Abstract
This study tests the hypothesis that dysfunction of transient receptor potential vanilloid type 1 (TRPV1) channels occurs and contributes to the decrease in the glomerular filtration rate (GFR) and sodium/water excretion in Dahl salt-sensitive hypertensive rats. Recirculating Krebs-Henseleit buffer added with inulin was perfused at a constant flow in the isolated kidneys of Dahl salt-sensitive (DS) or Dahl salt-resistant (DR) rats fed a high salt (HS) or low salt (LS) diet for three weeks. Perfusion pressures (PP) were pre-adjusted to three levels (~100, ~150, ~190 mmHg) with or without phenylephrine. Capsaicin (Cap), a selective TRPV1 agonist, in the presence or absence of capsazepine (Capz), a selective TRPV1 antagonist, was perfused. Basal GFR, urine flow rate (UFR) and Na+ excretion (UNaV) were significantly lower in DS-HS than in DR-HS, DS-LS and DR-LS rats. Cap caused pressure-dependent decreases in PP and increases in GFR, UFR and UNaV in all groups, with less magnitude of decreases in PP and increases in GFR, UFR and UNaV in DS-HS than in DR-HS, DS-LS and DR-LS rats. Capz fully blocked the effect of Cap on PP, GFR, UFR and UNaV in all groups. Thus, these results show that TRPV1 function is impaired in the kidney of DS rats fed a high salt diet, which may contribute to the decrease in GFR and renal excretory function in DS rats in face of salt challenge.
Keywords: transient receptor potential vanilloid type 1 channel, Dahl salt-sensitive rats, glomerular filtration rate
Introduction
The Dahl salt-sensitive (DS) rat is a genetic model of hypertension and renal disease that exhibits many phenotypic characteristics in common with human hypertension (Campese, 1994; Cowley and Roman, 1996). Similar to traits observed in black hypertensive subjects, DS rats is a low-renin, sodium-sensitive form of hypertension that is associated with a progressive decline in renal function (Tobian et al, 1979; Rostand et al, 1982). The prominent defect of renal function is the blunted pressure-diuresis and natriuresis response, which has been found to be ascribable to the decreased glomerular filtration rate (GFR) (Maude and Kao-Lo, 1982; Roman, 1986) and increased sodium reabsorption (Maude and Kao-Lo, 1982) among other abnormalities. However, the molecular basis for the decrease in GFR and increase in sodium reabsorption in DS rats has not been fully defined. We have previously shown that function and expression of the transient receptor potential vanilloid type 1 (TRPV1) channels expressed in sensory nerves innervating the kidney were impaired in DS rats fed a high salt diet (Wang and Wang 2006). As a result, blockade of TRPV1 led to an increase in blood pressure in DR-HS but not DS-HS rats, indicating that TRPV1 plays a compensatory role in preventing increased salt sensitivity in DR rats and that dysfunction of TRPV1 renders a DR rats salt sensitive (Wang and Wang, 2006). These data also suggest that decreases in GFR and increases in sodium reabsorption in DS rats may be due to, at least in part, TRPV1 dysfunction.
TRPV1, also known as a capsaicin receptor, is a nonselective cation channel that is mainly expressed in capsaicin-sensitive sensory nerves containing calcitonin gene-related peptide (CGRP) and substance P (SP) (Caterina and Julius, 2001). TRPV1 is activated not only by capsaicin and endovanilloids but also by a variety of physical and chemical stimuli that present challenges to homeostasis (Caterina and Julius, 2001; Li et al, 2003; Li and Wang 2003). Activation of TRPV1 induces the release of neuropeptides including CGRP and SP from sensory nerve endings, which are potent vasodilators in most vascular beds (Tippins, 1986; Withrington, 1992; Zygmunt, 1999; Katki et al, 2001). Intravenous infusion of CGRP resulted in a marked increase in renal plasma flow as well as GFR in normal rats (Amuchastegui et al, 1994). The kidney is well innervated by capsaicin-sensitive sensory nerves containing CGRP and SP (Knight et al, 1987; Kurtz et al, 1998). Our recent study showed that activation of TRPV1 by capsaicin increased CGRP and SP release, GFR and sodium/water excretion in the isolated perfused kidney of Wistar rats, suggesting that TRPV1 expressed in the kidney plays a role in regulating GFR and sodium/water excretion (Li and Wang, 2006). Thus, we hypothesized that impaired function/expression of TRPV1 in the kidney may underlie decreased GFR and sodium/water excretion in DS hypertensive rats. To test the hypothesis, the isolated perfused kidney was used to avoid the confounding systemic effect of TRPV1 activation.
Methods
Animal treatment
All procedures involving animals in this study were in accordance with the Michigan State University Animal Care and Use Committee. Five week-old Dahl salt-sensitive (DS) and – resistant (DR) rats purchased from the Charles River Laboratory were given a high salt diet (8% NaCI) or low salt diet (0.3% NaCI) for three weeks.
Isolated perfused rat kidney preparation
At the end of three-week dietary treatment, the rats were anesthetized with inactin (80 mg/kg i.p.) plus ketamine (30 mg/kg i.m.). The right jugular vein was cannulated for intravenous infusion of 0.9% sodium chloride during surgery and right carotid artery was cannulated for measurement of mean arterial pressure (MAP). Following a midline incision of abdomen, the left ureter was cannulated (PE-10) for collection of urine. The abdominal aorta and inferior vena cava were isolated 0.3–0.5 cm proximally and 1.5 cm distally to the left renal artery and vein. All visible branches from the isolated aorta and vena cava were ligated except for the left renal artery and vein. After heparinization (1000 U/kg IV), a PE-90 catheter was inserted retrogradely into the distal vena cava toward the left renal vein and a PE-90 catheter connected to the perfusion setup was inserted retrogradely into the distal aorta toward the left renal artery.
The left kidney was perfused with warmed (37°C), oxygenated Krebs-Henseleit solution of the following composition (in mmol/L): NaCl 118, KCl 4.7, KH2PO4 1.19, MgSO4 1.19, CaCl2 1.9, NaHCO3 25, and glucose 5.5 (Pomposiello et al, 2003). Inulin (60 mg/dL) was added in the perfusion solution for measurement of GFR and sodium/water excretion (Poola et al, 2002). Indomethacin (10 µmol/L) was added in perfusate to inhibit cyclooxygenase. Immediately afterward, the aorta and vena cava were tied off just above the left renal artery and vein, and the kidney was removed and transferred to 37°C chamber containing the Krebs-Henseleit solution. Kidneys were perfused for 5 to 8 minutes on an open circuit to remove all traces of blood before being transferred to a recirculating unit with a capacity of 110 ml. Immediately after recirculation of perfusate had been established, the perfusion flow rate was adjusted to achieve a basal perfusion pressure of 95–100 mmHg. The established perfusion flow rate was maintained constant throughout the entire procedure of the experiment. After 10 minutes of equilibration, the perfusion pressure was elevated to 145–150 or 185–190 mmHg by addition of phenylephrine in perfusate (Pomposiello et al, 2003). The effects of capsaicin on renal function were determined at these different levels of perfusion pressure. The perfusion pressure was continuously measured with a pressure transducer and workbench software (Kent Scientific Corporation, Torrington, CT, USA).
Experiment 1. Pressure-related activation of TRPV1 by capsaicin
Activation of TRPV1 by capsaicin in the isolated kidney was performed at three levels of perfusion pressure. The basal perfusion pressure of 95–100 mmHg was established by adjusting the perfusion flow rate, and two higher levels of perfusion pressure of 145–150 and 185–190 mmHg were established by addition of phenylephrine into the perfusion solution without changing the perfusion flow rate. Each kidney was used for only one perfusion pressure. Once perfusion pressure was stable, vehicle or capsaicin (10 µmol/L) was added to perfusate. Our preliminary data showed that capsaicin decreased the perfusion pressure and increased the urine flow rate and these values reached the greatest points at about 1 minute and recovered at about 5 minutes after capsaicin perfusion. Thus, the urine samples were collected for 2 minutes starting thirty seconds after capsaicin or vehicle administration when preexisting urine in the tube was emptied. The perfusate sample was collected from the renal vein for 1 minute starting thirty seconds after capsaicin or vehicle perfusion. The perfusion pressure was continuously monitored.
Experiment 2: Blockade of TRPV1 with capsazepine
The effects of TRPV1 blockade by capsazepine were examined at the perfusion pressure of 185–190 mmHg. Once perfusion pressures were stable, vehicle, capsazepine (Calbiochem, San Diego, CA), or capsazepine plus capsaicin was perfused. The urine samples were collected for 2 minutes while the perfusate samples collected from the renal vein for 1 minute starting thirty seconds after vehicle, capsazepine, or capsazepine plus capsaicin administration. Capsazepine, a selective antagonist of TRPV1, was added (30 µmol/L) alone or in combination with capsaicin (10 µmol/L) that was added 2 minutes after capsazepine administration. Preliminary experiments showed that capsazepine (30 µmol/L) caused a slight increase in perfusion pressure, which returned to the baseline within 2 minutes. The perfusion pressure was continuously monitored.
Measurement of sodium concentrations in urine and perfusate
Urine and perfusate samples were centrifuged at 3,200 × g for 5 minutes. Urine or perfusate at 50 µl was diluted with deionized water (1:4) for determination of sodium concentrations. Urine or perfusate at 200 µl was used for determination of sodium concentrations, which were determined by the use of a flame photometer (IL943, Instrumentation Laboratories, Lexington, MA, USA).
Colorimetric assay of inulin
Inulin was determined in perfusate and urine samples by colorimetric analysis using anthrone complexation as described previously (Poola et al, 2002). Briefly, 30 µL of perfusate samples were added to 30 µL of glucose oxidase (2U/µL) in 1.5 mL micro-centrifuge tubes (for urine analysis, 10µL of samples were added to 10µL of glucose oxidase). The tubes were vortexed for 5 seconds and placed in a shaker water bath at 37°C for 15 minutes. The tubes were then cooled to ambient temperature (25°C) and the sample volume adjusted to 250µL with deionized water. A total of 250µL of 1% (w/v) aqueous zinc sulfate solution was then added, and the mixture vortexed for 15 seconds. The tubes were then centrifuged for 10 min at 9000× g. Aliquots of 350µL were then transferred to 15-mL test tubes in an ice bath. Ice-cold anthrone reagent at 4 ml was added and vortex-mixed for 30 seconds. The tubes were then incubated in a shaker water bath at 57°C for 10 minutes. During this period, samples exhibited differences in color (green) as a function of inulin concentrations. The test tubes were then placed in ice-cold water for 5 minutes to stop the reaction. Within 40 minutes after stopping the reaction, samples were analyzed by an UV/Vis spectrophotometer at a wavelength of 620 nm.
Calculation
Renal clearance (C) was calculated by the standard formulae: C = U V/P Where U is the urine concentration, V is the urine flow rate, P is the plasma concentration. Renal clearance was normalized by the wet weight of the kidney to take into account of potential differences in kidney mass due to 3 weeks of high salt intake.
Statistical analysis
Statistical comparisons were performed by ANOVA analysis of variance followed by the Tukey-Kramer multiple comparison tests and t-tests. All results were expressed as means ± SE. Differences were considered statistical significant at p<0.05.
Results
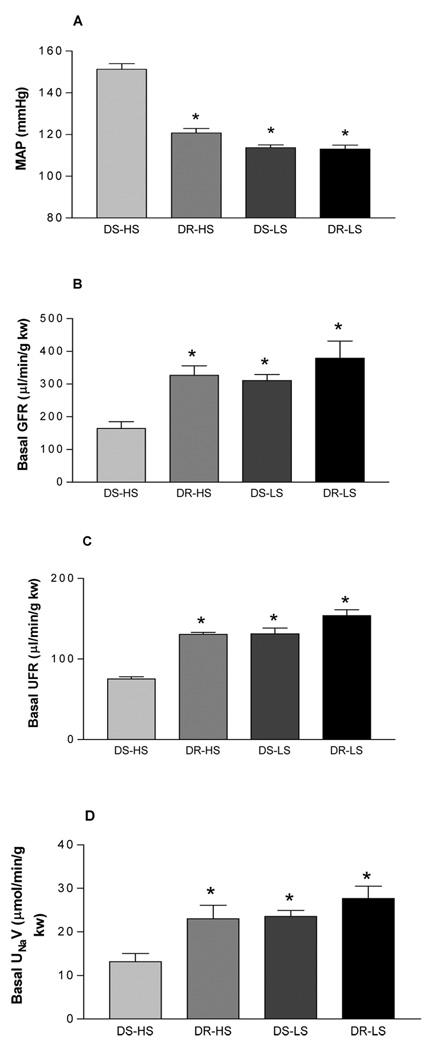
Although we have previously shown that TRPV1 protein expression in the kidney of DS-HS rats was decreased, it is unknown whether decreased TRPV1 confers a functional alternation of the kidney. The present study was designed to test the hypothesis that impaired function/expression of TRPV1 in the kidney may underlie decreased GFR and sodium/water excretion in DS rats. The isolated perfused kidney was used to avoid the confounding systemic effect of TRPV1 activation. We found that MAP was significant higher (p<0.001), and basal GFR, urine flow rate (UFR), and urine Na+ excretion (UNaV) were significant lower in DS-HS than in DR-HS, DS-LS and DR-LS rats (p<0.05) (Fig 1).
Figure 1.
Mean arterial pressure (MAP, panel A) in Dahl salt-sensitive rats fed a high salt (DS-HS) or a low salt diet (DS-LS) and Dahl salt-resistant rats fed a high salt (DR-HS) or a low salt (DR-LS) diet; basal glomerular filtration rate (GFR, panel B), urine flow rate (UFR, panel C), and sodium excretion (UNaV, panel D) in the isolated kidney perfused at a basal perfusion pressure of 95–100 mmHg. In panel A: n=16, *p<0.001 vs DS-HS. In panel B: n=5, *p<0.05 vs DS-HS. In panel C: n=5–10, *p<0.001 vs DS-HS. In panel D: n=5, *p<0.05 vs DS-HS.
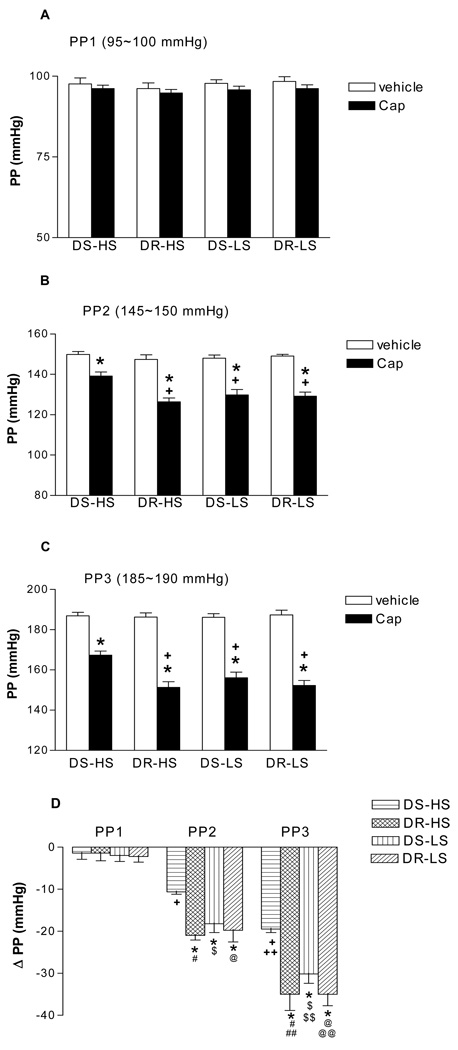
To control for different perfusion pressures in vivo between DS-HS, DS-NS, DR-HS, DR-NS kidneys, three independent perfusion pressures representing normal to high levels were used in all experimental kindeys, Capsaicin caused pressure-dependent decreases in perfusion pressure compared to vehicle in all groups, i.e., capsaicin was more potent when perfusion pressure was higher, indicating perfusion pressure may sensitize TRPV1 (Fig 2). The magnitude of the decrease in perfusion pressure induced by capsaicin was significant less in DS-HS than in DR-HS, DS-LS and DR-LS rats (p<0.05) (Fig 2). Given that the perfusion flow rate was constant throughout the entire procedure of the experiment for each individual kidney, these data indicate that capsaicin-induced decreases in renal vascular resistance, represented by decreases in perfusion pressure, were much less in DS-HS rats compared to other three groups of rats.
Figure 2.
The effects of capsaicin (Cap, 10µmol/L), a selective agonist of transient receptor potential vanilloid type 1 (TRPV1) channel, on renal perfusion pressure (PP) in the isolated kidneys of Dahl salt-sensitive rats fed a high salt (DS-HS) or a low salt (DS-LS) diet and Dahl salt-resistant rats fed a high salt (DR-HS) or a low salt (DR-LS) diet. The isolated kidneys were perfused at different perfusion pressures: PP1 = 95–100 mmHg (panel A), PP2 = 145–150 mmHg (panel B), PP3 = 185–190 mmHg (panel C). ΔPP representing the differences in PP between Cap and vehicle (panel D). N=5–6. In panel B: *p<0.01 vs corresponding vehicle, +p<0.05 vs DS-HS-Cap. In panel C: *p<0.001 vs corresponding vehicle, +p<0.01 vs DS-HS-Cap. In panel D: *p<0.05 vs corresponding DS-HS, + p<0.001 vs DS-HS(PP1), ++ p<0.001 vs DS-HS(PP2), # p<0.001 vs DR-HS(PP1), ## p<0.01 vs DR-HS(PP2), $ p<0.001 vs DS-LS(PP1), $$ p<0.01 vs DS-LS(PP2), @ p<0.001 vs DR-LS(PP1), @@ p<0.01 vs DR-LS(PP2).
Capsaicin caused pressure-dependent increases in GFR compared to vehicle in all groups indicating that increased perfusion pressure enhanced capsaicin-induced increases in GFR (Fig 3). Again, the magnitude of the increase in GFR induced by capsaicin was significant less in DS-HS than in DR-HS, DS-LS and DR-LS rats (p<0.05) (Fig 3). These results indicate capsaicin is less efficacious in elevating GFR in DS-HS rats compared to other three groups of rats.
Figure 3.
The effects of capsaicin (Cap, 10µmol/L), a selective agonist of transient receptor potential vanilloid type 1 (TRPV1) channel, on glomerular flow rate (GFR) in the isolated kidneys of Dahl salt-sensitive rats fed a high salt (DS-HS) or a low salt (DS-LS) diet and Dahl salt-resistant rats fed a high salt (DR-HS) or a low salt (DR-LS) diet. The isolated kidneys were perfused at different perfusion pressures: PP1 = 95–100 mmHg (panel A), PP2 = 145–150 mmHg (panel B), PP3 = 185–190 mmHg (panel C). ΔGFR representing the differences in GFR between Cap and vehicle (panel D). N=5. In panel A: *p<0.05 vs DS-HS-vehicle, + p<0.01 vs DS-HS-Cap. In panel B: *p<0.01 vs corresponding vehicle, +p<0.01 vs DS-HS-vehicle, # p<0.01 vs DS-HS-Cap. In panel C: *p<0.001 vs corresponding vehicle, +p<0.05 vs DS-HS-vehicle, # p<0.01 vs DS-HS-Cap. In panel D: *p<0.05 vs corresponding DS-HS, + p<0.05 vs DS-HS (PP1), # p<0.01 vs DR-HS (PP1), ## p<0.01 vs DR-HS (PP2), $ p<0.05 vs DS-LS (PP1), $$ p<0.001 vs DS-LS (PP2), @ p<0.001 vs DR-LS (PP1), @@ p<0.001 vs DR-LS (PP2).
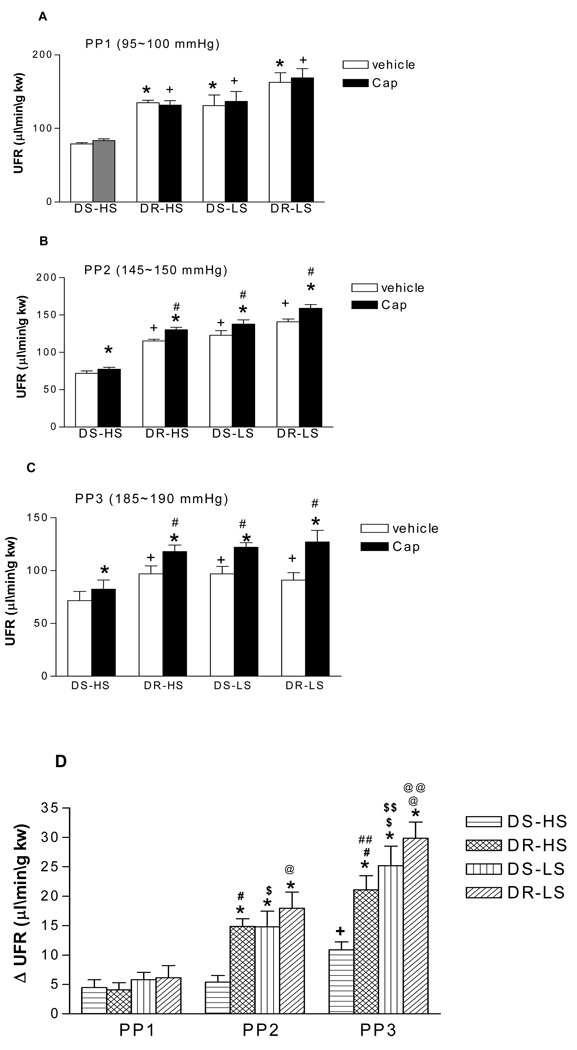
Capsaicin caused pressure-dependent increases in UFR compared to vehicle in all groups indicating that increased perfusion pressure enhanced capsaicin-induced increases in UFR (Fig 4). The magnitude of the increase in UFR induced by capsaicin was significant less in DS-HS than in DR-HS, DS-LS and DR-LS rats (p<0.05) (Fig 4). These data indicate that the diuresis of capsaicin is lower in DS-HS rats than in other three groups of rats.
Figure 4.
The effects of capsaicin (Cap, 10µmol/L), a selective agonist of transient receptor potential vanilloid type 1 (TRPV1) channel, on urine flow rate (UFR) in the isolated kidneys of Dahl salt-sensitive rats fed a high salt (DS-HS) or a low salt (DS-LS) diet and Dahl salt-resistant rats fed a high salt (DR-HS) or a low salt (DR-LS) diet. The isolated kidneys were perfused at different perfusion pressures: PP1 = 95–100 mmHg (panel A), PP2 = 145–150 mmHg (panel B), PP3 = 185–190 mmHg (panel C). ΔUFR representing the differences in UFR between Cap and vehicle (panel D). N=5. In panel A: *p<0.05 vs DS-HS-vehicle, + p<0.01 vs DS-HS-Cap. In panel B: *p<0.01 vs corresponding vehicle, +p<0.01 vs DS-HS-vehicle, # p<0.01 vs DS-HS-Cap. In panel C: *p<0.001 vs corresponding vehicle, +p<0.05 vs DS-HS-vehicle, # p<0.01 vs DS-HS-Cap. In panel D: *p<0.05 vs corresponding DS-HS, + p<0.05 vs DS-HS (PP1) and DS-HS (PP2), # p<0.01 vs DR-HS (PP1), ## p<0.05 vs DR-HS (PP2), $ p<0.05 vs DS-LS (PP1), $$ p<0.01 vs DS-LS (PP2), @ p<0.01 vs DR-LS (PP1), @@ p<0.01 vs DR-LS (PP2).
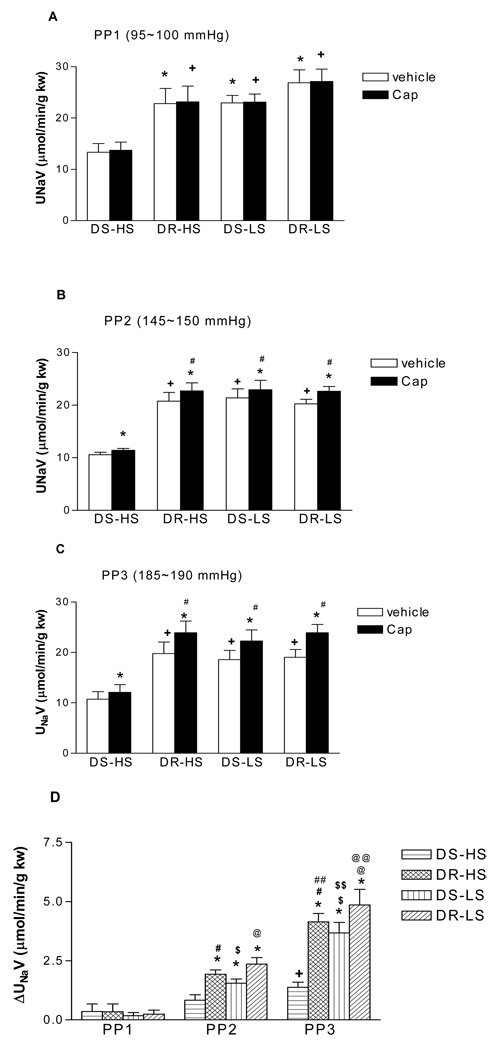
Capsaicin caused pressure-dependent increases in UNaV compared to vehicle in all groups indicating that increased perfusion pressure enhanced capsaicin-induced increases in UNaV (Fig 5). The magnitude of the increase in UNaV was significant less in DS-HS than in DR-HS, DS-LS and DR-LS rats (p<0.05) (Fig 5). These data indicate that the natriuresis of capsaicin is lower in DS-HS rats than in other three groups of rats.
Figure 5.
The effects of capsaicin (Cap, 10µmol/L), a selective agonist of transient receptor potential vanilloid type 1 (TRPV1) channel, on urine Na+ excretion (UNaV) in the isolated kidneys of Dahl salt-sensitive rats fed a high salt (DS-HS) or a low salt (DS-LS) diet and Dahl salt-resistant rats fed a high salt (DR-HS) or a low salt (DR-LS) diet. The isolated kidneys were perfused at different perfusion pressures: PP1 = 95–100 mmHg (panel A), PP2 = 145–150 mmHg (panel B), PP3 = 185–190 mmHg (panel C). ΔUNaV representing the differences in UNaV between Cap and vehicle (panel D). N=5. In panel A: *p<0.05 vs DS-HS-vehicle, + p<0.01 vs DS-HS-Cap. In panel B: *p<0.01 vs corresponding vehicle, +p<0.01 vs DS-HS-vehicle, # p<0.01 vs DS-HS-Cap. In panel C: *p<0.001 vs corresponding vehicle, +p<0.05 vs DS-HS-vehicle, # p<0.01 vs DS-HS-Cap. In panel D: *p<0.05 vs corresponding DS-HS, + p<0.05 vs DS-HS (PP1) and DS-HS (PP2), # p<0.01 vs DR-HS (PP1), ## p<0.05 vs DR-HS (PP2), $ p<0.05 vs DS-LS (PP1), $$ p<0.01 vs DS-LS (PP2), @ p<0.01 vs DR-LS (PP1), @@ p<0.01 vs DR-LS (PP2).
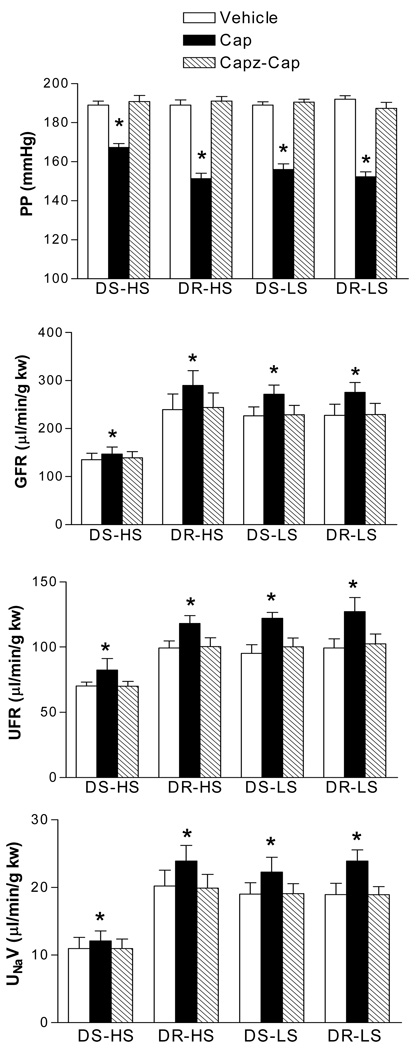
Capsazepine fully blocked the effects of capsaicin on perfusion pressure, GFR, UFR and UNaV in all groups (Fig 6), indicating that the capsaicin effect is TRPV1 specific.
Figure 6.
The blockade effects of capsazepine (Capz, 30 µmol/L), a selective antagonist of transient receptor potential vanilloid type 1 (TRPV1) channel on capsaicin (Cap, 10 µmol/L)-induced changes in perfusion pressure (PP, panel A), glomerular filtration rate (GFR, panel B), urine flow rate (UFR, panel C), and sodium excretion (UNaV, panel D) in the isolated kidneys of Dahl salt-sensitive rats fed a high salt (DS-HS) or a low salt (DS-LS) diet and Dahl salt-resistant rats fed a high salt (DR-HS) or a low salt (DR-LS) diet. The isolated kidneys were perfused at perfusion pressure of 185–190 mmHg. N=5. * p<0.01 vs corresponding vehicle and Capz-Cap.
At the basal perfusion pressure of 95–100 mmHg, capsaicin did not change perfusion pressure, GFR, UFR and UNaV compared to vehicles in all groups (Fig 2–5: A).
Discussion
The present study shows that (1) activation of TRPV1 by capsaicin, a selective TRPV1 agonist, caused pressure-dependent decreases in perfusion pressure (i.e. renal vascular resistance) and increases in GFR, UFR and UNaV in the kidney of both DS and DR rats, with a smaller magnitude of changes in these parameters in DS-HS compared to DR-HS, DS-LS and DR-LS rats; (2) capsazepine, a selective TRPV1 antagonist, fully blocked the effect of capsaicin on perfusion pressure, GFR, UFR and UNaV in all groups. The results indicated that, in the face of increased renal vascular resistance, activation of TRPV1 in the kidney caused renal vasodilatation, possibly mainly afferent arterials, leading to increases in GFR and diuresis/natriuresis in both DS and DR rats. These results suggest that TRPV1 plays a compensatory role protecting against the increase in renal vascular resistance. We have previously shown that TRPV1 protein expression in the kidney of DS-HS rats was decreased compared to that of DS-NS, DR-HS, and DR-NS rats (Wang and Wang, 2006), which may lead to impaired TRPV1-mediated vasodilatation and increases in renal excretory function. Indeed, the results from the present study show that TRPV1 activation by capsaicin led to attenuated responses of perfusion pressure, GFR, UFR and UNaV in the kidney of DS-HS rats, indicating that the protective effect of TRPV1 against the increase in renal vascular resistance was weakened probably due to impaired TRPV1 protein expression and therefore function in the kidney of DS-HS rats. These data support the notion that impaired TRPV1 protein expression and function in the kidney of DS-HS rats may contribute to increased salt sensitivity in DS rats.
The dose of capsaicin used was selected based on our previous study (Li et al, 2003), in which 100 µg/kg capsaicin given IV decreased blood pressure in WKY and SHR rats (assuming 100 µg/kg capsaicin resulting in approximately 10 µmol/L in circulation in rats weighing around 300g). This dose of capsaicin had no effect at lower perfusion pressure in the present study, but its effect on GFR, UFR and UNaV in all groups was significantly increased in parallel with increased perfusion pressure, albeit the magnitude of the increases in these parameters in DS-HS rats was significantly smaller than in that of DS-NS, DR-HS, and DR-NS rats. These results suggest that higher perfusion pressure may sensitize the renal response to capsaicin, possibly via altering local production of endovanilloid compounds. This notion was supported by a previous study showing that anandamide, an endogenous TRPV1 as well as cannabinoid CB1 receptor agonist, was present in the kidney where it exerted significant vasorelaxant and neuromodulatory effects (Deutsch et al 1997, Starowicz et al, 2007). Given that endovanilloid concentrations in the kidney under the physiological condition were much lower than that of capsaicin used, endogenous TRPV1 agonists may maintain an endovanilloid tone or potentiate TRPV1 effects when TRPV1 is activated by selective exogenous agonists. Ascertaining of this would require further future studies of TRPV1 activity combined with endovanilloid production/release at the lower and higher perfusion pressures as well as pathophysiological conditions such as salt loading.
Indeed, the present study was performed in isolated kidneys for which the nerves supplying the kidney have been cut. However, this surgical procedure would not change the facts that TRPV1 is expressed in sensory nerve terminals and that activation of TRPV1 in the tissues isolated from animals would activate sensory nerves (Wang et al, 2006). Moreover, the data obtained from the present study are consistent with our previous in vivo findings (Zhu et al, 2005). We have recently shown that capsaicin given unilaterally into the renal pelvis led to increases in urine flow rate and urinary sodium excretion bilaterally (Zhu et al, 2005). Furthermore, the TRPV1-induced sodium and water excretion appears to be mediated by increases in GFR but not by suppression of renal proximal and distal tubular reabsorption (Zhu et al, 2005). These results indicate that enhanced GFR may result from directly activation of TRPV1 in the kidney rather than as the result of autonomic reflex. Taken together, our data suggest a key role of TRPV1-mediated regulation of renal function in the maintenance of sodium and water homeostasis, which may justify for the future development of synthetic compounds targeting either TRPV1 receptors or the enzymes responsible for endovanilloid biosynthesis and degradation as potential new therapeutic drugs against renal function deterioration and hypertension.
Given that arachidonic acid metabolites of the cyclooxygenase pathway may sensitize TRPV1 (Planells-Cases et al, 2005), a cyclooxygenase inhibitor, indomethacin, was used in the perfusate to minimize TRPV1 interactions with vasoactive cyclooxygenase metabolites. The fact that capsaicin decreased perfusion pressure but increased GFR in the presence of cyclooxygenase inhibition indicate that capsaicin action was TRPV1-dependent. Indeed, regardless of the response to capsaicin under the vehicle condition, the falls in perfusion pressure and the increments in GFR, UFR and UNaV in response to capsaicin were significantly smaller in DS-HS compared to DS-NS, DR-HS, DR-NS rats (Figure 3D, 4D, 5D, 6D) at the higher perfusion pressure. Moreover, the fact that activation of TRPV1 decreased perfusion pressure but increased GFR while perfusion flow was constant indicates that TRPV1 activation may cause a greater vasodilation in afferent than efferent arterioles or constriction of efferent but minimally affecting afferent arterioles, leading to an increase in glomerular filtration pressure despite a fall in renal perfusion pressure. The heterogeneous effects on renal microvasculature induced by TRPV1 activation may play an important role in regulation of GFR and sodium/water excretion under physiological and pathological conditions. It is known that TRPV1 can be activated by a variety of physical and chemical stimuli that present challenges to homeostasis (Caterina and Julius, 2001; Li and Wang, 2003; Wang and Wang, 2006). Thus, TRPV1 may function as a molecular sensor to detect alterations in renal micro-environment and may respond with modification of GFR via its heterogeneous effects on renal microvasculature. If so, altered TRPV1 expression/activity in the kidney could impact sodium and water excretion and blood pressure. Indeed, our previous study showed that TRPV1 expression in the kidney of Wistar rats was upregulated by high salt intake, which may contribute to increased diuresis and natriuresis to prevent salt-induced increases in blood pressure (Li and Wang, 2003). In contrast, TRPV1 expression/function in the kidney of DS-HS rats was decreased by high salt intake, which may contribute to the development of hypertension in this model (Wang and Wang, 2006). These data indicate TRPV1 plays an important role in the regulation of renal function and blood pressure, and may serve as a target for defining pathogenesis of increased salt sensitivity of blood pressure.
Activation of TRPV1 induces vasodilatation in a variety of vascular beds, mainly via release of CGRP and SP from the sensory nerve endings (Li et al, 2003). We have shown in Wistar rats that the renal heterogeneous vasodilation and the increase in GFR induced by TRPV1 activation were the result of activation of CGRP and NK1 receptors following CGRP and SP release, respectively (Li and Wang, 2006), a result consisting with the finding that CGRP and SP exerted preferential endothelium-dependent vasodilation effect on afferent arterioles and thereby led to an increase in GFR (Cairns et al, 1991; Seikaly et al, 1992; Amuchastegui et al, 1994; Elhawary and Pang, 1995). We have shown TRPV1 and CGRP contents in perivascular sensory nerves were lower in DS-HS compared to DR-HS, DS-LS and DR-LS rats (Wang and Wang, 2006), suggesting that DS rat is genetically predisposed for salt-induced impairment of TRPV1 expression.
Pathological changes in the renal vascular structure in DS rats have been shown in previous studies (Ferguson and Bell, 1985; Hampton et al, 1989; Tomoda et al, 2000). Hypertrophic remodeling in preglomerular resistance vessels and structural defects in filtering in the glomeruli exist in prehypertensive DS rats (Tomoda et al, 2000), which provides a basis for the impairment of compensatory increases in GFR upon the circulatory plasma volume expanded by high salt intake. The structure defects may be exacerbated by high salt intake (Hampton et al, 1989), leading to decreased GFR. Thus, the decreased response of GFR to TRPV1 activation by capsaicin in DS-HS rat in the present study may be partially attributed to hypertrophic remodeling in preglomerular arteries. Alternatively, hypoperfusion due to initial perfusion at normal pressure for stabilizing the system may pose damage to DS-HS kidneys accustomed to high perfusion pressures in vivo in the present study. However, it is highly unlikely that a short period (10 min) of normal pressure perfusion would be sufficient for irreparable damage. Finally, the infusion of phenylephrine to increase perfusion pressure may influence signaling events up- or down-stream from TRPV1 such that effect of TRPV1 agonist is impaired. However, this is unlikely given that phenylephrine given to DS or DR rats fed a normal or high salt diet caused a similar degree of elevation in blood pressure while blockade of TRPV1 elevated blood pressure in DR but not DS rats fed a high salt diet (Wang and Wang 2006).
In conclusion, our data show that activation of TRPV1 in the isolated perfused kidney caused impaired regulation of renal hemodynamics and impaired increment of GFR and sodium/water excretion in DS-HS rats, suggesting the impaired TRPV1 expression/function in the kidney of DS-HS rats may contribute to dysfunction of the kidney and increased salt sensitivity in this genetic hypertension model.
Acknowledgments
This study was supported by National Institutes of Health of USA (grants HL-57853, HL-73287, and DK67620) and a grant from The Michigan Economic Development Corporation.
References
- Amuchastegui CS, Remuzzi G, Perico N. Calcitonin gene-related peptide reduces renal vascular resistance and modulates ET-1-induced vasoconstriction. AM J Physiol. 1994;267(5 Pt 2):F839–F844. doi: 10.1152/ajprenal.1994.267.5.F839. [DOI] [PubMed] [Google Scholar]
- Cairns HS, Rogerson ME, Westwick J, Neild GH. Regional heterogeneity of endothelium-dependent vasodilatation in the rabbit kidney. J Physiol. 1991;436:421, 9. doi: 10.1113/jphysiol.1991.sp018558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campese VM. Salt sensitivity in hypertension: renal and cardiovascular implications. Hypertension. 1994;23:531, 550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gate-way to the pain pathway. Annu Rev Neurosci. 2001;24:487, 517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Jr, Roman RJ. The role of the kidney in hypertension. JAMA. 1996;275:1581, 1589. [PubMed] [Google Scholar]
- Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HHO, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G, Moore LC. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538, 1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhawary AM, Pang CC. Renal vascular and tubular actions of calcitonin gene-related peptide: effect of NG-nitro-L-arginine methyl ester. J Pharmacol Exp Ther. 1995;273(1):56–63. [PubMed] [Google Scholar]
- Ferguson M, Bell C. Substance P-immunoreactive nerves in the rat kidney. Neurosci Lett. 1985;60(2):183–188. doi: 10.1016/0304-3940(85)90241-1. [DOI] [PubMed] [Google Scholar]
- Hampton JA, Bernardo DA, Khan NA, Lacher DA, Rapp JP, Gohara AF, Goldblatt PJ. Morphometric evaluation of the renal arterial system of Dahl salt-sensitive and salt-resistant rats on a high salt diet, II: interlobular arteries and intralobular arteries. Lab Invest. 1989;60:839, 846. [PubMed] [Google Scholar]
- Katki KA, Supowit SC, DiPette DJ. Role Of Calcitonin Gene-Related Peptide and Substance P in Dahl-Salt Hypertension. Hypertension. 2001;38(3):679–682. doi: 10.1161/hy09t1.095761. [DOI] [PubMed] [Google Scholar]
- Knight DS, Beal JA, Yuan ZP, Fournet TS. Substance P-immunoreactive nerves in the rat kidney. J Auton Nerv Syst. 1987;21(2–3):145–155. doi: 10.1016/0165-1838(87)90017-8. [DOI] [PubMed] [Google Scholar]
- Kurtz A, Muff R, Born W, Lundberg JM, Millberg BI, Gnadinger M, Uehlinger D, Weidmann P, Hokfelt T, Fischer JA. Calcitonin gene-related peptide is a stimulator of renin secretion. J Clin Invest. 1988;82:538, 543. doi: 10.1172/JCI113629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang DH. Function and regulation of the vanilloid receptor in rats fed a high salt diet. J Hypertens. 2003;21(8):1525–1530. doi: 10.1097/00004872-200308000-00017. [DOI] [PubMed] [Google Scholar]
- Li J, Kaminski NE, Wang DH. Anandamide-induced depressor effect in spontaneously hypertensive rats: role of the vanilloid receptor. Hypertension. 2003;41(3 Pt 2):757–762. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]
- Li J, Wang DH. Increased GFR and renal excretory function by activation of TRPV1 in the isolated perfused kidney (abstract) Hypertension. 2006;48(4):e60, 75. doi: 10.1016/j.phrs.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude DL, Kao-Lo G. Salt excretion and vascular resistance of perfused kidneys of Dahl rats. Hypertension. 1982;4:532, 537. doi: 10.1161/01.hyp.4.4.532. [DOI] [PubMed] [Google Scholar]
- Planells-Cases R, Garci` a-Sanz N, Morenilla-Palao C, Ferrer-Montiel A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch – Eur J Physiol. 2005;451:151, 159. doi: 10.1007/s00424-005-1423-5. [DOI] [PubMed] [Google Scholar]
- Pomposiello SI, Quilley J, Carroll MA, Falck JR, McGiff JC. 5,6– Epoxyeicosatrienoic Acid Mediates the Enhanced Renal Vasodilation to Arachidonic Acid in the SHR. Hypertension. 2003;42:548, 554. doi: 10.1161/01.HYP.0000090095.87899.36. [DOI] [PubMed] [Google Scholar]
- Poola NR, Bhuiyan D, Ortiz S, Savant IA, Sidhom M, Taft DR. A novel HPLC assay for pentamidine: comparative effects of creatinine and inulin on GFR estimation and pentamidine renal excretion in the isolated perfused rat kidney. J Pharm Pharmaceut Sci. 2002;5(2):135–145. [PubMed] [Google Scholar]
- Roman RJ. Abnormal renal hemodynamics and pressure-natriuresis relationship in Dahl salt-sensitive rats. Am J Physiol. 1986;251:F57, 65. doi: 10.1152/ajprenal.1986.251.1.F57. [DOI] [PubMed] [Google Scholar]
- Rostand GS, Kirk KA, Rutsky EA, Pate BA. Racial differences in the incidence of treatment for end-stage renal disease. N Engl J Med. 1982;306:1276, 1279. doi: 10.1056/NEJM198205273062106. [DOI] [PubMed] [Google Scholar]
- Seikaly MG, Eisner GM, Jose PA. Contrasting effect of substance P on renal function and dopamine excretion in hydropaenic and volume expanded dogs. J Auton Pharmacol. 1992;12(5):377–387. doi: 10.1111/j.1474-8673.1992.tb00386.x. [DOI] [PubMed] [Google Scholar]
- Spannow J, Thomsen K, Petersen JS, Haugan K, Christensen S. Influence of renal nerves and sodium balance on the acute antidiuretic effect of bendroflumethiazide in rats with diabetes insipidus. J Pharmacol Exp Ther. 1997;282(3):1152–1162. [PubMed] [Google Scholar]
- Starowicz K, Nigam S, Di Marzo V. Biochemistry and pharmacology of endovanilloids. Pharmacol Ther. 2007;114(1):13–33. doi: 10.1016/j.pharmthera.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Tippins JR. CGRP: a novel neuropeptide from the calcitonin gene is the most potent vasodilator known. J Hypertens. 1986;4(5):s102–s105. [PubMed] [Google Scholar]
- Tobian L, Lange J, Iwai J, Hiller K, Johnson MA, Goossens P. Prevention with thiazide of NaCl-induced hypertension in Dahl S rats. Hypertension. 1979;1:316, 323. doi: 10.1161/01.hyp.1.3.316. [DOI] [PubMed] [Google Scholar]
- Tomoda F, Takata M, Kinuno H, Tomita S, Yasumoto K, Inous H. Renal structural properties in prehypertensive Dahl salt-sensitive rats. Hypertension. 2000;36:68, 72. doi: 10.1161/01.hyp.36.1.68. [DOI] [PubMed] [Google Scholar]
- Wang LH, Luo M, Wang YP, Galligan JJ, Wang DH. Impaired vasodilation in response to perivascular nerve stimulation in mesenteric arteries of TRPV1-null mutant mice. J Hypertension. 2006;24(12):2399–2408. doi: 10.1097/01.hjh.0000251900.78051.56. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang DH. A novel mechanism contributing to development of Dahl saltsensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension. 2006;47(3):609–614. doi: 10.1161/01.HYP.0000197390.10412.c4. [DOI] [PubMed] [Google Scholar]
- Withrington PG. The actions of two sensory neuropeptides, substance P and calcitonin gene-related peptide, on the canine hepatic arterial and portal vascular beds. Br J Pharmacol. 1992;107(2):296–302. doi: 10.1111/j.1476-5381.1992.tb12741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wang YP, Wang DH. Natriuresis caused by activation of capsaicin-sensitive sensory nerves in renal pelvis of rats. Hypertension. 2005;46(4):992–997. doi: 10.1161/01.HYP.0000174603.27383.67. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wang DH. Segmental regulation of sodium and water excretion by a novel mechanism underlying activation of the vanilloid receptor in the kidney. Hypertension. 2005;46(5):860. [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452, 457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]