Uil JJ, Kokke FT, Mulder AM, van de Broek WG, Mulder CJ, Heymans HS. Repeatability of the sugar-absorption test, using lactulose and mannitol, for measuring intestinal permeability for sugars. J Pediatr Gastroenterol Nutr. 1995;20:184–188. doi: 10.1097/00005176-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Uil JJ, van Elburg RM, van Overbeek FM, Mulder CJ, VanBerge-Henegouwen GP, Heymans HS. Clinical implications of the sugar absorption test: intestinal permeability test to assess mucosal barrier function. Scand J Gastroenterol Suppl. 1997;223:70–78. [PubMed] [Google Scholar]
- 27.Katz KD, Hollander D, Vadheim CM, McElree C, Delahunty T, Dadufalza VD, Krugliak P, Rotter JI. Intestinal permeability in patients with Crohn’s disease and their healthy relatives. Gastroenterology. 1989;97:927–931. doi: 10.1016/0016-5085(89)91499-6. [DOI] [PubMed] [Google Scholar]
- 28.Hollander D. Intestinal permeability in health and disease. In: Joseph B Kirsner., editor. Inflammatory bowel disease. Philadelphia: Saunders; 2000. pp. 45–54. [Google Scholar]
- 29.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 30.Juby LD, Rothwell J, Axon AT. Lactulose/mannitol test: an ideal screen for celiac disease. Gastroenterology. 1989;96:79–85. doi: 10.1016/0016-5085(89)90767-1. [DOI] [PubMed] [Google Scholar]
- 31.Ukabam SO, Clamp JR, Cooper BT. Abnormal small intestinal permeability to sugars in patients with Crohn’s disease of the terminal ileum and colon. Digestion. 1983;27:70–74. doi: 10.1159/000198932. [DOI] [PubMed] [Google Scholar]
- 32.Munkholm P, Langholz E, Hollander D, Thornberg K, Orholm M, Katz KD, Binder V. Intestinal permeability in patients with Crohn’s disease and ulcerative colitis and their first degree relatives. Gut. 1994;35:68–72. doi: 10.1136/gut.35.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ainsworth M, Eriksen J, Rasmussen JW, Schaffalitzky de Muckadell OB. Intestinal permeability of 51Cr-labelled ethylenediaminetetraacetic acid in patients with Crohn’s disease and their healthy relatives. Scand J Gastroenterol. 1989;24:993–998. doi: 10.3109/00365528909089246. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins AP, Nukajam WS, Menzies IS, Creamer B. Simultaneous administration of lactulose and 51Cr-ethylenediaminetetraacetic acid. A test to distinguish colonic from small-intestinal permeability change. Scand J Gastroenterol. 1992;27:769–773. doi: 10.3109/00365529209011181. [DOI] [PubMed] [Google Scholar]
- 35.Turck D, Ythier H, Maquet E, Deveaux M, Marchandise X, Farriaux JP, Fontaine G. Intestinal permeability to [51Cr]EDTA in children with Crohn’s disease and celiac disease. J Pediatr Gastroenterol Nutr. 1987;6:535–537. doi: 10.1097/00005176-198707000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Joel B. Levine. Extraintestinal manifestations of inflammatory bowel disease. In: Joseph B Kirsner., editor. Inflammatory bowel disease. Philadelphia: W Saunders; 2000. pp. 45–54. [Google Scholar]
- 37.Murphy MS, Eastham EJ, Nelson R, Pearson AD, Laker MF. Intestinal permeability in Crohn’s disease. Arch Dis Child. 1989;64:321–325. doi: 10.1136/adc.64.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adenis A, Colombel JF, Lecouffe P, Wallaert B, Hecquet B, Marchandise X, Cortot A. Increased pulmonary and intestinal permeability in Crohn’s disease. Gut. 1992;33:678–682. doi: 10.1136/gut.33.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andre F, Andre C, Emery Y, Forichon J, Descos L, Minaire Y. Assessment of the lactulose-mannitol test in Crohn’s disease. Gut. 1988;29:511–515. doi: 10.1136/gut.29.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]