Abstract
A detailed molecular understanding of mitochondrial fusion and fission in mammalian cells is rapidly emerging. In this report, we demonstrate for the first time cross-species mitochondrial fusion between distantly related species using green and red fluorescent proteins targeted to the mitochondrial matrix. We found that mouse mitochondria were able to efficiently fuse to unmodified mitochondria of human cells and that the contents of the mitochondrial matrix were completely mixed in less than 4 h. We also observed that mitochondria from the mtDNA-less (ρ0) mouse cells can homogeneously fuse to the mitochondria of human cells. We were, however, unable to maintain human mitochondrial DNA in the mouse cells. These results indicate that mitochondrial fusion proteins in mouse and human cells have enough functional homology to mediate efficient cross-species mitochondrial fusion, but mouse nuclear and human mitochondrial genomes have not retained functional compatibility with one another.
Keywords: Mitochondrial fusion, Coevolution, Cybrid, Mitochondrial DNA
1. Introduction
The nuclear and mitochondrial genomes in eukaryotic cells have coevolved to optimize nuclear–mitochondrial interactions for the efficient generation of cellular energy (Gray et al., 1999). This co-evolution has led to species-specific compatibility between nuclear and mitochondrial genomes (Kenyon and Moraes, 1997; Bayona-Bafaluy et al., 2005). Although mitochondria from the same or from closely related species have been shown to have the ability to repopulate ρ0 cells (i.e., cells without mitochondrial DNA) following cell fusion, mitochondrial genomes from more distantly related species cannot provide mitochondrial functions in these cells (Kenyon and Moraes, 1997). Interspecies somatic hybrid cells between mouse and human have been obtained, but the maintenance of mitochondrial genomes, which are usually from only one species, has been shown to require essentially a complete set of cognate chromosomes (Wallace et al., 1976; De Francesco et al., 1980). Viable xenomitochondrial cybrids between cells from different rodent species (e.g., mouse and rat) (Dey et al., 2000; McKenzie and Trounce, 2000; Yamaoka et al., 2000; McKenzie et al., 2003) or human–humanoid primates (e.g., human and chimpanzee or gorilla or orangutan) (Kenyon and Moraes, 1997; Bayona-Bafaluy et al., 2005) have been reported, and transmitochondrial or xenomitochondrial mice have been produced (Pinkert and Trounce, 2000; Shoubridge, 2000; Sokolova et al., 2004), but these studies also suggest that the retention of nuclear–mitochondrial compatibility between species is essential for generating interspecies xenomitochondrial cybrids or animal models with a functional (but reduced) oxidative phosphorylation system.
Following intraspecies fusion of eukaryotic cells, the mitochondrial networks of the two cells rapidly fuse and the molecular contents of these networks are exchanged (Nunnari et al., 1997; Legros et al., 2002). The mitochondrial fusion process, which is balanced by the competing process of mitochondrial fission (Chen and Chan, 2005), is critical for transferring the mtDNA genomes both within and between these networks. Because only intraspecies mitochondrial fusion has been studied to date (e.g., human–human or yeast–yeast), we wanted to determine if interspecies mitochondrial fusion could occur after the fusion of cells from distantly related species. If the mitochondrial fusion process proved to be limited to exchanges between only closely related species, then we would know that this process is the first limiting step in interspecies mitochondrial exchange. If, on the other hand, we found that inter-species mitochondrial fusion can occur, we would know that mtDNA can be exchanged between distantly related species and that interspecies incompatibility was occurring at a down-stream process (e.g., mtDNA replication, transcription, or at the level of protein–protein binding incompatibility).
A detailed molecular understanding of mitochondrial fusion is emerging (Meeusen et al., 2004; Koshiba et al., 2004). Mitochondrial fusion proteins, which are nuclear-encoded, are associated with both the outer and inner mitochondrial membranes. In yeast, three components of the mitochondrial fusion machinery are known: Fzo1 (the outer-membrane GTPase), Mgm1 (the intermembrane-space GTPase) and Ugo1 (the outer-membrane protein that links both GTPases) (Mozdy and Shaw, 2003; Sesaki et al., 2003; Sesaki and Jensen, 2004). In mice and other mammals, homologs of two of these proteins have been identified: mitofusin 1 (Mfn1) and mitofusin 2 (Mfn2) are homologs of Fzo1, and Opa1 is homologous to Mgm1 (Legros et al., 2002; Cipolat et al., 2004). The carboxy-terminal cytosolic domains of Fzo1 or mitofusin proteins have been shown to form homotypic antiparallel dimers (Koshiba et al., 2004; Pfanner et al., 2004). Binding in trans between the Fzo1 or mitofusin proteins in adjacent mitochondria provides the mechanism for the first step of the fusion process (i.e., tethering). The amino-terminal GTPases of the mitofusins/Fzo1 are required for the fusion of the outer mitochondrial membranes. The intermembrane-space GTPases Opa1 and Mgm1 have not yet been as thoroughly characterized as mitofusins, but they are thought to be required for inner mitochondrial membrane fusion. Ugo1 has recently been shown to link Fzo1 to Mgm1 (Sesaki and Jensen, 2004) although a functional equivalent of Ugo1 in mammals has not yet been described.
In yeast and human cells, the mitochondrial fusion process has been analyzed by measuring the diffusion and/or mixing of differentially labeled matrix proteins (Nunnari et al., 1997; Legros et al., 2002). We used this experimental approach to characterize interspecies mitochondrial fusion directly between mouse and human mitochondrial networks. We monitored the fusion process by differentially labeling the mitochondria of these cells with the soluble matrix marker proteins GFP and DsRed. We report here that the mitochondrial fusion proteins in mice and humans have a high degree of functional homology to each other and readily mediate interspecies mitochondrial fusion and matrix content exchange between the mitochondrial networks of these species. In light of this result, we also performed experiments to determine if human mtDNA could functionally repopulate ρ0 mouse cells following cell and mitochondrial fusion between these species. As expected, transmitochondrial cybrid cells were obtained only from fusion with enucleated mouse cells and not from fusion with human cells, confirming that human mtDNA is functionally incompatible with the mouse nuclear genome.
2. Materials and methods
2.1. Media and strains
Mouse STO embryonic fibroblast (CRL-1503), SNL (G418-resistant derived from STO) and LL/2 (ATCC CRL-1642) (Bertram and Janik, 1980) cells were grown in DMEM (Life Technologies, Rockville, MD) in the presence of heat-inactivated 10% fetal bovine serum (FBS) at 37°C in a humidified 10% CO2 incubator. HeLa229 (ATCC CCL-2.1) was cultured in MEM alpha (Life Technologies) with 10% heat-inactivated FBS at 37 ° C in a humidified 5% CO2 incubator.
2.2. Construction of plasmids
The 24 N-terminal amino acids mitochondrial leader sequence of the mouse mitochondrial transcription factor A (Tfam) (Larsson et al., 1996) was cloned between the NheI and HindIII sites of pDsRed1-N1 (Clontech, Mountain View, CA), resulting in pmusTFAML-DsRed. The construction of pcDNA6-musTFAML-GFP was reported elsewhere (Yoon and Koob, 2005).
2.3. Generation of cell lines expressing mitochondria-targeted GFP or DsRed
To make a cell line expressing GFP or DsRed targeted to mitochondria, 10 µg of the expression vectors pcDNA6-musTFAML-GFP or pmusTFAML-DsRed was linearized with BglII or NotI and transfected into HeLa229 or STO cells. Transfection of the cells was performed using the calcium phosphate method (Kingston et al., 1987) and stable cell lines expressing mitochondria-targeted proteins were selected with blasticidin (5 µg/ml) or G418 (400 µg/ml) for 4 weeks. A clonal cell line was obtained by diluting these antibiotic-resistant cells to a single cell per well in 96-well plates. The cloned cells were then cultured in normal medium supplemented with 3 µg/ml of blasticidin or 400 µg/ml of G418. We have generated the HeLa229 and STO cell lines labeled with GFP and DsRed in their mitochondria, respectively.
2.4. Cell fusions
For cell fusion, cells carrying differently labeled mitochondria were mixed and plated on glass coverslips 16–40 h before cell fusion. Cycloheximide (20 µg/ml) was added 30 min before fusion and kept in all solutions used subsequently to inhibit protein synthesis. The protocol for PEG-mediated fusion of adherent cells was used (Legros et al., 2002; Rojo et al., 2002). Briefly, 70–100% confluent cells in a 35-mm culture dish are washed with minimal essential medium (MEM) without serum and incubated for 45–60 s with 750 µl of a prewarmed (37 °C) solution of PEG 1500 (50% [w/v] in DMEM). Cells were then washed extensively with MEM containing 10% serum and transferred to prewarmed culture medium.
2.5. Cloning of mtDNA-less (ρ0) SNL cells
To generate mtDNA-less ρ0 cell lines, mouse SNL cells were grown in the presence of ethidium bromide (5 µg/ml) for 4 weeks in medium supplemented with 50 µg/ml uridine and 0.1 mg/ml pyruvate (King and Attardi, 1996). Clonal ρ0 SNL cell lines were obtained by limiting dilution of these ethidium bromide-treated cells into 96-well plates in the presence of ethidium bromide. The cloned cells were then cultured in normal medium supplemented with 50 µg/ml uridine and 0.1 mg/ml pyruvate. The ρ0 state of the cloned cells was verified by a PCR assay using L-strand (5′-ACC CAA CGC GGC AAA CTA ACC-3′) and H-strand (5′-TCT TGT TCG TCT GCC AGG CT-3′) primers and by inability to grow in medium lacking uridine and pyruvate (King and Attardi, 1996).
2.6. Cybrid fusions
To transfer mitochondrial DNA to ρ0 cells, mitochondrial donor cells were chemically enucleated by treating with actinomycin D and fused with the SNLρ0 cells using PEG as described (Bayona-Bafaluy et al., 2003; Bayona-Bafaluy et al., 2005). Briefly, 3 × 105 cells were plated in 35 mm dishes, treated with actimomycin D for 15 h (0.5 µg/ml), and fused with SNL ρ0 cells with PEG 1500 (60 s). HeLa229 and LL/2 cells that were transfected with pcDNA6-musT-FAML-GFP were used as mitochondrial donor cells. Fused cells were grown 2 days in complete medium and then trypsinized and plated at low density on 10 mm dishes with selective medium (DMEM with 10% FBS, without uridine and pyruvate) (King and Attardi, 1996) containing 400 µg/ml of G418, in which the cells are required to have active mitochondrial function for growth and only G418-resistant SNL cells can be selected. Individual clones were isolated by the cloning ring method. Ten individual clones from LL/2+SNLρ0 fusion were further characterized to verify that they were transmitochondrial cybrids. The mouse mtDNA was confirmed by the mouse mtDNA-specific PCR using L-strand and H-strand primers.
3. Results
3.1. Human and mouse mitochondria fuse and their soluble matrix proteins rapidly mix
To investigate human and mouse cross-species mitochondrial fusion with an assay based on matrix content mixing, we generated stably transfected human HeLa229 cells expressing green fluorescent proteins and mouse STO cells expressing red fluorescent proteins, both targeted to their mitochondrial matrix (mtGFP and mtDsRed). The mitochondrial leader sequence from the mouse Tfam gene (Larsson et al., 1996), which was fused in frame with the GFP or DsRed coding sequences, selectively delivers the fluorescent proteins into the mitochondrial matrix of both cell types. The human and mouse cells with differently labeled mitochondria were fused with PEG following treatment with cycloheximide to inhibit protein synthesis. Cytoplasmic fusion and formation of polykaryons were easily observed by phase contrast microscopy after PEG-mediated fusion.
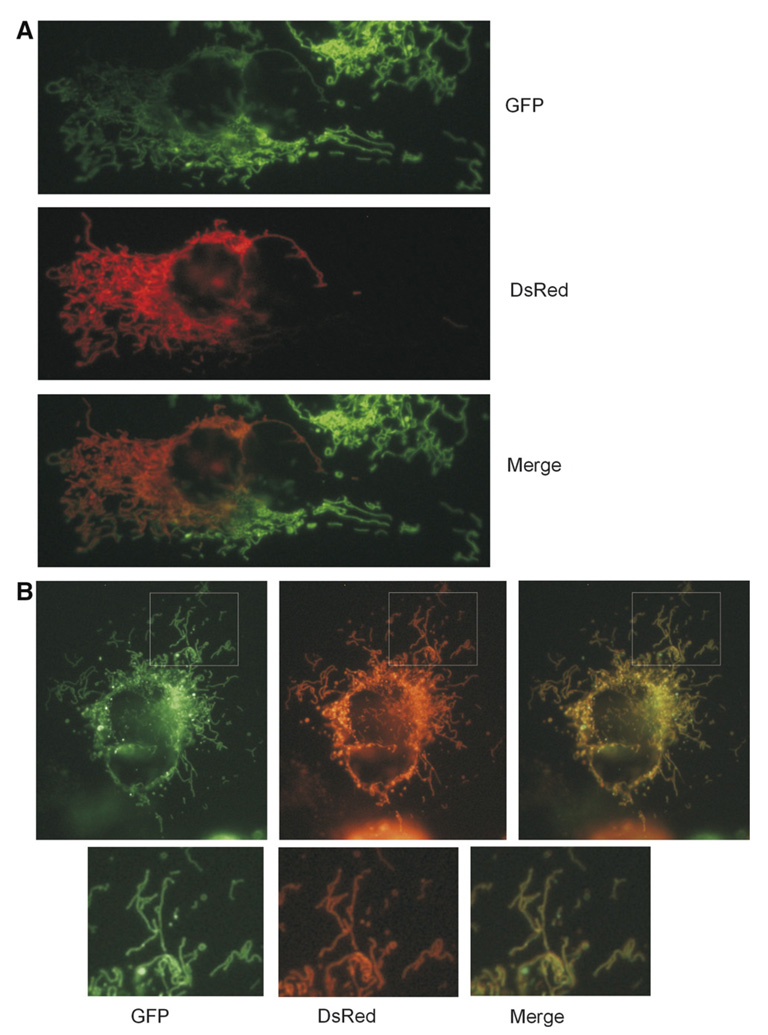
We observed the cells by microscope at various time points after fusion. In our first analyses at about 45 min after PEG treatment, we found that most mouse:human hybrids contained mitochondrial networks in which both of the fluorescent labels (mtGFP+mtDsRed) were present in a portion of network and only one marker was present in the rest of the network (Fig. 1A). In general, the double-labeled mitochondria were distributed in the hybrids near the point of cell:cell fusion and the single-labeled mitochondria were present in the peripheral regions of the polykaryon (Fig. 1A). The complete mixing of both fluorescent markers, in which the mtGFP and mtDsRed proteins are homogeneously distributed throughout the polykaryon mitochondria, was clearly observed in some hybrids analyzed even 45 min after fusion and was found in essentially all hybrids 4 h after cell fusion (Fig. 1B). We generated and microscopically observed several hundred mouse:human hybrids in these experiments and found even distribution of both fluorescent labels throughout the mitochondrial networks of all of the hybrids that we analyzed in detail at the 4 h time point.
Fig. 1.
Fusion between mouse and human mitochondrial networks. Differently labeled mouse STO cells (mtDsRed) and human HeLa229 cells (mtGFP) were coplated and fused with PEG in the presence of cycloheximide to stop protein synthesis. The presence of double-labeled mitochondria (yellow) demonstrates the exchange of fluorescent matrix proteins by mitochondrial fusion. (A) A partially mixed mitochondrial network in a mouse:human hybrid, photographed 45 min after PEG cell fusion. The distribution of doubly and singly labeled mitochondria indicates that the contents of both mitochondrial networks are diffusing and mixing throughout all of the mitochondrial network of the mouse:human hybrid. (B) Homogenous distribution of labeled proteins through a mitochondrial network, photographed 4 h after PEG cell fusion. Homogeneous labeling of mitochondria with mtDsRed and mtGFP indicates that mitochondrial fusion and diffusion have reached equilibrium within 4 h of cytoplasmic fusion. This homogenous labeling was typical of the several hundred cross-species hybrids observed microscopically at this time point. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Because the mitochondrial leader sequence of mouse Tfam is cleaved during import into mitochondria, these fluorescent proteins cannot be exchanged via the cytosol through successive export and import reactions. These experiments therefore demonstrate that the outer and the inner membranes of human and mouse cells do fuse with each other and allow the exchange of fluorescent matrix proteins between mitochondria from each species, and that these cross-species fusion events are relatively rapid and efficient.
3.2. Transmitochondrial cybrids can be obtained only from mouse–mouse fusion and not from mouse–human fusion
Because the human and mouse mitochondria were completely fused to each other, we tested whether the human mitochondrial DNA that would be transferred via this fusion could replace mouse mitochondrial function. We performed cybrid repopulation experiments with respiration-deficient mouse cells devoid of mtDNA (ρ0 cells) by fusing them either with human cells or, as a control, with mouse wild-type cells. Approximately 3 × 105 human HeLa229 and mouse LL/2 cells were treated with 0.5 µg/ml of actinomycin D for 15 h to chemically inactivate the nuclei of these cells. We calibrated the chemical enucleation process (Bayona-Bafaluy et al., 2003) by treating the human and mouse cells with different concentrations of actinomycin D for 4 and 15 h and by observing the cell viability (Table 1). As reported previously, higher concentrations (1–4 µg/ml) for a short time (4 h) were not able to kill all the cells whereas lower concentration (0.5 µg/ml) for 15 h was best for causing irreversible cell death (Table 1), which was in good agreement with published results (Bayona-Bafaluy et al., 2003; Bayona-Bafaluy et al., 2005). The chemically enucleated cells were fused to 1 × 106 SNLρ0 mouse cells and the fused cells were then recovered in complete medium for 2 days and plated at low density in G418 (400 µg/ml)-containing selective medium without uridine and pyruvate (King and Attardi, 1996).
Table 1.
Chemical enucleation with actinomycin D for the generation of transmitochondrial cybrids
| Actinomycin D (µg/ml) | Time (h) | Viability |
|
|---|---|---|---|
| HeLa229 | LL/2 | ||
| 0.5 | 4 | +++ | +++ |
| 1.0 | 4 | ++ | ++ |
| 1.5 | 4 | + | + |
| 4.0 | 4 | +/− | +/− |
| 0.5 | 15 | − | − |
Viability of cells was evaluated by the relative number of cells that recovered from the actinomycin D treatment: +++, 10–30% death; ++, 30–60% death; +, 60–80% death; +/−, 80–95% death; −, 100% death.
In order to confirm that the human mitochondria fused to the mouse ρ0 mitochondrial network, mouse SNLρ0 cells were transiently transfected with pmusTFAML-DsRed to label the ρ0 mitochondria. HeLa229 cells, in which the mitochondria were stably labeled with GFP, were then overlayed onto the transiently transfected SNLρ0 cells. Cycloheximide was applied to inhibit protein synthesis and PEG was added for 60 s to fuse both human and mouse ρ0 cells. Fig. 2 shows that the mitochondrial networks of fused cells were labeled both green and red within 4 h after cell fusion. This result indicates that the ρ0 mouse mitochondria can fuse to the mitochondria of human cells as well as ρ+ mitochondria. We therefore attempted to select the cells that have been potentially repopulated from these human–mouse fusions for mitochondrial activity. We cultured the fused cells for 3 weeks on the G418-containing medium without uridine and pyruvate, since SNLρ0 cells are G418-resistant and repopulated SNL cells would have active mitochondrial function. As expected, however, we did not obtain any repopulated colonies from this human and mouse ρ0 fusion.
Fig. 2.
Human mitochondria fuse to mouse mtDNA-less (ρ0) mitochondria but cannot restore mitochondrial functions. Differently labeled mouse SNLρ0 (mtDsRed) and human HeLa229 (mtGFP) cells were fused with PEG in the presence of cycloheximide. Homogeneous labeling of the mitochondrial networks in the xenocybrids with mtDsRed and mtGFP after 4 h indicates that fusion and diffusion of the fluorescent matrix proteins between ρ0 mouse and human mitochondria is efficient.
For the control experiment, we fused mouse SNLρ0 with actinomycin D-treated mouse LL/2 cells. We used LL/2 cells stably transfected with pcDNA6-musTFAML-GFP to visually confirm mitochondrial fusion. After 3 weeks of selection (above), we were able to obtain G418 resistant cells with mitochondrial activity from this mouse–mouse fusion. Ten of these clones were isolated by the cloning ring method and tested for blasticidin resistance and GFP expression to confirm that these clones were not derived from the mitochondrial donor cells (LL/2-pcDNA6-musT-FAML-GFP) and analyzed the mtDNA of each clone by mouse mtDNA-specific PCR. The SNLρ0 cell line used in these experiments is completely devoid of all mtDNA as measured by PCR analyses and does not generate an mtDNA product in this PCR assay. All of 10 individual cybrid clones tested did however generate strong mtDNA PCR products in this assay, indicating that these cybrids contained mtDNA derived from the LL/2 donor cells.
4. Discussion
We report that mouse mitochondria are able to efficiently fuse to the mitochondrial network in human cells (Fig. 1). The mitochondrial matrix in the human cells was labeled with GFP (green) and the mitochondrial matrix in the mouse cells was labeled with DsRed (red) and synthesis of these marker proteins was stopped prior to fusion with cycloheximide. As shown in Fig. 1A, the mitochondrial networks from these two species fused with one another and began to transfer the matrix marker proteins between the networks shortly after cell fusion. Fused cells examined approximately 4 h after PEG-mediated cell fusion contained both red and green fluorescent proteins distributed evenly throughout both networks (Fig. 1B). This analysis clearly shows that mitochondrial fusion does occur between mouse and human cells and that mixing of the matrix protein content is completed in a very short time (~4 h). This result indicates that mitochondrial fusion is a relatively promiscuous cellular process that does not require strictly identical mitochondrial fusion partners.
Fusion of mitochondria requires the sequential interaction of the outer and inner mitochondrial membranes (Pfanner et al., 2004; Malka et al., 2005). Formation of the Fzo1 or mitofusin homodimers between two adjacent mitochondria promotes their initial tethering (Koshiba et al., 2004) and subsequent outer- and inner-membrane fusion, which require GTP hydrolysis, proceed via other fusion proteins and regulatory factors (Meeusen et al., 2004). When we compared the amino acid sequence of each of the equivalent mitochondrial fusion proteins in mouse and human cells, we found that these fusion proteins have a high degree of homology to one another. Mfn1 and Mfn2 of mouse and human cells have 90.7% and 94.8% homology, respectively, and the Opa1 proteins are 97.5% homologous. Given this relatively high degree of protein homology, we are not surprised to find that these proteins appear to be highly functionally conserved as well.
We have confirmed that ρ0 mouse mitochondria are able to fuse to the mitochondria of human cells by labeling these mitochondria with two different colors (Fig. 2). However, the fused cells generated in this experiment did not survive on selective medium in which the cells are required to have active mitochondrial function for growth (King and Attardi, 1996). When mitochondria fuse, the contents of the mitochondrial segments, including the protein/mtDNA complexes known as nucleoids (Garrido et al., 2003), mix and become distributed throughout all of the mitochondrial segments fused to the network. During the mitochondrial fusion and subsequent homogenization between mouse and human mitochondrial networks, the human mtDNA would have been introduced into the mouse mitochondrial networks. Although we can now clearly see that this fusion between mouse and human mitochondria is an efficient process (Fig. 1 and Fig. 2), mouse ρ0 cells cannot be repopulated with human mtDNA donated through this process (Fig. 2). This experiment demonstrates that mitochondrial fusion is not a barrier to introducing human mtDNA into mouse cells and indicates rather that incompatibilities between the human mtDNA sequence and the nuclear-encoded mouse mitochondrial proteins are responsible for the absence of mitochondrial activity in these xenocybrid cells.
Acknowledgments
We would like to thank generous financial support from the Minnesota Medical Foundation, the Academic Health Center, and the Institute of Human Genetics of the University of Minnesota. This work was funded by National Institutes of Health (NINDS Grant No. NS052612).
References
- Bayona-Bafaluy MP, Manfredi G, Moraes CT. A chemical enucleation method for the transfer of mitochondrial DNA to ρ0 cells. Nucleic Acids Res. 2003;31:e98. doi: 10.1093/nar/gng100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayona-Bafaluy MP, Muller S, Moraes CT. Fast adaptive coevolution of nuclear and mitochondrial subunits of ATP synthetase in orangutan. Mol. Biol. Evol. 2005;22:716–724. doi: 10.1093/molbev/msi059. [DOI] [PubMed] [Google Scholar]
- Bertram JS, Janik P. Establishment of a cloned line of Lewis Lung Carcinoma cells adapted to cell culture. Cancer Lett. 1980;11:63–73. doi: 10.1016/0304-3835(80)90130-5. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum. Mol. Genet. 2005;14(Spec No 2):R283–R289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc. Natl. Acad. Sci. USA. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Francesco L, Attardi G, Croce CM. Uniparental propagation of mitochondrial DNA in mouse–human cell hybrids. Proc. Natl. Acad. Sci. USA. 1980;77:4079–4083. doi: 10.1073/pnas.77.7.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey R, Barrientos A, Moraes CT. Functional constraints of nuclear-mitochondrial DNA interactions in xenomitochondrial rodent cell lines. J. Biol. Chem. 2000;275:31520–31527. doi: 10.1074/jbc.M004053200. [DOI] [PubMed] [Google Scholar]
- Garrido N, Griparic L, Jokitalo E, Wartiovaara J, van der Bliek AM, Spelbrink JN. Composition and dynamics of human mitochondrial nucleoids. Mol. Biol. Cell. 2003;14:1583–1596. doi: 10.1091/mbc.E02-07-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- Kenyon L, Moraes CT. Expanding the functional human mitochondrial DNA database by the establishment of primate xenomitochondrial cybrids. Proc. Natl. Acad. Sci. USA. 1997;94:9131–9135. doi: 10.1073/pnas.94.17.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MP, Attardi G. Isolation of human cell lines lacking mitochondrial DNA. Methods Enzymol. 1996;264:304–313. doi: 10.1016/s0076-6879(96)64029-4. [DOI] [PubMed] [Google Scholar]
- Kingston RE, Chen CA, Okayama H. Calcium phosphate transfection. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Short Protocols in Molecular Biology. New York: Wiley; 1987. pp. 9.5–9.8. [Google Scholar]
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- Larsson NG, Garman JD, Oldfors A, Barsh GS, Clayton DA. A single mouse gene encodes the mitochondrial transcription factor A and a testis-specific nuclear HMG-box protein. Nat. Genet. 1996;13:296–302. doi: 10.1038/ng0796-296. [DOI] [PubMed] [Google Scholar]
- Legros F, Lombes A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol. Biol. Cell. 2002;13:4343–4354. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malka F, Guillery O, Cifuentes-Diaz C, Guillou E, Belenguer P, Lombes A, Rojo M. Separate fusion of outer and inner mitochondrial membranes. EMBO Rep. 2005;6:853–859. doi: 10.1038/sj.embor.7400488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie M, Trounce I. Expression of Rattus norvegicus mtDNA in Mus musculus cells results in multiple respiratory chain defects. J. Biol. Chem. 2000;275:31514–31519. doi: 10.1074/jbc.M004070200. [DOI] [PubMed] [Google Scholar]
- McKenzie M, Chiotis M, Pinkert CA, Trounce IA. Functional respiratory chain analyses in murid xenomitochondrial cybrids expose coevolutionary constraints of cytochrome b and nuclear subunits of complex III. Mol. Biol. Evol. 2003;20:1117–1124. doi: 10.1093/molbev/msg132. [DOI] [PubMed] [Google Scholar]
- Meeusen S, McCaffery JM, Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Science. 2004;305:1747–1752. doi: 10.1126/science.1100612. [DOI] [PubMed] [Google Scholar]
- Mozdy AD, Shaw JM. A fuzzy mitochondrial fusion apparatus comes into focus. Nat. Rev. Mol. Cell Biol. 2003;4:468–478. doi: 10.1038/nrm1125. [DOI] [PubMed] [Google Scholar]
- Nunnari J, Marshall WF, Straight A, Murray A, Sedat JW, Walter P. Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol. Biol. Cell. 1997;8:1233–1242. doi: 10.1091/mbc.8.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N, Wiedemann N, Meisinger C. Cell biology. Double membrane fusion. Science. 2004;305:1723–1724. doi: 10.1126/science.1104244. [DOI] [PubMed] [Google Scholar]
- Pinkert CA, Trounce IA. Production of transmitochondrial mice. Methods. 2000;26:348–357. doi: 10.1016/S1046-2023(02)00041-5. [DOI] [PubMed] [Google Scholar]
- Rojo M, Legros F, Chateau D, Lombes A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J. Cell Sci. 2002;115:1663–1674. doi: 10.1242/jcs.115.8.1663. [DOI] [PubMed] [Google Scholar]
- Sesaki H, Jensen RE. Ugo1p links the Fzo1p and Mgm1p GTPases for mitochondrial fusion. J. Biol. Chem. 2004;279:28298–28303. doi: 10.1074/jbc.M401363200. [DOI] [PubMed] [Google Scholar]
- Sesaki H, Southard SM, Yaffe MP, Jensen RE. Mgm1p, a dynamin-related GTPase, is essential for fusion of the mitochondrial outer membrane. Mol. Biol. Cell. 2003;14:2342–2356. doi: 10.1091/mbc.E02-12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge EA. A debut for mito-mouse. Nat. Genet. 2000;26:132–134. doi: 10.1038/79832. [DOI] [PubMed] [Google Scholar]
- Sokolova VA, Kustova ME, Arbuzova NI, Sorokin AV, Moskaliova OS, Bass MG, Vasilyev VB. Obtaining mice that carry human mitochondrial DNA transmitted to the progeny. Mol. Reprod. Dev. 2004;68:299–307. doi: 10.1002/mrd.20075. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Pollack Y, Bunn CL, Eisenstadt JM. Cytoplasmic inheritance in mammalian tissue culture cells. In Vitro. 1976;12:758–776. doi: 10.1007/BF02835451. [DOI] [PubMed] [Google Scholar]
- Yamaoka M, Isobe K, Shitara H, Yonekawa H, Miyabayashi S, Hayashi JI. Complete repopulation of mouse mitochondrial DNA-less cells with rat mitochondrial DNA restores mitochondrial translation but not mitochondrial respiratory function. Genetics. 2000;155:301–307. doi: 10.1093/genetics/155.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YG, Koob MD. Transformation of isolated mammalian mitochondria by bacterial conjugation. Nucleic Acids Res. 2005;33:e139. doi: 10.1093/nar/gni140. [DOI] [PMC free article] [PubMed] [Google Scholar]