Abstract
Functional nanofibrous scaffolds produced by electrospinning have great potential in many biomedical applications, such as tissue engineering, wound dressing, enzyme immobilization and drug (gene) delivery. For a specific successful application, the chemical, physical and biological properties of electrospun scaffolds should be adjusted to match the environment by using a combination of multi-component compositions and fabrication techniques where electrospinning has often become a pivotal tool. The property of the nanofibrous scaffold can be further improved with innovative development in electrospinning processes, such as two-component electrospinning and in-situ mixing electrospinning. Post modifications of electrospun membranes also provide effective means to render the electrospun scaffolds with controlled anisotropy and porosity. In this review, we review the materials, techniques and post modification methods to functionalize electrospun nanofibrous scaffolds suitable for biomedical applications.
Keywords: Electrospinning, nanofiber, scaffold, biomedical applications, copolymers, mixtures, modifications
1. Introduction
Electrospinning is a unique technology that can produce non-woven fibrous articles with fiber diameters ranging from tens of nanometers to microns, a size range that is otherwise difficult to access by conventional non-woven fiber fabrication techniques [1,2]. Electrospun nanofibrous scaffolds possess an extremely high surface-to-volume ratio, tunable porosity, and malleability to conform over a wide variety of sizes and shapes. In addition, the scaffold composition can be controlled to achieve desired properties and functionality. Due to these advantages, electrospun nanofibrous scaffolds have been widely investigated in the past several years with materials of different compositions [3–10] for applications of varying end-uses, such as filtration [11–13], optical and chemical sensors [14–19], electrode materials [20–23], and biological scaffolds [24–27].
For small-scale productions (i.e., on a laboratory scale), electrospinning is a simple method to generate nanoscale fibers. A basic electrospinning system usually consists of three major components: a high voltage power supply, a spinneret (e.g. a pipette tip) and a grounded collecting plate (usually a metal screen, plate, or rotating mandrel). When a charged polymer solution is fed through the spinneret under an external electric field, a suspended conical droplet is formed, whereby the surface tension of the droplet is in equilibrium with the electric field. When the applied electric field is strong enough to overcome the surface tension, a tiny jet is ejected from the surface of the droplet and drawn toward the collecting plate. During the jet propagation toward the collecting plate, the solvent in the jet stream gradually evaporates. The resulting product is a non-woven fibrous scaffold with a large surface area-to-volume ratio and a small pore size (in microns). The fiber thickness and morphology can be controlled by many parameters, such as solution properties (viscosity, elasticity, conductivity and surface tension), electric field strength, distance between the spinneret and the collecting plate, temperature and humidity. These parameters have been well studied and summarized in a recent review [28]. With very small fiber diameters, the yield per spinneret of the electrospinning process is extremely low. Recently, multi-jet electrospinning [29,30] and blowing-assisted electrospinning technology [30–32] have been developed, demonstrating the production capability for fabricating nanofibrous articles on an industrially relevant scale.
The usage of electrospun nanofibrous scaffolds for biomedical applications has attracted a great deal of attention in the past several years. For examples, nanofibrous scaffolds have been demonstrated as suitable substrates for tissue engineering [24–27], immobilized enzymes and catalyst [33–36], wound dressing [37,38] and artificial blood vessels [39,40]. They have also been used as barriers for the prevention of post-operative induced adhesion [41,42] and vehicles for controlled drug (gene) delivery [43–47]. For a successful application to a specific target, the nanofibrous scaffold must exhibit suitable physical and biological properties closely matching the desired requirements. For example, in tissue engineering, the electrospun scaffold should physically resemble the nanofibrous features of extracellular matrix (ECM) with suitable mechanical properties. It should also be able to promote cell adhesion, spreading and proliferation. For wound dressing, the nanofibrous scaffold should not only serve as a substrate for tissue regeneration, but also may deliver suitable bioactive agents, including drugs (e.g. antibiotic agent), within a controlled manner during healing. The fabrication of such functional nanofibrous scaffolds for biomedical applications often requires an interdisciplinary approach combining physics, chemistry, biology and engineering.
For electrospun nanofibrous scaffolds in biomedical applications, its physical and biological properties, such as hydrophilicity, mechanical modulus and strength, biodegradability, biocompatibility, and specific cell interactions, are largely determined by the materials’ chemical compositions. Based on polymer physics, copolymerization and polymer blending are two effective means to combine different polymers to yield new materials properties. Thus, by selecting a combination of proper components and by adjusting the component ratio, properties of electrospun scaffolds can be tailored with desired new functions. For example, many kinds of copolymers and polymer mixtures, such as poly(lactide-co-glycolide) [41], poly(ethylene-co-vinyl alcohol) [48], mixtures of collagen with elastin [49], and mixtures of chitosan with poly(ethylene oxide) (PEO or PEG when the molecular weight is small, say less than 5000 Da) [50], have been electrospun to fabricate nanofibrous scaffolds for biomedical applications, but with varying degrees of success.
Besides taking advantage of the materials compositions, the fabrication process, through which the fiber diameter, morphology and scaffold porosity can be manipulated, also plays an important role on the scaffold property and functionality. For example, the two-phase electrospinning process provides a new pathway to incorporate drugs or biopolymers inside the fiber core that will be suitable for the controlled release over a prolonged period of time [51]. Physical and chemical modifications of the scaffolds after electrospinning are also able to render the scaffolds with enhanced properties and suitable functionality for specific applications. For example, the grafting of gelatin onto the surface of a polyethylene terephthalate (PET) scaffold after electrospinning could increase the biocompatibility and make the scaffold more suitable for cell adhesion and proliferation [40].
This review is concerned with the recent progress on the use of electrospun scaffolds for biomedical applications, with emphasis on materials, technology, and post treatment of the scaffolds: (1) rational polymer material design, including copolymers and polymer mixtures, (2) new innovative electrospinning techniques, and (3) post-electrospinning modifications. In practice, the three considerations can be combined together to generate new functional nanofibrous scaffolds with enhanced physical and biological properties.
2. Rational design of polymeric materials
2.1 Homopolymers
2.1.1 Natural polymers
Compared with synthetic polymers, naturally occurring polymers normally exhibit better biocompatibility and low immunogenicity, when used in biomedical applications. All four major classes of biopolymers: proteins, polysaccharides, DNAs and lipids, have been fabricated into electrospun scaffolds. Protein fibers, mainly from collagen, gelatin, elastin and silk fibroin, have been well studied in recent years [52–55]. For example, collagen is the principal structural element of the extracellular matrix (ECM) in tissues, where three types of collagen, types I, II and III, have been fabricated into nanofibrous scaffolds for studies of cell growth and penetration [56–58]. Wnek et al. have electrospun human or bovine fibrinogen fraction I, dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFP) with minimal essential medium (Earle’s salts), and used the resulting scaffolds for tissue-engineering applications [59]. Min et al. have prepared silk fibroin (SF) electrospun scaffolds with fiber diameters of around 80 nm [60]. They found that normal human keratinocytes and fibroblasts seeded on the SF nanofibers were able to attach and grow, indicating that the SF nanofibers may be a good candidate for wound dressing and tissue engineering [60,61]. The treatment of the scaffold by water vapor induced a conformational transition of SF from random coil to beta-sheet structures, thereby the mechanical strength and the cellular compatibility were improved [62,63]. In addition, Huang et al. have electrospun gelatin into nanofibers with diameters ranging from 100 to 340 nm using 2,2,2-trifluoroethanol as the solvent [64].
Recently, our group has demonstrated the successful electrospinning of hyaluronic acid (HA) in aqueous solutions [65]. HA is essentially an associating polymer in aqueous solution, often exhibiting very high solution viscosity. Consequently, typical electrospinning processes could not be used successfully to develop a steady jet stream. The sample has to be spun with the assistance of air flow at elevated temperatures, thereby broadening the processing window. This process is termed “blowing-assisted” electrospinning, which has been described elsewhere [32,65] and will not be elaborated on in this review. The electrospun HA nanofibrous scaffolds with a suitable degree of post-crosslinking will be suitable for cartilage repair, since hyaluronan is an abundant polysaccharide found almost exclusively in articular joints, allowing the cells to attach for cartilage regeneration. Other polysaccharides, such as dextran [66], chitosan (chitin) [67–70] and cellulose acetate [71–75], have also been fabricated to form nanofibers by electrospinning. Besides proteins and polysaccharides, calf thymus Na-DNA in an aqueous solution was electrospun to form nanofibers with diameters of around 50–80 nm [76]. However, no specific biomedical applications of such DNA nanofibers have been reported. Recently, McKee M. et al. reported the non-woven membranes from electrospinning lecithin solutions in a single processing step [77]. At concentrations above the critical concentration for entanglement, Ce, electrospun fibers with diameters ranging from 1 to 5 micrometers were fabricated (Figure 1). Such scaffolds offered many potential applications, such as tissue growth and engineering vehicles, as well as drug-delivery platforms.
Figure 1.

Field-emission scanning electron microscopic images of lecithin fibers prepared at different solution concentrations (from below to above the critical concentration for entanglement). (From Ref. [77] with permission)
2.1.2 Synthetic polymers
Synthetic polymers often offer many advantages over natural polymers in that they can be tailored to give a wider range of properties and predictable lot-to-lot uniformity. Moreover, synthetic polymers are cheaper and represent a more reliable source of raw materials. Typical synthetic polymers used in biomedical applications are hydrophobic biodegradable polyesters, such as polyglycolide (PGA)[76, 78, 79], polylactide (PLA)[10, 80–83] and poly(ε-caprolactone) (PCL) [84–87], which have all been electrospun into nanofibrous scaffolds. Table 1 lists the physical properties of some popular biodegradable polyesters and their copolymers [88]. Other hydrophilic biodegradable polymers, such as polyurethane [89, 90], poly(vinyl alcohol) [91, 92], PEO [93], polydioxanone [94] and polyphosphazene derivatives [95, 96] have also been electrospun into nanofibrous scaffolds for biomedical applications.
Table 1.
Biodegradable polymers for biomedical applications
| Polymer name | Melting point (°C) | Glass transition temperature(°C) | Modulus (Gpa)a | Degradation time (Month)b |
|---|---|---|---|---|
| PGA | 225–230 | 35–40 | 7.0 | 6 to 12 |
| L-PLA | 173–178 | 60–65 | 2.7 | >24 |
| D, L-PLA | Amorphous | 55–60 | 1.9 | 12 to 16 |
| PCL | 58–63 | (−65)–(−60) | 0.4 | > 24 |
| PDO | N/A | (−10)–0 | 1.5 | 6 to 12 |
| 85/15 PLGA | Amorphous | 50–55 | 2.4 | 5 to 6 |
| 75/25 PLGA | Amorphous | 50–55 | 2.0 | 4 to 5 |
| 65/35 PLGA | Amorphous | 45–50 | 2.0 | 3 to 4 |
| 50/50 PLGA | Amorphous | 45–50 | 2.0 | 1 to 2 |
tensile or flexural modulus
time to complete mass loss. Rate also depends on geometry.
PGA: poly(glycolide); PLA: poly(lactide); PCL: poly(ε-caprolactone); PDO: poly(dioxanone); PLGA: copolymer of PGA and DL-PLA, ratio is PLA/PGA.
2.2 Synthetic copolymers
The use of copolymers is a viable scheme to generate new materials of desirable properties. When properly implemented, the performance of electrospun scaffolds based on copolymers can be significantly improved when compared to that of homopolymers. For example, biodegradable hydrophobic polyesters generally have good mechanical properties but lack cell affinity for tissue engineering. The incorporation of a proper hydrophilic polymer segment can increase the cell affinity. Besides the cell affinity, the mechanical properties, morphology, structure, pore size and distribution, biodegradability and other physical properties can also be tailored by using copolymers. Moreover, with amphiphilic copolymers as protecting molecules to encapsulate drug molecules, electrospun scaffolds can be used for drug release in a controlled manner.
PLGA, the random copolymer of glycolide (G) and lactide (L), is a popular and well-studied system that has been broadly used as electrospun scaffolds for biomedical applications. As listed in Table 1, the mechanical properties and the degradation rate of PLGA, being dependent on the L/G ratio, are quite different from PGA and PLA homopolymers. The in vitro degradation rate of electrospun PLGA scaffold at different L/G composition has been investigated by our group. The nanofibrous PLGA scaffolds generally degrade faster than the regular casting film with the same dimensions and composition, mainly due to the high surface area-to-volume ratio and the high water adsorption ability (both decrease the induction time during hydrolysis) [41]. Recently, Laurencin et al. have studied the potential use of PLGA nanofibrous scaffolds as an antibiotic delivery vehicle for the treatment of wounds [44]. They demonstrated that PLGA nanofibers could be tailored to desired diameters through modifications in processing parameters, such as orifice diameter (needle gauge), polymer solution concentration and voltage per unit length, where the antibiotic drugs, such as cefazolin, could be incorporated into the nanofibers.
The lactide component can also be copolymerized with ε-caprolactone. The degradation rate of the copolymer, P(LA-CL), is between those of the two homopolymers (PLA and PCL), which are significantly longer than that of PGA. Its degradation rate can also be controlled by the composition ratio. The potential use of electrospun P(LA-CL) scaffolds in tissue engineering has been investigated by several groups [97–100]. For example, Kwon et al. [97] electrospun P(LA-CL) scaffolds using different L/CL molar ratios (70/30, 50/50, 30/70) and systematically investigated the scaffold structure, mechanical properties and cell adhesion ability. They found that the human umbilical vein endothelial cells (HUVECs) could adhere and proliferate on the P(LA-CL) nanofibers with the average diameter ranging from 300 nm to 1.2 μm. Mo et al. [99] studied the interactions of smooth muscle cells and endothelial cells with P(LA-CL) nanofibrous scaffolds. They found that both cell adhesion and proliferation took place after 7 days on the electrospun P(LA-CL) scaffold with a LA/CL ratio of 75/25. Their results indicated that P(LA-CL) nanofibrous scaffolds have excellent biocompatibility and they are potentially very useful in tissue engineering applications.
Electrospun scaffolds based on DegraPol®, a degradable block polyester-urethane, containing crystalline blocks of poly((R)-3-hydroxybutyric acid)-diol and blocks of poly(ε-caprolactone-co-glycolide)-diol linked with a diisocyanate, was studied as a potential scaffold for skeletal muscle tissue engineering [26]. As a block copolymer, DegraPol® combined the characteristics of traditional polyesters with good processibility and distinct elasticity of polyurethanes; it also exhibited good affinity with tissue cells. Electrospun DegraPol® nanofibrous scaffolds showed satisfactory mechanical properties and promising cellular response in preliminary cell adhesion and differentiation experiments. It has now been considered as one of the most promising scaffolds for skeletal muscle tissue engineering [26].
Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) is a biodegradable and biocompatible copolymer derived from microbial polyesters; it has also been fabricated into nanofibrous scaffolds recently. By controlling the electrospinning parameters, nanofibers with an average diameter of around 185 nm were fabricated. Compared with the PHBV cast films, electrospun PHBV nanofibrous scaffolds provided a much more suitable environment for the attachment and growth of chondrocytes derived from rabbit ears [101]. Choi et al. found that the fiber diameter of PHBV was decreased by addition of a small amount of benzyl trialkylammonium chlorides in the solution before electrospinning, and the degradation rate of PHBV fiber was also accelerated, probably due to a significant increase in the surface area of PHBV nanofibers [102].
Bhattarai et al. [103, 104] developed a novel block copolymer based on poly(p-dioxanone-co-L-lactide)-block-poly(ethylene glycol) (PPDO/PLLA-b-PEG) that could be electrospun into scaffolds for applications of tissue engineering and drug-release. The random disposition of the PPDO and PLLA segments, as well as the incorporation of PEG oligomers, significantly improved the biodegradability and hydrophilicity of the electrospun scaffolds. For example, NIH 3T3 fibroblast cells were found to grow and proliferate on the scaffold after 10 days, which showed a six-fold increase in the cell population after incubation when compared with the same environment without the scaffold [104]. Kenawy et al. [48] combined the hydrophilicity of the vinyl-alcohol repeating unit with the hydrophobicity of the ethylene repeating unit in electrospun poly(ethylene-co-vinyl alcohol) nanofibrous scaffolds, also resulting in improved biocompatibility. The hydroxyl group in the vinyl-alcohol repeating unit could offer opportunities for chemical modifications either before or after electrospinning. Without modification, the electrospun poly(ethylene-co-vinyl alcohol) scaffold was found to be readily able to support the culturing of smooth muscle cells and fibroblasts. In addition, the derivatives of poly(ethylene-co-vinyl alcohol), poly(ethylene-co-vinylacetate) [105], as well as poly(L-lactic acid-co-succinic acid-co-1,4-butane diol) [106] have also been fabricated into electrospun nanofibers that appeared to be suitable for varying tissue engineering applications.
2.3 Polymer mixtures
2.3.1 Blends of natural polymers
Polymer mixtures (or blends) have an advantage over copolymers in that they are not limited by suitable synthetic schemes. Therefore, nanofibrous scaffolds formed by mixing different polymers become an appealing option, which is especially true for natural polymers, as their chemical monomers are often more difficult to modify. Blending of natural polymers may provide a straightforward pathway to combine different bioactivities for biomedical applications. For example, Boland et al. [49] demonstrated the electrospinning of micro- and nano-fibrous scaffolds based on collagen and elastin mixtures in order to develop viable vascular tissue engineered constructs. Collagen/elastin scaffolds could replicate the complex architecture of a blood vessel wall and withstand high pressures under the pulsatile environment induced by the bloodstream. Therefore, such scaffolds could have the potential to create a suitable environment for in vitro generation of vascular replacements [49]. The thermal stability of the collagen/elastin scaffolds was able to be improved by crosslinking of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide (NHS) [107].
2.3.2 Blends of natural and synthetic polymers
As regenerated natural polymers usually possess weak mechanical properties, blends of natural and synthetic polymers can overcome this problem and combine two desired characteristics, i.e., the strength and durability of a synthetic polymer, and the specific cell affinity of a natural polymer. Electrospun scaffolds based on blends of natural and synthetic polymers can enhance both physical properties and biological functionality. One example was the electrospinning of casein or lipase suspensions, mixed with synthetic PEO or PVA [108]. The stand-alone suspensions of casein and lipase were not suitable for electrospinning. However, their mixtures with PEO or PVA could significantly facilitate the electro-spinning process. Figure 2 shows the scanning electron microscopic (SEM) images of electrospun scaffolds from PEO/casein mixtures. With the PEO (Mv, 600 KDa) concentration below 5%, a non-fibrous scaffold was obtained (Figure 2e). However, this situation could be significantly improved by increasing the PEO content to 80%, as illustrated in Figure 2b [108]. To maintain casein concentration as high as 80%, an increase in the total mixture concentration to 10% appeared to be capable of producing fine fibers by electrospinning (Figure 2f). Their study also showed that lipase could be electrospun together with PEO or PVA, and the catalytic activity on the hydrolysis of olive oil in lipase/PVA electrospun scaffolds was 6 times higher than that in cast film with similar compositions [108]. Furthermore, the crosslinking of polymer/lipase scaffolds using a dialdehyde could significantly improve the water stability. The pH level of the reaction media during crosslinking was found to play an important role in the activity of the immobilized lipase [34].
Figure 2.
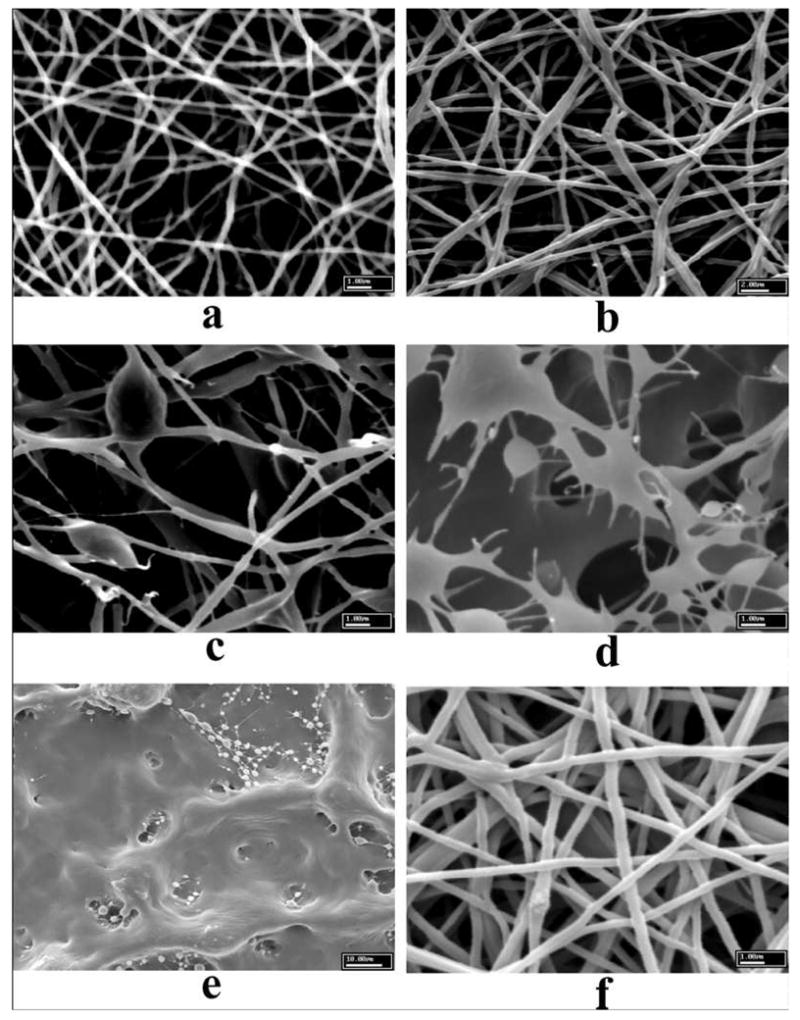
Electrospun scaffolds from 5 wt% total concentration of mixtures with PEO to casein ratio at (a) 100:0, (b) 80:20, (c) 50:50, (d) 20:80, (e) 5:95, and (f) 20:80 at 10% concentration. (From Ref. [108] with permission)
Zhang et al. [109] mixed 10 % w/v gelatin with 10 % w/v PCL in 2,2,2-trifluoroethanol (TFE) at a ratio of 50:50 to produce a gelatin/PCL nanofibrous scaffold by electrospinning. The scaffold showed enhanced mechanical properties and more favorable wetability than those obtained from either PCL or gelatin scaffolds alone. Bone-marrow stromal cells (BMSC) were found to attach and grow well on the surface of the blend nanofibrous scaffold. In addition, BMSCs were found to be able to migrate inside the scaffold up to a depth of 114 microns within 1 week of culture, suggesting the potential use of composite gelatin/PCL fibrous scaffolds for preparation of the three-dimensional tissue construct.
The mixture of heparin and PEG was also electrospun to prepare nanofibrous scaffolds [110]. The presence of PEG in the electrospun scaffolds prolonged the release of heparin, which could closely match the time scale needed for use in wound dressings. The composition of the scaffold is also suitable for drug delivery. It is evident that the mixtures of type I collagen and PEO can provide a convenient, non-toxic and non-denaturing way to generate collagen-containing nanofibrous scaffolds that may have good potential in biomedical applications [111]. A blend of wool keratin and PEO in aqueous solutions was also fabricated into nanofibers in a similar fashion [112].
Electrospinning of synthetic polymers followed by the coating of natural material has also been demonstrated as a practical approach to yield desired functional features. For example, He et al fabricated the collagen-coated poly(L-lactic acid)-co-poly(ε-caprolactone) (P(LLA-CL) 70:30) scaffold with a porosity of 64–67% and a fiber diameter of 470 nm by electrospinning followed by plasma treatment and collagen coating [113]. The coating of collagen was found to improve the biocompatibility of the scaffold, thus enhancing the spreading, viability and attachment of the human coronary artery endothelial cells and preserving the cells’ phenotype [113] in the scaffold. The properties of the electrospun collagen-coated poly(L-lactic acid)-co-poly(ε-caprolactone) scaffold are quite suitable for engineered vascular graft.
2.3.3 Synthetic polymer blends based on PLGA
Blends of synthetic polymers have been routinely used in electrospinning to produce new scaffolding materials. As PLGA has been widely used in biomedical applications from sutures, medical devices to tissue regeneration, its mixtures with other synthetic polymers are reviewed here. By mixing PLGA with another polymer material, the physical properties of PLGA, such as hydrophobicity, degradation rate, shrinkage behavior in body fluids and mechanical modulus, can be altered to specific biomedical applications, such as carriers for drugs or DNA with controlled-release capability.
2.3.3.1 PLGA with dextran
PLGA is a hydrophobic polymer while dextran is a hydrophilic polymer that is highly soluble in an aqueous medium. By mixing PLGA and dextran together at a 1 to 1 ratio, Jiang et al. [66] produced a hydrophobic/hydrophilic electrospun composite scaffold. With a portion of dextran methacrylated in advance, they could also photo-crosslink the dextran phase in the solid state to fabricate water-resistant nanofibrous scaffolds with improved hydrophilicity. The crosslinked PLGA/dextran scaffolds may be used as substrates for tissue engineering. However, no further information on the structure control and cell growth has been reported on this system yet.
2.3.3.2 PLGA with PEG-g-CHN
It is difficult to incorporate hydrophilic drugs into the hydrophobic scaffolds (e.g. PLGA) by electrospinning. Jiang et al. [43] synthesized a graft copolymer, poly(ethylene glycol)-g-chitosan (PEG-g-CHN) that could encapsulate most hydrophilic drugs, such as ibuprofen (an anti-inflammatory agent), and was also compatible with the PLGA matrix. The unique structure of PEG-g-CHN also showed the controlled release capability of hydrophilic drugs from electrospun PLGA scaffolds. In their study [43], mixtures of PEG-g-CHN and PLGA with varying ratios were used to fabricate medicated electrospun scaffolds. It was found that the addition of PEG-g-CHN decreased the glass transition temperature of PLGA, resulting in a decrease in the tensile strength at break and an increase in the tensile strain of the scaffold. The shrinkage behavior of the electrospun composite scaffold at 37°C in the body fluid was also improved when compared with that of the pure PLGA scaffold (e.g. when the content of PEG-g-CHN reached 30 wt%, only a 3% decrease in the area of the composite scaffold was detected, while the shrinkage change of the pure PLGA electrospun scaffold could be more than 50% in some cases [43]). More importantly, the presence of PEG-g-CHN significantly slowed down the initial release rate of ibuprofen from the scaffold and prolonged the release of ibuprofen for over two weeks. Specifically, at 5 wt% loading of ibuprofen to scaffold, the initial amount of drug release reached ~ 45% after day 4, and continued gradually up to ~ 70% over the next two weeks. This data was in contrast with the same weight percentage of ibuprofen in PLGA alone, which rapidly reached ~ 85% after day 4. Because of its desired sustained release rate, Jiang et al. concluded that these polymer scaffolds, being mechanically strong and compliant, could be suitable candidates for the prevention of post-surgery induced atrial fibrillation when applied to the surface of the heart [43].
Co-electrospinning of PLGA/1,1,1,3,3,3-hexafluoro-2-propanol (HFP) solution and chitin/formic acid solution at a weight ratio of 80/20, Min et al. [114] generated a composite nanofibrous scaffold with chitin nanoparticles evenly distributed and strongly adhered to the PLGA nanofibers. Both normal human keratinocytes and fibroblasts were used to test the efficacy of this unique scaffold for tissue engineering. The PLGA/chitin composite scaffolds showed better results than pure PLGA scaffolds on normal human keratinocytes. However, on fibroblasts, PLGA/chitin and PLGA showed similar performance, with no improvement observed on the PLGA/chitin electrospun scaffold [114].
2.3.3.3 PLGA with PEG-PLA copolymers
Amphiphilic block copolymers, containing hydrophobic PLA blocks and hydrophilic poly(ethylene glycol) (PEG) blocks, have shown great promise in the applications of drug delivery. These copolymers (e.g. diblock PEG-PLA, triblock PEG-PLA-PEG or PLA-drug PEG-PLA) are suitable to encapsulate and protect drug or DNA molecules, whereby the encapsulated drug (gene)/polymer aggregates can be incorporated into the nanofibrous PLGA scaffolds by electrospinning. Since PLGA and PEG-PLA are compatible with each other, the addition of even a small amount of PLA-PEG block copolymer can significantly change the hydrophobicity and the degradation rate of electrospun PLGA-based scaffolds [115]. Blending PEG-PLA copolymers is, thus, an effective way to fine-tune the properties of PLGA-based scaffolds for different biomedical applications.
For drug delivery, our group has recently demonstrated that the release of cefoxitin sodium (Mefoxin®), a hydrophilic antibiotic drug, could be modulated by the addition of a diblock PEG-b-PLA copolymer (Mw of PEG and PLA are 5 K and 4.6 K, respectively) in an electrospun PLGA scaffold [45]. Figure 3 shows the effect of PEG-b-PLA on the cefoxitin sodium release profile. Without the block copolymer, about 75% of cefoxitin sodium was released in one day; while with 15 wt% of PEG-b-PLA, only about 60% of cefoxitin sodium was released in one day. The rapid initial burst release was designed to prevent bacteria infection immediately after surgery, but the prolonged secondary delivery profile was also desirable in order to minimize potential bacteria growth. The efficacy of released cefoxitin sodium was checked by an inhibition study using S. aureus bacteria culture. The results showed that the process of electrospinning did not compromise the efficacy of this drug. In other words, the structure and bioactivity of cefoxitin sodium was retained during the processes of drug incorporation and electrospinning [45].
Figure 3.
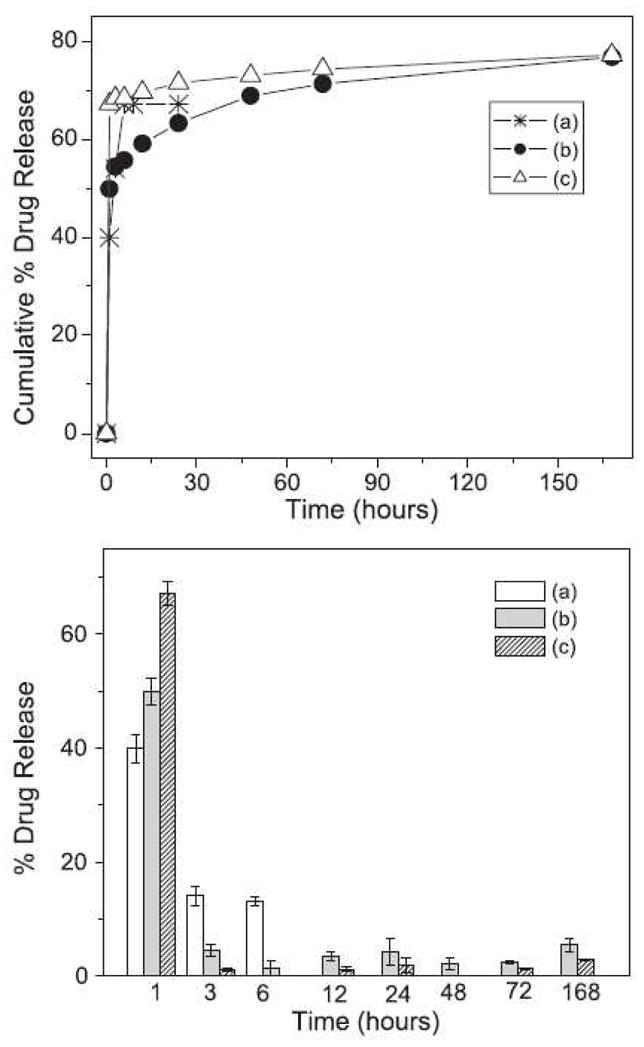
Drug (cefoxitin sodium) release profiles (cumulative curve-top and differential curve-bottom) from medicated electrospun scaffolds. The data represents the mean ± S.D. (n = 5 scaffolds): (a) medicated PLGA with 1 wt% drug, (b) medicated PLGA/PLA/PEG-b-PLA blend with 5 wt% drug, and (c) medicated PLGA with 5 wt% drug. (From Ref. [45] with permission)
Using a triblock PLA-PEG-PLA copolymer (Mw of PEG and PLA are 3.4 K and 0.6 K, respectively), our group also reported that DNA molecules could be incorporated and then released from electrospun scaffolds in a controlled manner [47]. With addition of 10–15 wt% PLA-PEG-PLA, the release of β-galactosidase encoding plasmid DNA from the gene-containing electrospun PLGA scaffold was sustained over 20 days, with the maximum amount of release occurring within about 2 h. The cumulative release profiles indicated that the amount of DNA released was approximately 68–80% of the initial load. Figure 4 shows the transfection activity of the released DNA from the electrospun scaffold. Results indicated that DNA released directly from the PLGA scaffolds was indeed intact, capable of cellular transfection and successfully encoding the protein β-galactosidase [47].
Figure 4.
Bioactivity of released DNA in the transfection of MC3T3 cells. (a) Naked DNA added directly to cell medium, (b) cells transfected with control DNA complex (Fugene 6), (c) DNA containing scaffold incubated with cell for 4 h, then removed, (d) released DNA from scaffold complexed with Fugene 6. Scale bar 100 μm. (From Ref. [47] with permission)
Our group also evaluated the potential use of a composite scaffold containing PLGA, PEG-PLA diblock copolymer and cefoxitin sodium to prevent surgery-induced adhesion [42]. Acting as a physical barrier but with drug delivery capability, this electrospun medicated PLGA-based scaffold was able to completely prevent any adhesion formation after 28 days using an objective rat model. The combined advantages of the composition adjustment, drug-loading capability, and easy placement handling ability in the body (the material is relatively hydrophobic) have made these scaffolds potential candidates for further clinical evaluations [42].
A composite scaffold formed by electrospinning of a multi-component mixture containing PLA, PLGA, triblock copolymer of PLA-b-PEG-b-PLA and lactide was fabricated by our group [115]. The objective of choosing multi-components was to precisely control the physical and biological properties of the scaffold, with each component providing a different function. For example, PLA of high molecular weight provided the overall mechanical strength, PLA-PEG-PLA affected the hydrophilicity, PLGA coarse-tuned and lactide fine-tuned the degradation rate[115]. We found that a scaffold containing 40 wt% high molecular weight PLA, 25 wt% low molecular weight PLGA, 20 wt% PLA-PEG-PLA and 15 wt% lactide showed a suitable degradation profile, good hydrophilicity, and stable mechanical properties in aqueous solution (and body fluids) for the prevention of post-operative adhesion [115].
2.3.3.4 PLGA with other polymers
Many other biocompatible and biodegradable polymers have been mixed together with PLGA based polymers to form nanofibrous scaffolds by electrospinning. For example, mixtures of PLA with poly(vinylpyrrolidone) [116], and of PLA with poly(ethylene-co-vinylacetate) [117] were fabricated into nanofibrous scaffolds by electrospinning for biomedical applications.
2.3.4 Synthetic polymer blends containing PEO/PEG
Poly(ethylene oxide) (PEO) or poly(ethylene glycol) (PEG, when the molecular weight is small, say less than 5000 Da) is a unique polyether diol, which is amphiphilic and can be dissolved in both organic solvents and aqueous solutions, including pure water. PEO/PEG is non-toxic and can be eliminated by renal and hepatic pathways, making it suitable for many biomedical applications. Thus far, PEO/PEG has been used as the electrospun scaffold mainly for two reasons: (1) to improve the fiber property and functions (e.g. hydrophilicity), and (2) to facilitate electrospinning of other more difficult to process biomaterials as a processing aid For example, in first applications, PEG has been incorporated in the electrospun scaffolds in the form of copolymers, such as PEG-g-CHN [43] and PEG-PLA [45, 118] described earlier. Duan et al. [50] fabricated nanofibrous scaffolds by co-electrospinning mixtures of chitosan and PEO in aqueous solutions containing 2 wt% acetic acid. With the PEO/chitosan mass ratio of 2:1 or 1:1, fine fibers with two diameter distributions (the diameter ranged from 80 nm to 180 nm) were obtained from solutions of 4–6 wt% chitosan/PEO concentrations. They found that thick and thin fibers were formed mainly by PEO and chitosan, respectively [50]. Spasova et al. [119] applied electrospun chitosan/PEO scaffolds for delivery of potassium 5-nitro-8-quinolinolate(K5N8Q), an antimicrobial and antimycotic drug. They showed that the drug had an effect on the production of fiber diameter and fiber morphology. With 1 wt% K5N8Q loading in the scaffold based on the chitosan/PEO ratio of 1:1, the resulting nanofibrous mat showed antibacterial and antimycotic activity against E. coli, S. aureus and C. albicans [119]. The molecular weight of PEG as a processing aid for electrospinning was relatively high, usually larger than 5,000 Da. This is because the lower molecular weight PEG has the form of a liquid. For example, Xie et al. used oligomeric PEG to facilitate the electrospinning of two natural proteins: casein and lipase enzyme [108]. Jin et al. also showed that the addition of a small amount of oligomeric PEG was able to improve the processibility of silkworm fibroin solutions [120].
2.3.5 Other multi-component polymer systems
Other mixtures of biocompatible and biodegradable polymers have also been electrospun into nanofibrous scaffolds, such as polyether imide/poly(3-hydroxybutyrate-co-3-hydroxy valerate) [6]. In addition to polymer blends, blends of synthetic polymers and inorganic particles, such as silver particles [74, 121], calcium carbonate [122], calcium phosphate [123], and hydroxy-apatite [124, 125] were also used to prepare nanofibrous scaffolds, which were found to be useful in biomedical applications. Since elemental silver and silver salts have been used for decades as antimicrobial agents in curative and preventive health care, Son et al. [74] electrospun cellulose acetate fibers containing AgNO3, which was further reduced to silver nanoparticles by a photo-reduction technique using UV irradiation. Silver nanoparticles in cellulose acetate fibers were stabilized by interactions with carbonyl oxygen groups on cellulose acetate and showed very strong antimicrobial activity. Recently, Melaiye et al. [121] prepared Tecophilic nanofibers (a family of hydrophilic polyether-based thermoplastic aliphatic polyurethanes), containing up to 75 wt% silver-imidazole cyclophane gem-diol complex by electrospinning. The nanofibrous mat encapsulated the silver particles and released them in a sustained profile over a long period of time. Therefore, the rate of bactericidal activity of the silver particles was greatly improved and the amount of silver used was much reduced. Such electrospun organic/inorganic hybrid scaffolds were found to be very effective against E. coli, P. aeruginosa, S. aureus, C. albicans, A. niger and S. cerevisiae [121].
Fujihara et al. demonstrated that the incorporation of calcium carbonate (CaCO3) in the electrospun PCL scaffold was able to assist the bone cell regeneration [122]. To achieve the desired mechanical stability, two layered structures, one formed by neat PCL and one formed by the mixture of PCL and CaCO3 at different compositions, were employed. Good cell attachment and proliferation was observed in such composite scaffolds. Fan et al. incorporated b-tertiary calcium phosphate (b-TCP) into electrospun PLA scaffolds [123]. Compared with pure PLA scaffold, the incorporation of b-TCP increased the hydrophilicity of the scaffold and improved cell adhesion and proliferation, greatly improving its potential for use in tissue engineering.
Bioceramic hydroxy-apatite and PLA were fabricated into nanocomposite nanofibers by electrospinning [124]. A surfactant, hydroxysteric acid (HSA), was added in the system to effectively disperse hydrophilic hydroxy-apatite powders in the PLA solutions in chloroform. As a result, continuous and uniform nanofibers with diameters about 1–2 μm were generated. Cellular assay experiments indicated that this scaffold had excellent cell attachment and proliferation properties as well as enhanced expression of alkaline phosphatase at 7 days of culturing. To mimic the human bone matrix, Kim et al. fabricated a nanocomposite nanofibrous scaffold by electrospinning mixtures of gelation and hydroxy-apatite nanocrystals [125]. The hydroxy-apatite/gelatin mixture was lyophilized and dissolved in the organic solvent HFP and then electrospun under controlled conditions. With this method, the hydroxy-apatite nanocrystals were well distributed within the gelatin fibers. Compared to pure gelatin, the nanocomposite nanofibers significantly improved the bone-derived cellular activity, thus having good potential in the application of guided tissue (bone) regeneration [125].
3. New innovations in electrospinning for biomedical applications
Using the schemes of copolymerization and polymer mixtures, desirable physical and biological properties of electrospun nanofibrous scaffolds can be obtained. However, the performance of the electrospun scaffold can be further controlled by adjusting the diameter and morphology of the nanofibers, desirable 3D patterns (e.g., layered structures) and the porosity through the electrospinning processing technology. In this section, we describe several innovative electrospinning techniques to enhance the functions and properties of electrospun nanofibers. The new development includes multilayered electrospinning, core-shelled electrospinning, two-phase electrospinning, blowing-assisted electrospinning and post-alignment methods. Furthermore, some of these techniques are highly complementary in nature and can be combined to generate new hybrid materials in the platform of nanofibrous scaffolds with specific and desired properties. The selected examples represent only a snapshot of current activities reported in the community of electrospinning. Without a doubt, there will be many more innovative developments on the fabrication methods based on electrospinning technology in the future.
3.1 Scaffolds with oriented fiber alignment
During electrospinning, as the velocity of the fiber jet near the collector is very high (e.g. near a fraction of the speed of sound), the resulting nanofiber is usually collected in a random fashion without preferred orientation (i.e., non-woven structure). For certain applications in tissue engineering, scaffolds with aligned fibers are often more desirable to guide the cell growth with desired anisotropy [27, 53, 100, 126, 127]. Several fiber collection methods, including (1) auxiliary electrode/electrical field [128–130], (2) thin wheel with sharp edge collector [131], and (3) frame collector [28], have been developed to align the fibers on the collector. The most practical method to align the electrospun scaffold is perhaps by mechanical drawing (e.g. uniaxial drawing or sequential biaxial drawing) [132]. However, it has been found that with the increase in the stretching extension ratio, the porosity of the scaffold would decrease correspondingly. If this is a concern, the sequential biaxial stretching process with asymmetric draw ratios can be effectively used to control both orientation and porosity of the electrospun scaffold.
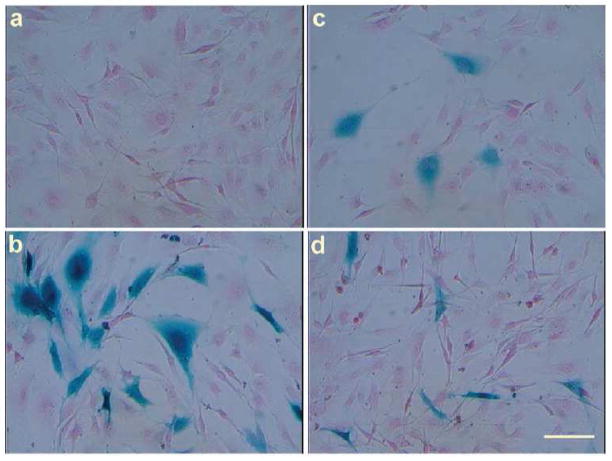
The use of oriented electrospun scaffolds has been demonstrated in several studies. For example, Xu et al. [100] investigated the P(LA-CL) nanofibrous scaffold with aligned fibrous structure and found that human coronary artery smooth muscle cells (SMCs) attached and migrated along the axis of the aligned nanofibers and expressed a spindle-like contractile phenotype. Figure 5 illustrates that the distribution and organization of smooth muscle cytoskeleton proteins inside SMCs were parallel to the direction of nanofibers. They also found that the adhesion and proliferation rate of SMCs on the aligned nanofibrous scaffold was significantly improved when compared with those on solid polymer films [100].
Figure 5.
Confocal micrographs of immunostained myosin filaments in SMCs after 1 day of culture; (a) on aligned nanofibrous scaffold, (b) on aligned nanofibrous scaffold, overlay image on the aligned fiber, and (c) on tissue culture polystyrene as control. (From Ref. [100] with permission)
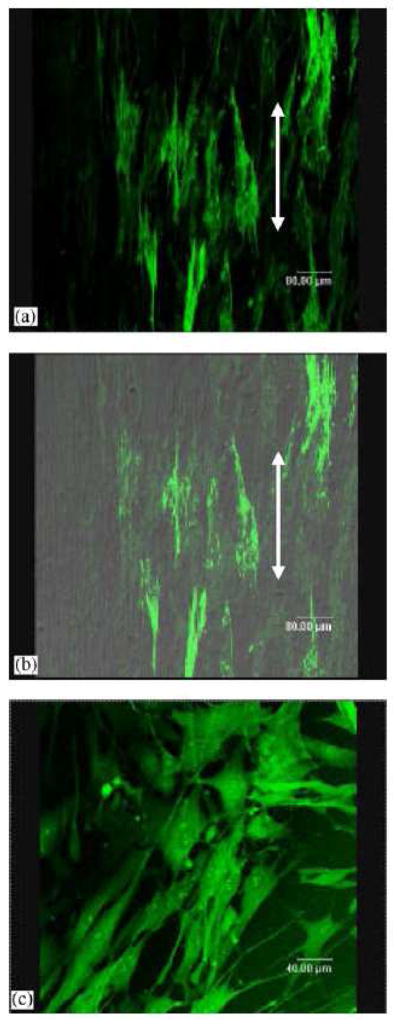
Our group has investigated the structural and functional effects of oriented electrospun scaffolds on the growth of cardiac myocytes (CM) [133]. The orientation was achieved by using a post-drawing process after electrospinning. Scanning electron microscopy (SEM) revealed that the fine fiber architecture of the non-woven matrix allowed the cardiomyocytes to make extensive use of provided external cues for isotropic or anisotropic (oriented) growth, and to some extent to crawl inside and pull on fibers (Figure 6a). Structural analysis by confocal microscopy indicated that CM had a preference for relatively hydrophobic surfaces (Figure 6b). Cardiac myocytes on electrospun poly(L-lactide) (PLLA) scaffolds developed mature contractile machinery (sarcomeres). Functionality (excitability) of the engineered constructs was confirmed by optical imaging of electrical activity using voltage-sensitive dyes (Figure 6c). The study clearly indicates that engineered cardiac tissue structure and function could be modulated by the chemistry and geometry of the provided nano- and micro-textured surfaces.
Figure 6.
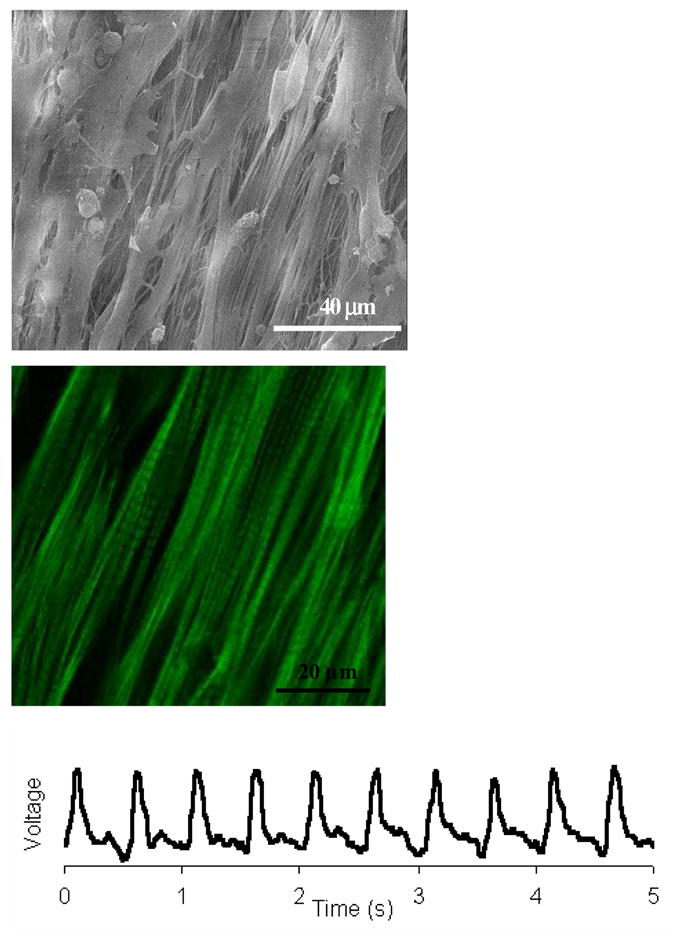
(a) SEM images of cardiac myocytes cultured on uniaxially stretched aligned PLLA electrospun scaffolds, (b) corresponding confocal micrograph of (a); (c) electrical response of cardiac myocytes on electrospun scaffolds (action potentials were measured using a voltage-sensitive dye di-8-ANEPPS and a micro scale optical recording system). (From Ref. [133] with permission)
3.2 Multilayer electrospinning and mixing electrospinning
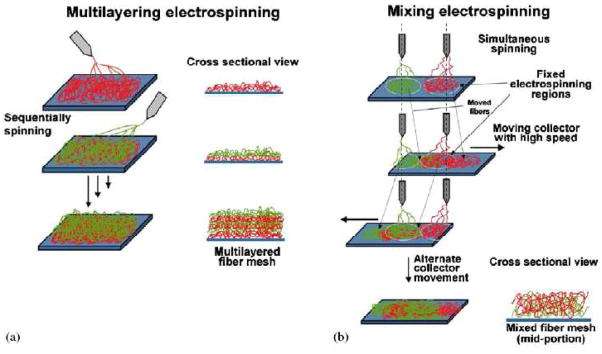
Recently, Kidoaki et al. [134] demonstrated two novel electrospinning techniques: (1) multilayer electrospinning and (2) mixing electrospinning (Figure 7), to fabricate composite scaffolds containing different polymers. In multilayer electrospinning, each polymer was electrospun to form its individual layer and was sequentially collected on the same target collector. As shown in Figure 7a, such a process could produce a multilayered non-woven nanofibrous mesh, in which a hierarchically ordered structure composed of different polymer meshes could be obtained. In mixing electrospinning, two different polymer solutions were simultaneously electrospun from different syringes under different processing conditions. The spun polymer fibers were mixed on the same target collector, resulting in the formation of mixed fiber mesh (Figure 7b). Three layered scaffolds, containing segmented polyurethane, styrenated gelatin and type I collagen, fabricated by using the multilayered electrospinning technique, and co-mingled nanofibrous scaffold, containing segmented polyurethane and poly(ethylene oxide), fabricated by using the mixing electrospinning technique, have been demonstrated [134]. The multilayer electrospun scaffolds have been further used for guided bone regeneration [122] and biohemostat [135] studies.
Figure 7.
Schematic diagram of (a) multilayer electrospinning and (b) mixing electrospinning. (From Ref. [134] with permission)
3.3 Fabrication of dual-porosity scaffolds
The presence of clay nanoparticles is capable of enhancing the strength, stiffness, resistance to heat, and mechanical and physical properties of the polymer matrix. Lee et al. [136] used the combination of electrospinning, based on suspensions containing PLA, solvent and clay nanoparticles, and salt addition, to fabricate a nanofibrous composite. After the salt leaching/gas forming process, a unique dual-porosity nanofibrous scaffold based on PLA/clay nanocomposites was generated. As shown in Figure 8, the scaffold exhibited a 3-D structure with nano-sized pores at the interstices of the entangled fibers (Figure 8a) and micro-sized (50–300 μm) pores formed by the salt particles and gas bubbles (Figure 8b). Such morphology is desired for scaffolding in tissue regeneration, as the large holes will enable the transportation of typical cells (in tens of microns) and the small holes will enable the perfusion of smaller size molecules (e.g. nutrients, growth factors). The biological activity of the dual-porosity scaffold, however, has not been reported.
Figure 8.
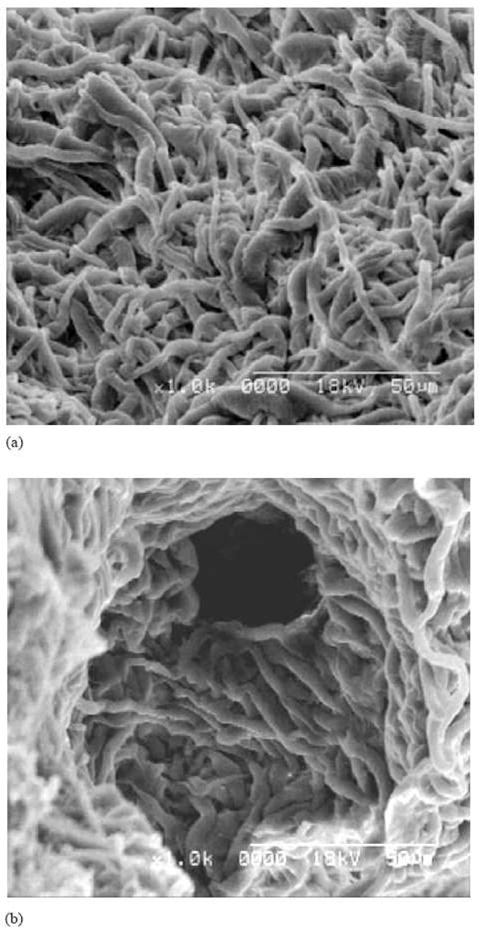
SEM image of PLA/clay nanocomposite scaffold by electrospinning and salt leaching/gas foaming methods. (From Ref. [136] with permission)
3.4 Two-phase electrospinning
Immiscible polymer solutions, such as poly(ethylene-co-vinyl acetate) in dichloromethane and bovine serum albumin (BSA) in phosphate-buffered saline (PBS) at a 40:1 ratio, have been electrospun to form a fibrous mat, containing a distinct two-phase structure in the resulting fibers [51]. The morphology of such fibers is illustrated in Figure 9 (left). The incompatible water phase (BSA in PBS) was encapsulated in the matrix of poly(ethylene-co-vinyl acetate). In Figure 9 (right), the florescent-labeled protein in PBS could be visualized directly by both visible and ultraviolet light, exhibiting different spectroscopic properties from the polymer matrix [51]. The two-phase electrospinning process, using a single spinneret, provides a viable means to incorporate small molecules and/or macromolecules, including drugs and proteins in the nanofibrous scaffolds, provided that the molecules could withstand the electrospinning process. The encapsulated bioactive molecules could be immobilized for a long time and then released in a controlled manner. Therefore, the techniques may offer potentially useful advantages over other electrospinning techniques in the applications of drug delivery and tissue engineering.
Figure 9.
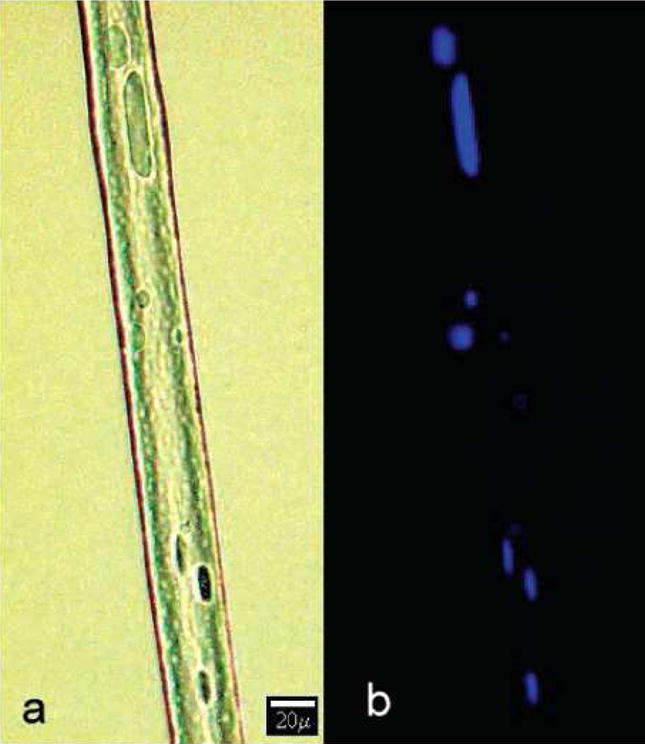
Visualization of fluorescently labeled protein encapsulated in polymer fibers using visible (a) and ultraviolet (b) light. (From Ref. [51] with permission)
3.5 Fabrication of core-shelled nanofibers
The fabrication of core-shelled nanofibers by electrospinning was first reported by Sun et al. [137]. Using this technology, some difficult-to-process polymer solutions could be co-electrospun to form an ultra-fine core within the shell of other polymer materials [138]. Figure 10 shows a typical setup used to generate the core-shelled structures by electrospinning. Basically, two polymer solutions were co-electrospun without direct mixing. Zhang et al. [139] reported the fabrication of a biodegradable core-shelled structure with PCL being the shell and gelatin being the core. Transmission electron microscopy (TEM) images and x-ray photoelectron spectroscopy (XPS) analysis confirmed the encapsulation of the gelatin within the PCL phase. This technique can be particularly useful in producing surface-modified nanofibers, functional nanocomposites, and even continuous hollow fibers.
Figure 10.
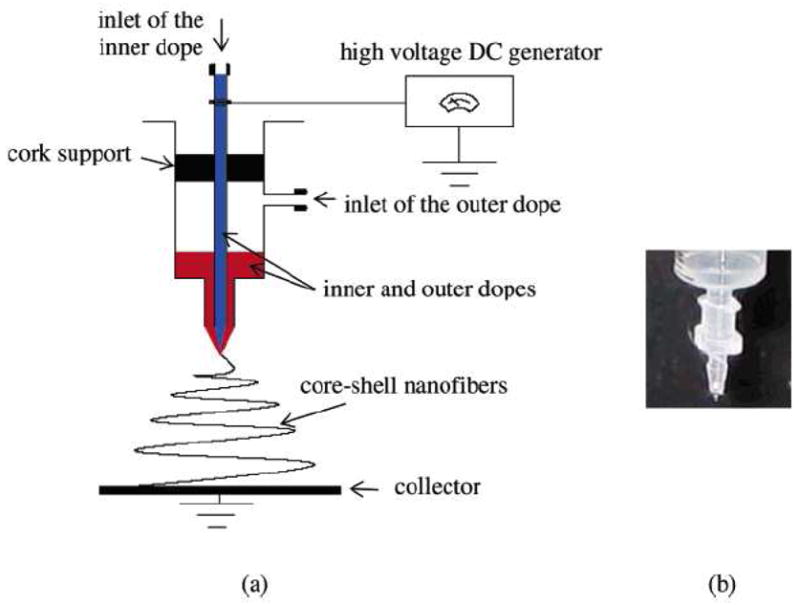
A setup used to generate core-shelled structure by electrospinning. (From Ref. [138] with permission)
Another advantage of the core-shelled fiber is that the shell protects the material in the core during the electrospinning process. This feature is even more attractive when bio-related materials are employed to form nanofibrous scaffolds. For example, Jiang et al. electrospun a fiber with poly(ε-caprolactone) as the shell and BSA together with dextran as the core [140]. With the help of dextran and the protection of the shell, BSA was nearly intact during the electrospinning process. A release of BSA in a controlled manner was achieved by the formation of the core-shelled fiber [140]. Besides proteins, the shell has the ability to protect even living cells. Recently, Townsend-Nicholson and Jayasinghe [141] demonstrated that, with poly(dimethylsiloxane) (PDMS) forming the shell, the cell suspension inside the core suffered almost no cellular damage during the fabrication process. Figure 11 shows the fluorescent micrographs of core-shelled fibers obtained at two different flow rates. Obviously, the Rhodamine 6G labeled cells, which are red in color under the microscope, were safely encapsulated by PDMS after electrospinning [141].
Figure 11.
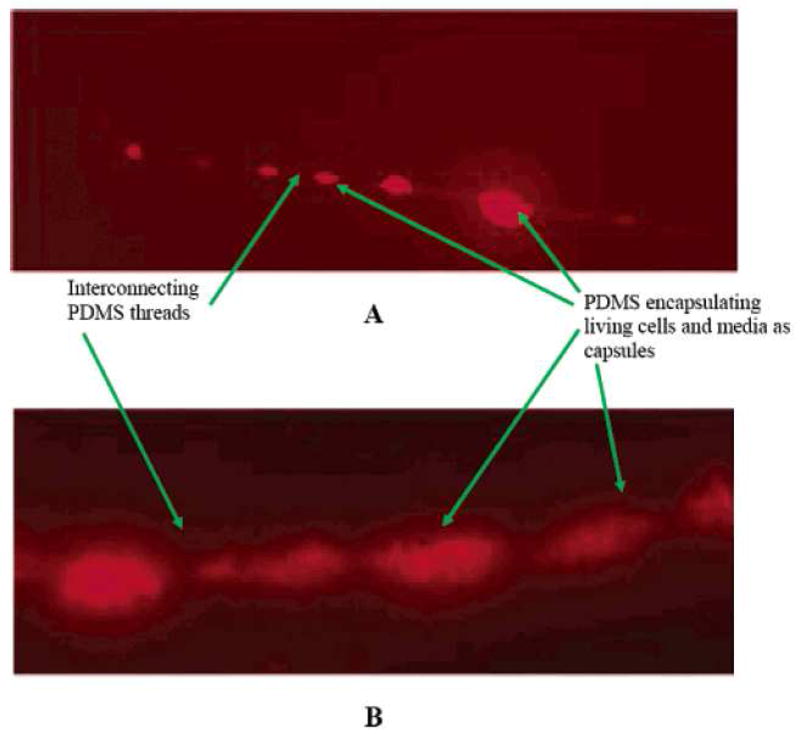
Characteristic fluorescent micrographs showing the variation in fiber diameter that results from cell encapsulation. Flow rate conditions: (A) cell suspension, 10−12 m3/s, polymer solution, 10−11 m3/s; (B) cell suspension, 10−8 m3/s, polymer solution 10−7 m3/s. (From Ref. [141] with permission)
3.6 Blowing-assisted electrospinning technique
To date, it is believed that nearly one hundred different polymers have been successfully electro-spun [28]. However, there are many more polymers that could not be electro-spun successfully. One of them is hyaluronic acid (HA), a naturally occurring polysaccharide, commonly found in connective tissues in the body, such as vitreous, umbilical cord, and joint fluid, due to its very high solution viscosity and high surface tension, even at fairly low solution concentrations.
Our research group has successfully demonstrated the fabrication of high molecular weight HA nanofibers using the blowing-assisted electro-spinning technique, which combined the process of electro-spinning with air blowing capability around the spinneret (a schematic diagram of the setup is shown in Figure 12) [32,65]. In this study, the effects of various experimental parameters, such as air-blowing rate, HA concentration, feeding rate of HA solution, applied electric field, and type of collector on the performance of blowing-assisted electro-spinning of HA solution were investigated. With the assistance of air-blowing, the HA solution feeding rate could be increased to 40 μl/min/spinneret and the applied electric field could be decreased to 2.5 kV/cm. The optimum conditions for consistent fabrication of HA (with a molecular weight about 3.5 million) nanofibers involved the use of an air blowing rate of around 70 ft3/hr and a concentration range between 2.5 to 2.7 wt% in aqueous solution.
Figure 12.
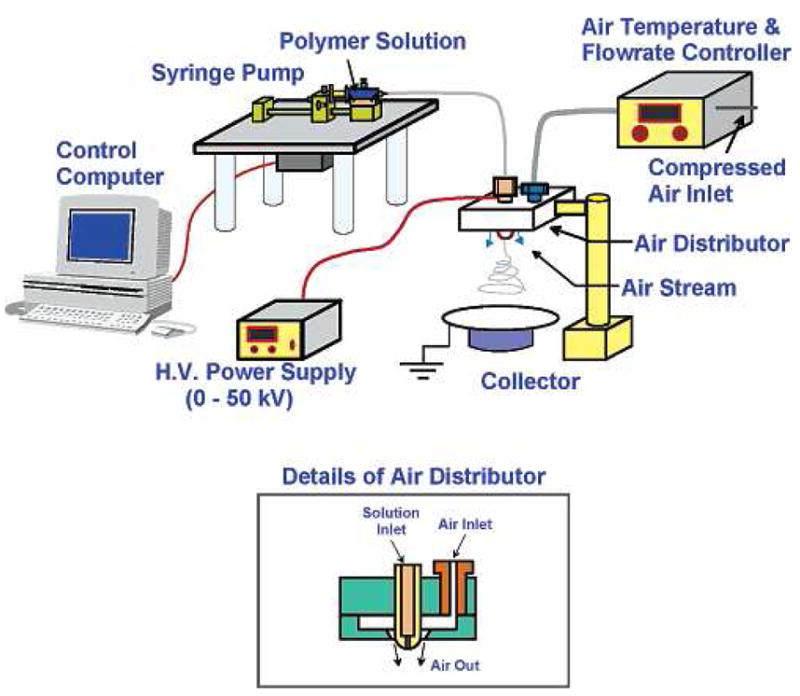
A setup of blowing-assisted electrospinning device that can process materials usually difficult to be electrospun. (From Ref. [32] with permission)
We demonstrated that there were at least four advantages in the electro-blowing process [32]. (1) The combination of air blowing force and the applied electric field is capable of overcoming the high viscosity, as well as the high surface tension, of the polymer solution. (2) The use of elevated temperature of the blown air can further decrease the HA solution viscosity at the spinneret, facilitating the jet formation of the HA solution at the spinneret. (3) The blowing air can accelerate the solvent evaporation process, a necessary condition for the fiber formation before the solution jet stream reaches the ground collector during the process. (4) The fiber diameter, which is one of the key factors to control the physical properties of nanofibrous membranes, can be tailored by controlling the air temperature and the air flow rate. With these advantages, it is expected that many useful polymers, which could not be electro-spun until now, can be processed by using the new electro-blowing approach. Furthermore, the electro-blowing process shall significantly increase the production rate and thus can lead to practical mass production schemes.
4. Modifications of post-electrospun Scaffolds
Although the combined use of different polymer preparation schemes (e.g. copolymers and mixtures) and innovative electrospinning techniques can significantly improve physical and biological properties of nanofibrous scaffolds, further modifications on the surface of electrospun nanofibers are often needed in order to refine their in vivo or clinical usage. In other words, surface modifications of electrospun scaffolds with suitable bioactive molecules are often essential to render the materials with more desirable biological features for biomedical applications.
Chua et al. [142] reported an approach to modify the poly(ε-caprolactone-co-ethyl ethane phosphate) (PCLEEP) nanofiber surface by grafting a hepatocyte-specified galactose ligand for hepatocyte culture. Figure 13 schematically shows the surface modification procedure. In brief, electrospun PCLEEP nanofibers, having an average diameter of about 760 nm, were first cleaned by ethanol and grafted with poly(acrylic acid) (PAA) by photo-induced polymerization. 1-O-(6′-aminohexyl)-D-galactopyranoside (AHG) and then conjugated to PAA chains in a sodium phosphate buffer containing N-hydroxysulfosuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC). During cell culture, hepatocyte was able to adhere to the surface through the galactose-asialoglycoprotein receptor (ASGPR). Besides the electrospun nanofibrous scaffolds, spin-coating films containing the same material were also surface-modified using the same procedure. The authors showed that hepatocytes cultured on the electrospun galactosylated scaffolds clearly exhibited superior biological properties, including cell attachment, albumin synthesis and 3-methylcholanthrene-induced cytochrome P450 function, to hepatocytes cultured on unmodified electrospun scaffolds [142]. Selected SEM images of hepatocytes after 8 days of culture on galactosylated spin-cast films and electrospun scaffolds are shown in Figure 14. Hepatocytes, cultured on modified electrospun scaffolds, formed spheroids that engulfed the galactosylated nanofibers (Figure 14d–f); the spheroids were immobilized on the scaffold and would not detach from the scaffold upon agitation, which was quite different from that on modified spin-cast films (Figure 14a–c). Based on these results, they concluded that hepatocyte spheroid immobilization and stabilization strategy through the use of galactosylated nanofibrous scaffolds would be advantageous in the design of a bioartificial liver-assist device [142].
Figure 13.
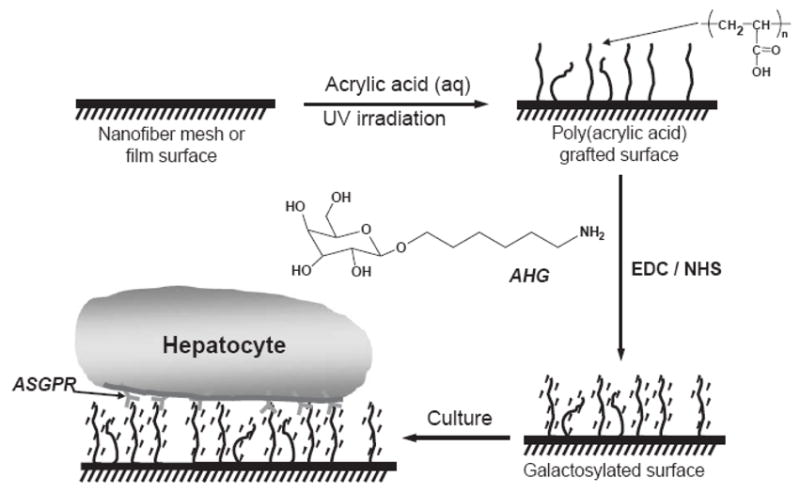
Surface modification scheme for galactose conjugation to PCLPEEP nanofiber mesh and spin-cast film. (From Ref. [142] with permission)
Figure 14.
SEM images of hepatocytes after 8 days of culture: (a–c) hepatocytes cultured on galactosylated spin-cast film formed around spheroids; (d–f) in contrast, hepatocytes cultured on galactosylated electrospun scaffold showed aggregates engulfed the functional nanofibers. (From Ref. [142] with permission)
Using a similar strategy, Ma et al. [40] grafted gelatin onto the electrospun poly(ethylene terephthalate) (PET) nanofibrous scaffolds by using a chemical scheme to overcome the chemical and biological inertness of the PET surface. The scheme is as follows. The electrospun PET scaffold was fixed on a piece of glass with glue and then treated with formaldehyde to introduce hydroxyl groups. Methacrylic acid was polymerized on the surface with Ce(IV) as the initiator, followed by the grafting of gelatin with carbodiimide as the coupling agent. They tested the bioactivity of the gelation-modified electrospun PET scaffold by using endothelial cells (ECs). Compared with the unmodified PET scaffold, the gelatin-modified PET scaffold showed a clear improvement in cell adhesion, spreading and proliferation. Moreover, the modified scaffold preserved the EC’s phenotype [40].
Wang et al. [35] demonstrated that the surface of an electrospun cellulose acetate scaffold was able to immobilize enzymes after being modified with PEG spacers. The modification scheme is as follows. The electrospun cellulose acetate nanofibrous scaffold was hydrolyzed and followed by grafting of PEG diacylchloride. Lipase enzyme was then attached to the scaffold surface through the coupling with PEG spacers. Their result showed that the bound lipase exhibited much better retention ability of catalytic activity after exposure to cyclohexane (81%), toluene (62%) and hexane (34%) than the activity of the free lipase (25%). More impressively, the bound lipase showed significant catalytic activity, up to 8–10 more times at 60 ~ 70 °C than that of the free form [35].
Surface modifications of electrospun scaffolds can significantly improve the biological performance while retaining all nanostructure features and properties. The modification scheme will be application specific and material dependent. In general, electrospun nanofibers should first be functionalized by a reactive spacer, which can then couple with other bioactive molecules to modify the physical or biological properties. As the electrospun nanofibrous scaffold has a very large surface area-to-volume ratio, such modifications will be extremely useful to generate new nanostructured materials with novel functionality for biomedical applications.
5. Conclusion
Electrospun nanofibrous scaffolds showed great promise and potential for many biomedical applications, such as tissue engineering, wound dressing, immobilized enzymes and controlled-delivery of drugs (genes). As described in this review, a successful creation of nanofibrous scaffolds must start with the proper selection of materials, a judicious and realistic fabrication pathway, and possible post-modification with functional reagent. The polymer material selection plays a key role in the fabrication of scaffolds. Many desirable properties can be achieved by polymer mixing (natural and/or synthetic polymers), copolymerization or a hybrid of materials and processing techniques. Multi-component mixtures can be miscible or immiscible, containing different phases (liquid or solid). Several newly developed innovative electrospinning methods have been described, including oriented scaffolds, multilayer electrospinning, mixing electrospinning, fabrication of dual-porosity scaffolds, two-phase electrospinning, fabrication of core-shelled nanofibers and blowing-assisted electrospinning. The processing schemes can be further combined or/and modified to generate new morphology based on the platform of nanofiber technology. Finally, the surface modification of electrospun scaffolds with suitable bioactive agents is an effective means to fine-tune the functionality of nanofibers for specific biomedical applications. The electrospinning technology platform can indeed offer the versatility and unique nanostructure features beyond most existing technologies.
Acknowledgments
Financial support of this work was provided by a National Institutes of Health-SBIR grant (GM63283-03), administered by Stonybrook Technology and Applied Research, Inc, and the National Science Foundation (DMR 0454887). The assistance of Dr. Dufei Fang in the preparation of this review is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li D, Xia YN. Electrospinning of nanofibers: Reinventing the wheel? Advanced Materials. 2004;16:1151–1170. [Google Scholar]
- 2.Reneker DH, Chun I. Nanometer diameter fibers of polymer, produced by electrospinning. Nanotechnology. 1996;7:216–223. [Google Scholar]
- 3.Zhang CX, Yuan XY, Wu LL, Han Y, Sheng J. Study on morphology of electrospun poly(vinyl alcohol) mats. European Polymer Journal. 2005;41:423–432. [Google Scholar]
- 4.Yang QB, Li ZY, Hong YL, Zhao YY, Qiu SL, Wang C, Wei Y. Influence of solvents on the formation of ultrathin uniform poly(vinyl pyrrolidone) nanofibers with electrospinning. Journal Of Polymer Science Part B-Polymer Physics. 2004;42:3721–3726. [Google Scholar]
- 5.Lin T, Wang HX, Wang HM, Wang XG. The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology. 2004;15:1375–1381. [Google Scholar]
- 6.Han SO, Son WK, Cho D, Youk JH, Park WH. Preparation of porous ultra-fine fibers via selective thermal degradation of electro spun polyether imide/poly(3-hydroxybutyrate-co-3-hydroxy valerate) fibers. Polymer Degradation and Stability. 2004;86:257–262. [Google Scholar]
- 7.Gupta P, Trenor SR, Long TE, Wilkes GL. In situ photo-cross-linking of cinnamate functionalized poly(methyl methacrylate-co-2-hydroxyethyl acrylate) fibers during electrospinning. Macromolecules. 2004;37:9211–9218. [Google Scholar]
- 8.Chen H, Hsieh YL. Ultrafine hydrogel fibers with dual temperature- and pH-responsive swelling behaviors. Journal Of Polymer Science Part A-Polymer Chemistry. 2004;42:6331–6339. [Google Scholar]
- 9.Xu H, Yarin AL, Reneker DH. Characterization of fluid flow in jets during electrospinning. Abstracts Of Papers Of The American Chemical Society. 2003;226:U424–U424. [Google Scholar]
- 10.Jun Z, Hou HQ, Schaper A, Wendorff JH, Greiner A. Poly-L-lactide nanofibers by electrospinning - Influence of solution viscosity and electrical conductivity on fiber diameter and fiber morphology. E-Polymers. 2003 [Google Scholar]
- 11.Schreuder-Gibson HL, Gibson P, Tsai P, Gupta P, Wilkes G. Cooperative charging effects of fibers from electrospinning of electrically dissimilar polymers. International Nonwovens Journal. 2004;13:39–45. [Google Scholar]
- 12.Schreuder-Gibson H, Gibson P, Wadsworth L, Hemphill S, Vontorcik J. Effect of filter deformation on the filtration and air flow for elastomeric nonwoven media. Advances in Filtration and Separation Technology. 2002;15:525–537. [Google Scholar]
- 13.Gibson P, Schreuder-Gibson H, Rivin D. Transport properties of porous membranes based on electrospun nanofibers, Colloids and Surfaces. A: Physicochemical and Engineering Aspects. 2001;187–188:469–481. [Google Scholar]
- 14.Wannatong L, Sirivat A. Electrospun fibers of polypyrrole/polystyrene blend for gas sensing applications. PMSE Preprints. 2004;91:692–693. [Google Scholar]
- 15.Wang XY, Kim YG, Drew C, Ku BC, Kumar J, Samuelson LA. Electrostatic assembly of conjugated polymer thin layers on electrospun nanofibrous membranes for biosensors. Nano Letters. 2004;4:331–334. [Google Scholar]
- 16.Ding B, Kim J, Miyazaki Y, Shiratori S. Electrospun nanofibrous membranes coated quartz crystal microbalance as gas sensor for NH3 detection, Sensors and Actuators. B: Chemical. 2004;B101:373–380. [Google Scholar]
- 17.Ding B, Kim J, Fujimoto K, Shiratori S. Electrospun nanofibrous polyelectrolyte membranes for advanced chemical sensors. Chemical Sensors. 2004;20:264–265. [Google Scholar]
- 18.Wang XY, Drew C, Lee SH, Senecal KJ, Kumar J, Sarnuelson LA. Electrospun nanofibrous membranes for highly sensitive optical sensors. Nano Letters. 2002;2:1273–1275. [Google Scholar]
- 19.Liu HQ, Kameoka J, Czaplewski DA, Craighead HG. Polymeric nanowire chemical sensor. Nano Letters. 2004;4:671–675. [Google Scholar]
- 20.Kim C, Park SH, Lee WJ, Yang KS. Characteristics of supercapacitor electrodes of PBI-based carbon nanofiber web prepared by electrospinning. Electrochimica Acta. 2004;50:877–881. [Google Scholar]
- 21.Kim C, Choi YO, Lee WJ, Yang KS. Supercapacitor performances of activated carbon fiber webs prepared by electrospinning of PMDA-ODA poly(amic acid) solutions. Electrochimica Acta. 2004;50:883–887. [Google Scholar]
- 22.Kim C, Yang KS. Electrochemical properties of carbon nanofiber web as an electrode for supercapacitor prepared by electrospinning. Applied Physics Letters. 2003;83:1216–1218. [Google Scholar]
- 23.Kim C, Yang KS, Lee WJ. The Use of Carbon Nanofiber Electrodes Prepared by Electrospinning for Electrochemical Supercapacitors. Electrochemical and Solid-State Letters. 2004;7:A397–A399. [Google Scholar]
- 24.Khil MS, Bhattarai SR, Kim HY, Kim SZ, Lee KH. Novel fabricated matrix via electrospinning for tissue engineering. Journal of Biomedical Materials Research, Part B: Applied Biomaterials. 2005;72B:117–124. doi: 10.1002/jbm.b.30122. [DOI] [PubMed] [Google Scholar]
- 25.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of Nanofiber Matrix as Tissue-Engineering Scaffolds. Tissue Engineering. 2005;11:101–109. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 26.Riboldi SA, Sampaolesi M, Neuenschwander P, Cossu G, Mantero S. Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials. 2005;26:4606–4615. doi: 10.1016/j.biomaterials.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 27.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 28.Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Science and Technology. 2003;63:2223–2253. [Google Scholar]
- 29.Chu B, Hsiao BS, Fang D. Application: WO. The Research Foundation of State University of New York; USA: 2004. p. 55. [Google Scholar]
- 30.Ding B, Kimura E, Sato T, Fujita S, Shiratori S. Fabrication of blend biodegradable nanofibrous nonwoven mats via multi-jet electrospinning. Polymer. 2004;45:1895–1902. [Google Scholar]
- 31.Chu B, Hsiao BS, Hadjiargyrou M, Fang D, Zong X, Kim K. Application: US. The Research Foundation At State University of New York; USA: 2003. [Google Scholar]
- 32.Um IC, Fang DF, Hsiao BS, Okamoto A, Chu B. Electro-spinning and electro-blowing of hyaluronic acid. Biomacromolecules. 2004;5:1428–1436. doi: 10.1021/bm034539b. [DOI] [PubMed] [Google Scholar]
- 33.Jia H, Zhu G, Vugrinovich B, Kataphinan W, Reneker DH, Wang P. Enzyme-Carrying Polymeric Nanofibers Prepared via Electrospinning for Use as Unique Biocatalysts. Biotechnology Progress. 2002;18:1027–1032. doi: 10.1021/bp020042m. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Hsieh YL. Enzyme immobilization via electrospinning of polymer/enzyme blends, Polymer Preprints (American Chemical Society, Division of Polymer Chemistry) 2003;44:1212–1213. [Google Scholar]
- 35.Wang Y, Hsieh YL. Enzyme immobilization to ultra-fine cellulose fibers via amphiphilic polyethylene glycol spacers. Journal of Polymer Science, Part A: Polymer Chemistry. 2004;42:4289–4299. [Google Scholar]
- 36.Wu L, Yuan X, Sheng J. Immobilization of cellulase in nanofibrous PVA membranes by electrospinning. Journal of Membrane Science. 2005;250:167–173. [Google Scholar]
- 37.Khil M-s, Cha D-i, Kim H-y, Kim I-s, Bhattarai N. Electrospun nanofibrous polyurethane membrane as wound dressing. Journal of Biomedical Materials Research, Part B: Applied Biomaterials. 2003;67B:675–679. doi: 10.1002/jbm.b.10058. [DOI] [PubMed] [Google Scholar]
- 38.Kim HY, Lee BM, Kim IS, Jin TH, Ko KH, Ryu YJ. Fabrication of triblock copolymer of poly(r-dioxanone-co-L-lactide)-block-poly(ethylene glycol) nonwoven mats by electrospinning and applications for wound dressing. PMSE Preprints. 2004;91:712–713. [Google Scholar]
- 39.Buttafoco L, Kolkman NG, Poot AA, Dijkstra PJ, Vermes I, Feijen J. Electrospinning collagen and elastin for tissue engineering small diameter blood vessels. Journal Of Controlled Release. 2005;101:322–324. [PubMed] [Google Scholar]
- 40.Ma Z, Kotaki M, Yong T, He W, Ramakrishna S. Surface engineering of electrospun polyethylene terephthalate (PET) nanofibers towards development of a new material for blood vessel engineering. Biomaterials. 2005;26:2527–2536. doi: 10.1016/j.biomaterials.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 41.Zong X, Fang D, Kim K, Ran S, Hsiao BS, Chu B, Brathwaite C, Li S, Chen E. Nonwoven nanofiber membranes of poly(lactide) and poly(glycolide-co-lactide) via electrospinning and application for anti-adhesions, Polymer Preprints (American Chemical Society, Division of Polymer Chemistry) 2002;43:659–660. [Google Scholar]
- 42.Zong X, Li S, Chen E, Garlick B, Kim K-S, Fang D, Chiu J, Zimmerman T, Brathwaite C, Hsiao Benjamin S, Chu B. Prevention of post-surgery-induced abdominal adhesions by electrospun bioabsorbable nanofibrous poly(lactide-co-glycolide)-based membranes. Ann Surg FIELD Full Journal Title: Annals of Surgery. 2004;240:910–915. doi: 10.1097/01.sla.0000143302.48223.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang HL, Fang DF, Hsiao BJ, Chu BJ, Chen WL. Preparation and characterization of ibuprofen-loaded poly(lactide-co-glycolide)/poly(ethylene glycol)-g-chitosan electrospun membranes. Journal Of Biomaterials Science-Polymer Edition. 2004;15:279–296. doi: 10.1163/156856204322977184. [DOI] [PubMed] [Google Scholar]
- 44.Katti DS, Robinson KW, Ko FK, Laurencin CT. Bio-resorbable nanofiber-based systems for wound healing and drug delivery: Optimization of fabrication parameters. Journal Of Biomedical Materials Research Part B-Applied Biomaterials. 2004;70B:286–296. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- 45.Kim K, Luu YK, Chang C, Fang DF, Hsiao BS, Chu B, Hadjiargyrou M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. Journal Of Controlled Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Kim KS, Chang C, Zong XH, Fang DF, Hsiao BS, Chu B, Hadjiargyrou M. Incorporation of an antibiotic drug in electrospun poly(lactide-co-glycolide) non-woven nanofiber scaffolds. Abstracts of Papers of The American Chemical Society. 2003;226:U437–U437. [Google Scholar]
- 47.Luu YK, Kim K, Hsiao BS, Chu B, Hadjiargyrou M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. Journal of Controlled Release. 2003;89:341–353. doi: 10.1016/s0168-3659(03)00097-x. [DOI] [PubMed] [Google Scholar]
- 48.Kenawy ER, Layman JM, Watkins JR, Bowlin GL, Matthews JA, Simpson DG, Wnek GE. Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials. 2003;24:907–913. doi: 10.1016/s0142-9612(02)00422-2. [DOI] [PubMed] [Google Scholar]
- 49.Boland ED, Matthews JA, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Frontiers in Bioscience. 2004;9:1422–1432. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 50.Duan B, Dong C, Yuan X, Yao K. Electrospinning of chitosan solutions in acetic acid with poly(ethylene oxide) Journal of Biomaterials Science, Polymer Edition. 2004;15:797–811. doi: 10.1163/156856204774196171. [DOI] [PubMed] [Google Scholar]
- 51.Sanders EH, Kloefkorn R, Bowlin GL, Simpson DG, Wnek GE. Two-phase electrospinning from a single electrified jet: Microencapsulation of aqueous reservoirs in poly(ethylene-co-vinyl acetate) fibers. Macromolecules. 2003;36:3803–3805. [Google Scholar]
- 52.Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 2005;26:5999–6008. doi: 10.1016/j.biomaterials.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 53.Zhong S, Teo WE, Zhu X, Beuerman RW, Ramakrishna S, Yung LYL. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. Journal of Biomedical Materials Research, Part A. 2006;79A:456–463. doi: 10.1002/jbm.a.30870. [DOI] [PubMed] [Google Scholar]
- 54.Li J, He A, Zheng J, Han CC. Gelatin and Gelatin-Hyaluronic Acid Nanofibrous Membranes Produced by Electrospinning of Their Aqueous Solutions. Biomacromolecules. 2006;7:2243–2247. doi: 10.1021/bm0603342. [DOI] [PubMed] [Google Scholar]
- 55.Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S. Crosslinking of the electrospun gelatin nanofibers. Polymer. 2006;47:2911–2917. [Google Scholar]
- 56.Matthews JA, Boland ED, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen type II: A feasibility study. Journal of Bioactive and Compatible Polymers. 2003;18:125–134. [Google Scholar]
- 57.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 58.Shields KJ, Beckman MJ, Bowlin GL, Wayne JS. Mechanical properties and cellular proliferation of electrospun collagen type II. Tissue Engineering. 2004;10:1510–1517. doi: 10.1089/ten.2004.10.1510. [DOI] [PubMed] [Google Scholar]
- 59.Wnek GE, Carr ME, Simpson DG, Bowlin GL. Electrospinning of nanofiber fibrinogen structures. Nano Letters. 2003;3:213–216. [Google Scholar]
- 60.Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004;25:1289–1297. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 61.Min BM, Jeong L, Nam YS, Kim JM, Kim JY, Park WH. Formation of silk fibroin matrices with different texture and its cellular response to normal human keratinocytes. International Journal Of Biological Macromolecules. 2004;34:281–288. doi: 10.1016/j.ijbiomac.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 62.Min BM, Jeong L, Lee KY, Park WH. Regenerated silk fibroin nanofibers: Water vapor-induced structural changes and their effects on the behavior of normal human cells. Macromolecular Bioscience. 2006;6:285–292. doi: 10.1002/mabi.200500246. [DOI] [PubMed] [Google Scholar]
- 63.Jeong L, Lee KY, Liu JW, Park WH. Time-resolved structural investigation of regenerated silk fibroin nanofibers treated with solvent vapor. International Journal Of Biological Macromolecules. 2006;38:140–144. doi: 10.1016/j.ijbiomac.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Huang ZM, Zhang YZ, Ramakrishna S, Lim CT. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer. 2004;45:5361–5368. [Google Scholar]
- 65.Wang X, Um IC, Fang D, Okamoto A, Hsiao BS, Chu B. Formation of water-resistant hyaluronic acid nanofibers by blowing-assisted electro-spinning and non-toxic post treatments. Polymer. 2005;46:4853–4867. [Google Scholar]
- 66.Jiang HL, Fang DF, Hsiao BS, Chu B, Chen WL. Optimization and characterization of dextran membranes prepared by electrospinning. Biomacromolecules. 2004;5:326–333. doi: 10.1021/bm034345w. [DOI] [PubMed] [Google Scholar]
- 67.Min BM, Lee SW, Lim JN, You Y, Lee TS, Kang PH, Park WH. Chitin and chitosan nanofibers: electrospinning of chitin and deacetylation of chitin nanofibers. Polymer. 2004;45:7137–7142. [Google Scholar]
- 68.Ohkawa K, Cha DI, Kim H, Nishida A, Yamamoto H. Electrospinning of chitosan. Macromolecular Rapid Communications. 2004;25:1600–1605. [Google Scholar]
- 69.Subramanian A, Lin HY, Vu D, Larsen G. Synthesis and evaluation of scaffolds prepared from chitosan fibers for potential use in cartilage tissue engineering. Biomedical Sciences Instrumentation. 2004;40:117–122. [PubMed] [Google Scholar]
- 70.Noh HK, Lee SW, Kim JM, Oh JE, Kim KH, Chung CP, Choi SC, Park WH, Min BM. Electrospinning of chitin nanofibers: Degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials. 2006;27:3934–3944. doi: 10.1016/j.biomaterials.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 71.Khil MS, Kim HY, Kang YS, Bang HJ, Lee DR, Doo JK. Preparation of electrospun oxidized cellulose mats and their in vitro degradation behavior. Macromolecular Research. 2005;13:62–67. [Google Scholar]
- 72.Liu HQ, Hsieh YL. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. Journal Of Polymer Science Part B-Polymer Physics. 2002;40:2119–2129. [Google Scholar]
- 73.Son WK, Youk JH, Lee TS, Park WH. Electrospinning of ultrafine cellulose acetate fibers: Studies of a new solvent system and deacetylation of ultrafine cellulose acetate fibers. Journal Of Polymer Science Part B-Polymer Physics. 2004;42:5–11. [Google Scholar]
- 74.Son WK, Youk JH, Lee TS, Park WH. Preparation of antimicrobial ultrafine cellulose acetate fibers with silver nanoparticles. Macromolecular Rapid Communications. 2004;25:1632–1637. [Google Scholar]
- 75.Son WK, Youk JH, Park WH. Preparation of ultrafine oxidized cellulose mats via electrospinning. Biomacromolecules. 2004;5:197–201. doi: 10.1021/bm034312g. [DOI] [PubMed] [Google Scholar]
- 76.Fang X, Reneker DH. DNA fibers by electrospinning. Journal of Macromolecular Science, Physics. 1997;B36:169–173. [Google Scholar]
- 77.McKee MG, Layman JM, Cashion MP, Long TE. Phospholipid nonwoven electrospun membranes. Science. 2006;311:353–355. doi: 10.1126/science.1119790. [DOI] [PubMed] [Google Scholar]
- 78.Boland ED, Telemeco TA, Simpson DG, Wnek GE, Bowlin GL. Utilizing acid pretreatment and electrospinning to improve biocompatibility of poly(glycolic acid) for tissue engineering. Journal of Biomedical Materials Research, Part B: Applied Biomaterials. 2004;71B:144–152. doi: 10.1002/jbm.b.30105. [DOI] [PubMed] [Google Scholar]
- 79.Boland ED, Wnek GE, Simpson DG, Pawlowski KJ, Bowlin GL. Tailoring tissue engineering scaffolds using electrostatic processing techniques: A study of poly(glycolic acid) electrospinning. Journal Of Macromolecular Science-Pure And Applied Chemistry. 2001;38:1231–1243. [Google Scholar]
- 80.Bognitzki M, Frese T, Wendorff JH, Greiner A. Submicrometer shaped polylactide fibers by electrospinning. Abstracts Of Papers Of The American Chemical Society. 2000;219:U491–U491. [Google Scholar]
- 81.Yang F, Xu CY, Kotaki M, Wang S, Ramakrishna S. Characterization of neural stem cells on electrospun poly(L-lactic acid) nanofibrous scaffold. Journal of Biomaterials Science, Polymer Edition. 2004;15:1483–1497. doi: 10.1163/1568562042459733. [DOI] [PubMed] [Google Scholar]
- 82.Zeng J, Chen X, Xu X, Liang Q, Bian X, Yang L, Jing X. Ultrafine fibers electrospun from biodegradable polymers. Journal of Applied Polymer Science. 2003;89:1085–1092. [Google Scholar]
- 83.Jing Z, Xu XY, Chen XS, Liang QZ, Bian XC, Yang LX, Jing XB. Biodegradable electrospun fibers for drug delivery. Journal Of Controlled Release. 2003;92:227–231. doi: 10.1016/s0168-3659(03)00372-9. [DOI] [PubMed] [Google Scholar]
- 84.Hsu CM, Shivkumar S. N,N-dimethylformamide additions to the solution for the electrospinning of poly(e-caprolactone) nanofibers. Macromolecular Materials and Engineering. 2004;289:334–340. [Google Scholar]
- 85.Hsu CM, Shivkumar S. Nano-sized beads and porous fiber constructs of poly(vepsiln.-caprolactone) produced by electrospinning. Journal of Materials Science. 2004;39:3003–3013. [Google Scholar]
- 86.Ohkawa K, Kim H, Lee K, Yamamoto H. Electro spun non-woven fabrics of poly(e-caprolactone) and their biodegradation by pure cultures of soil filamentous fungi. Macromolecular Symposia. 2004;216:301–306. [Google Scholar]
- 87.Tan EPS, Ng SY, Lim CT. Tensile testing of a single ultrafine polymeric fiber. Biomaterials. 2005;26:1453–1456. doi: 10.1016/j.biomaterials.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 88.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as medical devices. Medical Plastics and Biomaterial. 1998 doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 89.Rockwood D, Fromstein J, Woodhouse K, Chase B, Rabolt JF. Electrospinning of a biodegradable polyurethane for use in tissue engineering, Polymer Preprints (American Chemical Society. Division of Polymer Chemistry) 2004;45:406. [Google Scholar]
- 90.Verreck G, Chun I, Rosenblatt J, Peeters J, Van Dijck A, Mensch J, Noppe M, Brewster ME. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. Journal of Controlled Release. 2003;92:349–360. doi: 10.1016/s0168-3659(03)00342-0. [DOI] [PubMed] [Google Scholar]
- 91.Ding B, Kim HY, Lee SC, Shao CL, Lee DR, Park SJ, Kwag GB, Choi KJ. Preparation and characterization of a nanoscale poly(vinyl alcohol) fiber aggregate produced by an electrospinning method. Journal Of Polymer Science Part B-Polymer Physics. 2002;40:1261–1268. [Google Scholar]
- 92.Yao L, Haas TW, Guiseppi-Elie A, Bowlin GL, Simpson DG, Wnek GE. Electrospinning and Stabilization of Fully Hydrolyzed Poly(Vinyl Alcohol) Fibers. Chemistry of Materials. 2003;15:1860–1864. [Google Scholar]
- 93.Son WK, Youk JH, Lee TS, Park WH. The effects of solution properties and polyelectrolyte on electrospinning of ultrafine poly(ethylene oxide) fibers. Polymer. 2004;45:2959–2966. [Google Scholar]
- 94.Boland Eugene D, Coleman Branch D, Barnes Catherine P, Simpson David G, Wnek Gary E, Bowlin Gary L. Electrospinning polydioxanone for biomedical applications. Acta Biomater FIELD Full Journal Title: Acta biomaterialia. 2005;1:115–123. doi: 10.1016/j.actbio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 95.Nair LS, Bhattacharyya S, Bender JD, Greish YE, Brown PW, Allcock HR, Laurencin CT. Fabrication and optimization of methylphenoxy substituted polyphosphazene nanofibers for biomedical applications. Biomacromolecules. 2004;5:2212–2220. doi: 10.1021/bm049759j. [DOI] [PubMed] [Google Scholar]
- 96.Bhattacharyya S, Nair LS, Singh A, Krogman NR, Greish YE, Brown PW, Allcock HR, Laurencin CT. Electrospinning of poly[bis(ethyl alanato) phosphazene] nanofibers. Journal of Biomedical Nanotechnology. 2006;2:36–45. [Google Scholar]
- 97.Kwon IK, Kidoaki S, Matsuda T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: structural characteristics, mechanical properties and cell adhesion potential. Biomaterials. 2005;26:3929–3939. doi: 10.1016/j.biomaterials.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 98.Mo X, Xu C, Kotaki M, Ramakrishna S, Weber HJ. Electrospinning P(LLA-CL) nanofiber: Structure characterization and properties determination, Polymer Preprints (American Chemical Society. Division of Polymer Chemistry) 2003;44:128–129. [Google Scholar]
- 99.Mo XM, Xu CY, Kotaki M, Ramakrishna S. Electrospun P(LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 2004;25:1883–1890. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 100.Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2003;25:877–886. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 101.Lee IS, Kwon OH, Meng W, Kang IK. Nanofabrication of microbial polyester by electrospinning promotes cell attachment. Macromolecular Research. 2004;12:374–378. [Google Scholar]
- 102.Choi JS, Lee SW, Jeong L, Bae SH, Min BC, Youk JH, Park WH. Effect of organosoluble salts on the nanofibrous structure of electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate) International Journal of Biological Macromolecules. 2004;34:249–256. doi: 10.1016/j.ijbiomac.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 103.Bhattarai N, Cha DI, Bhattarai SR, Khil MS, Kim HY. Biodegradable electrospun mat: Novel block copolymer of poly(p-dioxanone-co-L-lactide)-block-poly(ethylene glycol) Journal of Polymer Science, Part B: Polymer Physics. 2003;41:1955–1964. [Google Scholar]
- 104.Bhattarai SR, Bhattarai N, Yi HK, Hwang PH, Cha DI, Kim HY. Novel biodegradable electrospun membrane: scaffold for tissue engineering. Biomaterials. 2004;25:2595–2602. doi: 10.1016/j.biomaterials.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 105.Stitzel JD, Bowlin GL, Mansfield K, Wnek GE, Simpson DG. Electrospraying and electrospinning of polymers for biomedical applications. Poly(lactic-co-glycolic acid) and poly(ethylene-co-vinylacetate) International SAMPE Technical Conference. 2000;32:205–211. [Google Scholar]
- 106.Jin HJ, Hwang MO, Yoon JS, Lee KH, Chin IJ, Kim MN. Preparation and characterization of electrospun poly(L-lactic acid-co-succinic acid-co-1,4-butane diol) fibrous membranes. Macromolecular Research. 2005;13:73–79. [Google Scholar]
- 107.Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes I, Feijen J. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2005;27:724–734. doi: 10.1016/j.biomaterials.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 108.Xie JB, Hsieh YL. Ultra-high surface fibrous membranes from electrospinning of natural proteins: casein and lipase enzyme. Journal Of Materials Science. 2003;38:2125–2133. [Google Scholar]
- 109.Zhang Y, Ouyang H, Lim CT, Ramakrishna S, Huang ZM. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. Journal of Biomedical Materials Research, Part B: Applied Biomaterials. 2005;72B:156–165. doi: 10.1002/jbm.b.30128. [DOI] [PubMed] [Google Scholar]
- 110.Casper CL, Yamaguchi N, Chase B, Rabolt JF, Kiick KL. Functionalized electrospun fibers for potential use in biomedical applications. PMSE Preprints. 2004;91:1030. [Google Scholar]
- 111.Huang L, Nagapudi K, Apkarian RP, Chaikof EL. Engineered collagen-PEO nanofibers and fabrics. Journal of Biomaterials Science, Polymer Edition. 2001;12:979–993. doi: 10.1163/156856201753252516. [DOI] [PubMed] [Google Scholar]
- 112.Aluigi A, Varesano A, Montarsolo A, Vineis C, Ferrero F, Mazzuchetti G, Tonin C. Electrospinning of keratin/poly(ethylene oxide) blend nanofibers. Journal of Applied Polymer Science. 2007;104:863–870. [Google Scholar]
- 113.He W, Ma Z, Yong T, Teo WE, Ramakrishna S. Fabrication of collagen-coated biodegradable polymer nanofiber mesh and its potential for endothelial cells growth. Biomaterials. 2005;26:7606–7615. doi: 10.1016/j.biomaterials.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 114.Min BM, You Y, Kim JM, Lee SJ, Park WH. Formation of nanostructured poly(lactic-co-glycolic acid)/chitin matrix and its cellular response to normal human keratinocytes and fibroblasts. Carbohydrate Polymers. 2004;57:285–292. [Google Scholar]
- 115.Kim K, Yu M, Zong XH, Chiu J, Fang DF, Seo YS, Hsiao BS, Chu B, Hadjiargyrou M. Control of degradation rate and hydrophilicity in electrospun non-woven poly(D,L-lactide) nanofiber scaffolds for biomedical applications. Biomaterials. 2003;24:4977–4985. doi: 10.1016/s0142-9612(03)00407-1. [DOI] [PubMed] [Google Scholar]
- 116.Bognitzki M, Frese T, Steinhart M, Greiner A, Wendorff JH, Schaper A, Hellwig M. Preparation of fibers with nanoscaled morphologies: Electrospinning of polymer blends. Polymer Engineering And Science. 2001;41:982–989. [Google Scholar]
- 117.Kenawy ER, Bowlin GL, Mansfield K, Layman J, Sanders E, Simpson DG, Wnek GE. Release of tetracycline hydrochloride from electrospun polymers, Polymer Preprints (American Chemical Society, Division of Polymer Chemistry) 2002;43:457–458. doi: 10.1016/s0168-3659(02)00041-x. [DOI] [PubMed] [Google Scholar]
- 118.Bhattarai SR, Bhattarai N, Viswanathamurthi P, Yi HK, Hwang PH, Kim HY. Hydrophilic nanofibrous structure of polylactide; fabrication and cell affinity. Journal Of Biomedical Materials Research Part A. 2006;78A:247–257. doi: 10.1002/jbm.a.30695. [DOI] [PubMed] [Google Scholar]
- 119.Spasova M, Manolova N, Paneva D, Rashkov I. Preparation of chitosan-containing nanofibers by electrospinning of chitosan/poly(ethylene oxide) blend solutions. e-Polymers. 2004 No pp given. [Google Scholar]
- 120.Jin HJ, Fridrikh SV, Rutledge GC, Kaplan DL. Electrospinning Bombyx mori Silk with Poly(ethylene oxide) Biomacromolecules. 2002;3:1233–1239. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- 121.Melaiye A, Sun ZH, Hindi K, Milsted A, Ely D, Reneker DH, Tessier CA, Youngs WJ. Silver(I)-imidazole cyclophane gem-diol complexes encapsulated by electrospun tecophilic nanofibers: Formation of nanosilver particles and antimicrobial activity. Journal of The American Chemical Society. 2005;127:2285–2291. doi: 10.1021/ja040226s. [DOI] [PubMed] [Google Scholar]
- 122.Fujihara K, Kotaki M, Ramakrishna S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials. 2005;26:4139–4147. doi: 10.1016/j.biomaterials.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 123.Fan HS, Wen XT, Tan YF, Wang R, Cao HD, Zhang XD. Compare of electrospinning PLA and PLA/b-TCP scaffold in vitro. Materials Science Forum. 2005;475–479:2379–2382. [Google Scholar]
- 124.Kim HW, Lee HH, Knowles JC. Electrospinning biomedical nanocomposite fibers of hydroxyapatite/poly(lactic acid) for bone regeneration. Journal Of Biomedical Materials Research Part A. 2006;79A:643–649. doi: 10.1002/jbm.a.30866. [DOI] [PubMed] [Google Scholar]
- 125.Kim HW, Song JH, Kim HE. Nanofiber generation of gelatin-hydroxyapatite biomimetics for guided tissue regeneration. Advanced Functional Materials. 2005;15:1988–1994. [Google Scholar]
- 126.Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, Shin JW. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 2004;26:1261–1270. doi: 10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 127.Mo XM, Weber HJ. Electrospinning P(LLA-CL) nanofiber: A tubular scaffold fabrication with circumferential alignment. Macromolecular Symposia. 2004;217:413–416. [Google Scholar]
- 128.Kameoka J, Orth R, Yang Y, Czaplewski D, Mathers R, Coates GW, Craighead HG. A scanning tip electrospinning source for deposition of oriented nanofibres. Nanotechnology. 2003;14:1124–1129. [Google Scholar]
- 129.Li D, Wang YL, Xia YN. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Letters. 2003;3:1167–1171. [Google Scholar]
- 130.Kakade MV, Givens S, Gardner K, Lee KH, Chase DB, Rabolt JF. Electric Field Induced Orientation of Polymer Chains in Macroscopically Aligned Electrospun Polymer Nanofibers. Journal of the American Chemical Society. 2007;129:2777–2782. doi: 10.1021/ja065043f. [DOI] [PubMed] [Google Scholar]
- 131.Theron A, Zussman E, Yarin AL. Electrostatic field-assisted alignment of electrospun nanofibres. Nanotechnology. 2001;12:384–390. [Google Scholar]
- 132.Zong XH, Ran SF, Fang DF, Hsiao BS, Chu B. Control of structure, morphology and property in electrospun poly (glycolide-co-lactide) non-woven membranes via post-draw treatments. Polymer. 2003;44:4959–4967. [Google Scholar]
- 133.Zong X, Bien H, Chung CY, Yin L, Fang D, Hsiao BS, Chu B, Entcheva E. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials. 2005;26:5330–5338. doi: 10.1016/j.biomaterials.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 134.Kidoaki S, Kwon IK, Matsuda T. Mesoscopic spatial designs of nano- and microfiber meshes for tissue-engineering matrix and scaffold based on newly devised multilayering and mixing electrospinning techniques. Biomaterials. 2004;26:37–46. doi: 10.1016/j.biomaterials.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 135.Layman JM, Kenawy ER, Watkins JR, Carr ME, Jr, Bowlin G, Wnek GE. Development of the biohemostat - a treatment modality for high pressure bleeding based on super-absorbent polymers and electrospun membranes, Polymer Preprints (American Chemical Society. Division of Polymer Chemistry) 2003;44:94–95. [Google Scholar]
- 136.Lee YH, Lee JH, An IG, Kim C, Lee DS, Lee YK, Nam JD. Electrospun dual-porosity structure and biodegradation morphology of Montmorillonite reinforced PLLA nanocomposite scaffolds. Biomaterials. 2005;26:3165–3172. doi: 10.1016/j.biomaterials.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 137.Sun ZC, Zussman E, Yarin AL, Wendorff JH, Greiner A. Compound core-shell polymer nanofibers by co-electrospinning. Advanced Materials. 2003;15:1929-+. [Google Scholar]
- 138.Yu JH, Fridrikh SV, Rutledge GC. Production of submicrometer diameter fibers by two-fluid electrospinning. Advanced Materials (Weinheim, Germany) 2004;16:1562–1566. [Google Scholar]
- 139.Zhang YZ, Huang ZM, Xu XJ, Lim CT, Ramakrishna S. Preparation of core-shell structured PCL-r-gelatin Bi-component nanofibers by coaxial electrospinning. Chemistry Of Materials. 2004;16:3406–3409. [Google Scholar]
- 140.Jiang H, Hu Y, Zhao P, Li Y, Zhu K. Modulation of protein release from biodegradable core-shell structured fibers prepared by coaxial electrospinning. Journal of Biomedical Materials Research, Part B: Applied Biomaterials. 2006;79B:50–57. doi: 10.1002/jbm.b.30510. [DOI] [PubMed] [Google Scholar]
- 141.Townsend-Nicholson A, Jayasinghe SN. Cell electrospinning: a unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules. 2006;7:3364–3369. doi: 10.1021/bm060649h. [DOI] [PubMed] [Google Scholar]
- 142.Chua KN, Lim WS, Zhang P, Lu H, Wen J, Ramakrishna S, Leong KW, Mao HQ. Stable immobilization of rat hepatocyte spheroids on galactosylated nanofiber scaffold. Biomaterials. 2005;26:2537–2547. doi: 10.1016/j.biomaterials.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 143.Lee IS, Kwon OH, Meng W, Kang IK, Ito Y. Nanofabrication of microbial polyester by electrospinning promotes cell attachment. Macromolecular Research. 2004;12:374–378. [Google Scholar]
- 144.Xie J, Hsieh YL. Ultra-high surface fibrous membranes from electrospinning of natural proteins: casein and lipase enzyme. Journal of Materials Science. 2003;38:2125–2133. [Google Scholar]
- 145.Zhang Y, Ouyang H, Lim Chwee T, Ramakrishna S, Huang Z-M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res B Appl Biomater. 2005;72:156–165. doi: 10.1002/jbm.b.30128. [DOI] [PubMed] [Google Scholar]
- 146.Casper CL, Yamaguchi N, Chase B, Rabolt JF, Kiick KL. Functionalized electrospun fibers for potential use in biomedical applications. Polymeric Materials: Science and Engineering. 2004;91:1030. [Google Scholar]
- 147.Jiang H, Fang D, Hsiao B, Chu B, Chen W. Preparation and characterization of ibuprofen-loaded poly(lactide-co-glycolide)/poly(ethylene glycol)-g-chitosan electrospun membranes. Journal of Biomaterials Science, Polymer Edition. 2004;15:279–296. doi: 10.1163/156856204322977184. [DOI] [PubMed] [Google Scholar]
- 148.Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, Hadjiargyrou M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. Journal of Controlled Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 149.Zong X, Li S, Chen E, Garlick B, Kim K-S, Fang D, Chiu J, Zimmerman T, Brathwaite C, Hsiao Benjamin S, Chu B. Prevention of post-surgery-induced abdominal adhesions by electrospun bioabsorbable nanofibrous poly(lactide-co-glycolide)-based membranes. Annals of surgery. 2004;240:910–915. doi: 10.1097/01.sla.0000143302.48223.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Melaiye A, Sun Z, Hindi K, Milsted A, Ely D, Reneker DH, Tessier CA, Youngs WJ. Silver(I)-Imidazole Cyclophane gem-Diol Complexes Encapsulated by Electrospun Tecophilic Nanofibers: Formation of Nanosilver Particles and Antimicrobial Activity. Journal of the American Chemical Society. 2005;127:2285–2291. doi: 10.1021/ja040226s. [DOI] [PubMed] [Google Scholar]