Abstract
While brainstem serotonergic (5-HT) systems are involved in the protective responses to hypoxia, abnormalities of 5-HT function are strongly implicated in SIDS, and the neurochemical mechanisms by which 5-HT receptors influence brainstem cardiorespiratory responses to hypoxia remains unclear. This study focuses on the role of excitatory neurotransmission, including 5-HT3 signaling, to cardiac vagal neurons (CVNs) that dominate the control of heart rate. Excitatory synaptic inputs to CVNs, located in the nucleus ambiguus (NA), were recorded simultaneously with respiratory activity in in-vitro brainstem slices. During control conditions excitatory inputs to CVNs were blocked by application of NMDA and AMPA/kainate glutamatergic receptor antagonists, while the 5-HT3 and purinergic receptor antagonists ondansetron and pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), respectively, had no effect. However, during hypoxia ondansetron inhibited excitatory neurotransmission to CVNs. In recovery from hypoxia, spontaneous and respiratory-related excitatory events were blocked by glutamatergic and purinergic receptor blockers, respectively, while ondancetron had no effect. These results demonstrate that hypoxia recruits a 5-HT pathway to CVNs that activates 5-HT3 receptors on CVNs to maintain parasympathetic cardiac activity during hypoxia. Exaggeration of this 5-HT neurotransmission could increase the incidence of bradycardia and risk of sudden infant death during hypoxia.
Episodes of apnea and bradycardia are common in infants who succumb to SIDS (1, 2). Although a specific cause in a majority of SIDS death is unknown, developmental abnormalities of serotonin (5-HT) function in the ventral medulla have recently been closely correlated with SIDS (3). These abnormalities involve multiple elements of 5-HT function including increased number of 5-HT neurons, reduction of 5-HT1A receptor binding, and relative reduction of 5-HT transporter function (4). In agreement with these findings the study of cerebrospinal fluids of SIDS victims showed a significant increase of the metabolites of 5-HT (5, 6). Medullary 5-HT abnormalities and enhanced 5-HT activity may result in exaggeration of responses to hypoxia including deleterious bradyarrhythmias.
Respiratory responses to hypoxia include initial an increase, followed by a decrease, in the respiratory frequency in most mammals (7, 8). Similarly, hypoxia evokes an initial increase in heart rate followed by a parasympathetically mediatedbradycardia and ultimately, cessation of cardiac contractions (9–11). This biphasic response to hypoxia is likely partly due to the biphasic increase followed by decrease in inhibitory GABAergic and glycinergic inputs to cardiac vagal neurons (CVNs) located within the nucleus ambiguus (NA) (12). However, the role of excitatory neurotransmission to CVNs in cardiorespiratory responses to hypoxia remains unclear.
Hypoxia evokes neurotransmitter release in the brainstem including 5-HT (13), ATP (14, 15), and glutamate (13). 5-HT neurons in the midline raphe (16, 17) and glutamatergic neurons in the retrotrapezoid nucleus (18) are postulated to be central neuronal chemoreceptors. In addition, purinergic neurons and receptors are likely involved in chemosensitivity of the ventral medullary surface (14).
Within the NA premotor neurons receive a high number of axosomatic 5-HT contacts, and the 5-HT contacts surrounding neurons in the NA are among the most dense in the brainstem (19). 5-HT fibers also specifically surround CVNs, which have been described as “ensheathed in 5-HT immunoreactive axonal boutons” (20). 5-HT may act on different receptor subtypes including 5-HT3 receptors, which have been shown to play an important role in cardiovascular regulation. Intravenous administration of 5-HT evokes a reflex bradycardia and hypotension via 5-HT3 receptor activation (21–23). In the nucleus tractus solitarii (NTS), activation of 5-HT3 receptors blocks the chemoreflex bradycardia and inhibits both baroreflex and Bezold –Jarisch reflex responses (24–27). In the dorsal vagal motor nucleus, another brainstem site that contains CVNs, activation of 5-HT3 receptors mediates excitation of CVNs (28, 29). Furthermore, 5-HT3 receptors are involved in physiological responses to hypoxia in the peripheral nerve system. Hypoxia elicits 5-HT secretion from intact neuroepithelial body cells, presumed airway chemoreceptors, via positive feedback activation of 5-HT3 autoreceptors (30). In the brainstem, 5-HT3 receptors mediate excitation of CVNs post hypoxia-hypercapnia (31). However, it remains unknown if 5-HT3 receptors are involved in the central cardio-respiratory responses evoked by hypoxia alone.
Here we studied the role of excitatory neurotransmission, including 5-HT3 signaling, to CVNs in central cardiorespiratory responses to hypoxia. More specifically, the relative role of 5-HT3, glutamate, and purinergic receptors were examined before, during, and inrecovery from hypoxia.
METHODS
To identify cardiac vagal neurons in vitro, a two-stage procedure was utilized. In an initial surgery, Sprague–Dawley rats (postnatal days 2–6; Hilltop, Scottdale, PA, USA) were anesthetized with hypothermia and received a right thoracotomy. 0.05 mL of 1–5% rhodamine (XRITC, Molecular Probes) was injected into the pericardial sac to retrogradely label CVNs. The location and identification of these neurons, particularly in juxtaposition to other cholinergic neurons in the NA, was previously described (32). Specificity of the cardiac vagal labeling was confirmed by the absence of any labeled neurons in the brainstem when rhodamine is injected either outside the pericardial sac or within the pericardial sac if the cardiac branch of the vagus nerve is sectioned (n = 4). Recent work demonstrated this method identifies cardiac vagal neurons localized in the external formation of the NA (32). In other control experiments (n = 10), i.v. injection of up to 10 mg of rhodamine failed to label any neurons in the medulla except for rare labeling of neurons in the area postrema, an area with a deficient blood–brain barrier. On the day of experiment (2–4 days later), the animals were anesthetized with isoflurane and sacrificed by rapid cervical dislocation. The brain was submerged in cold (4°C) buffer composed of 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 5 mM glucose, and 10 mM Hepes and continually gassed with 100% O2. A single slice of the medulla (800-μm thickness) that included CVNs, the rostral hypoglossal nucleus and rootlets and the pre-Botzinger complex was obtained and submerged in a recording chamber, which allowed perfusion (5–10 mL/min) of ACF at room temperature (24–25°C) containing 125 mM NaCl, 3 mM KCl, 2 mM CaCl2, 26 mM NaHCO3, 5 mM glucose, and 5 mM Hepes equilibrated with carbogen (95% O2 and 5% CO2, pH 7.4). All animal procedures were performed in compliance with the institutional guidelines at George Washington University and are in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association and the National Institutes of Health publication Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
The thick medullary slice preparation generates rhythmic inspiratory-related motor discharge in hypoglossal cranial nerves. Spontaneous inspiratory-related activity was recorded by monitoring motorneuron population activity from hypoglossal nerve rootlets using a suction electrode. Hypoglossal rootlet activity was amplified 50,000 times and filtered (10–300 HZ bandpass; CWE, Ardmore, PA, USA).
Individual CVNs in the NA were identified by the presence of the fluorescent tracer using a Zeiss Axioskop upright microscope (Carl Zeiss Inc., Thornwood, NY, USA) using a 40× water immersion objective. These identified CVNs were then imaged with differential interference contrast optics, infrared illumination, and infrared-sensitive video detection cameras to gain better spatial resolution. Patch pipettes (2.5–3.5 MΩ) were filled with a solution consisting of 135 mM K-gluconicacid, 10 mM HEPES, 10 mM ethylene glucol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraaceticacid (EGTA), 1 mM CaCl2, and 1 mM MgCl2, pH 7.35 and guided to the surface of individual CVNs. Voltage clamp whole-cell recordings were made at a holding potential of −80 mV with an Axopatch 200B and pClamp 8 software (Axon Instruments, Union City, CA, USA).
All drugs used in these experiments were applied using a pneumatic picopump pressure system (WPI, Sarasota, FL, USA). Drugs were focally released using a picrospritzer and pressure ejected from a patch pipette positioned within 30 μm of the patched CVN. The maximum range of drug application was determined previously to be 100–120 μm downstream from the drug pipette and was considerably less behind the drug pipette (33). Excitatory postsynaptic currents (EPSCs) were isolated by continuous focal application of strychnine (1 μM) and gabazine (25 μM) to block glycine and GABAergic receptors, respectively. Other drugs used included ondansetron (100 μM) to block 5-HT3 receptors, pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS, 100 μM) to block purinergic receptors; and finally d-2-amino-5-phosphonovalerate (AP-5, 50 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 50 μM) were used to block NMDA and AMPA/kainate glutamatergic neurotransmission, respectively. All drugs were purchased fromSigma-Aldrich (St. Louis, MO).
Rhythmic inspiratory-related activity and EPSCs of CVNs were recorded simultaneously for 10 min in control ACSF, equilibrated with 95% O2, and 5% CO2 (normoxia). Slices were then exposed for 10-min to hypoxia by changing control ACSF to ACSF equilibrated with 5% CO2, 20% O2, and 75% N2, and then slices were reoxygenated by returning the perfusate to initial control ACSF equilibrated with 95% O2, and 5% CO2 (posthypoxia). Only one experiment was conducted per preparation.
Synaptic events were detected using MiniAnalysis (version 5.6.12; Synaptosoft, Decatur, GA, USA). Threshold was set at root-mean-square noise multiplied by 5. The frequency of EPSCs that occurred in CVNs was grouped in 1-s bins and cross-correlated with onset of inspiratory-related hypoglossal activity. Data were analyzed from all bursts during the last 2 min of the control period, during the last 2 min of the hypoxia period, during the last 2 min of the 10-min posthypoxia period, and from minutes 6–8 during each 8-min drug regimen application period. Results were presented as means ± SEM and were statistically compared using Student’s t-test to examine spontaneous activity before and after drug application within a condition. One-way ANOVA with repeated measures and Dunnett’s posttest were utilized to examine the differences between spontaneous and respiratory related EPSCs within a condition. To examine time-dependent differences in response to various drug application periods the results were analyzed by two-ways ANOVA test with repeated measures, following by Bonferroni posttest. Significant differences for all data were set at P < 0.05.
RESULTS
Central cardiorespiratory responses to hypoxia
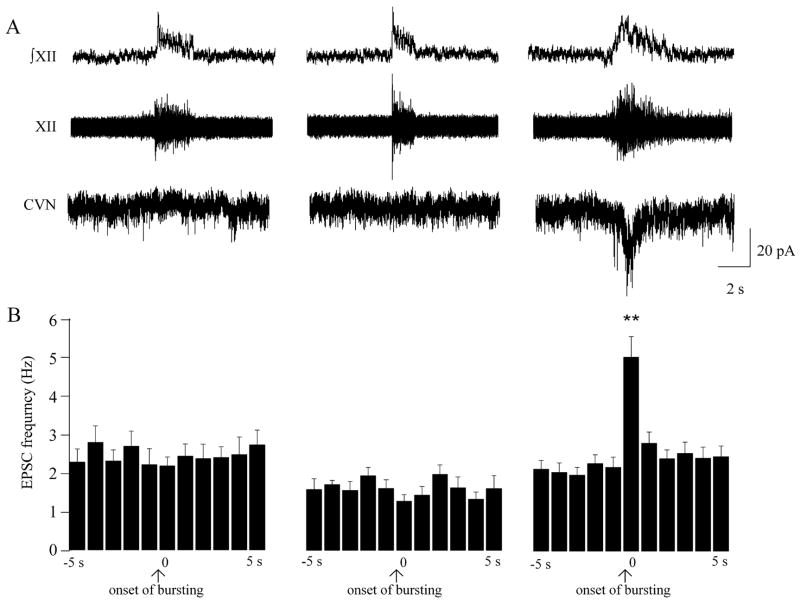
In agreement with previously published data (34), the frequency of excitatory neurotransmission to CVNs was not altered by respiratory activity under either normoxic or hypoxic conditions (spontaneous 2.3 ± 0.3 Hz, respiratory-related 2.2 ± 0.2 Hz, n = 9; P > 0.05, and spontaneous 1.6 ± 0.3 Hz, respiratory-related 1.3 ± 0.2 Hz, n = 9; P > 0.05, respectively, see Fig. 1). However, during recovery from hypoxia respiratory activity elicited a significant increase in the frequency of EPSCs from 2.1 ± 0.2 Hz to 5.0 ± 0.5 Hz that occurred at the onset of respiratory activity (n = 9; P < 0.01, see Fig. 1).
Figure 1.
Central cardiorespiratory responses to hypoxia. Respiratory-related bursting activity was recorded from the hypoglossal rootlet (XII; ∫XII – the integrated activity of the nerve rootlet) simultaneously with activity of fluorescently identified and patch clamped CVNs within the NA (here and in all subsequent figures). As shown in the left panel, under control conditions there was no excitatory respiratory-related inputs to CVNs. Changing the perfusate from ACSF, equilibrated with 95% O2, and 5% CO2 to ACSF equilibrated with 5% CO2, 20% O2, and 75% N2, did not alter excitatory neurotransmission to CVNs (middle panel). However, on recovery from hypoxia (right panel) the frequency of EPSCs was significant increased and correlated with the inspiratory burst activity as shown in a typical experiment (A) and in the summary data (n = 9, B). ** denotes P < 0.01, using one-way ANOVA with repeated measures.
Glutamatergic receptors, but neither purinergic nor 5-HT3 receptors, mediate excitation of CVNs under normoxic conditions
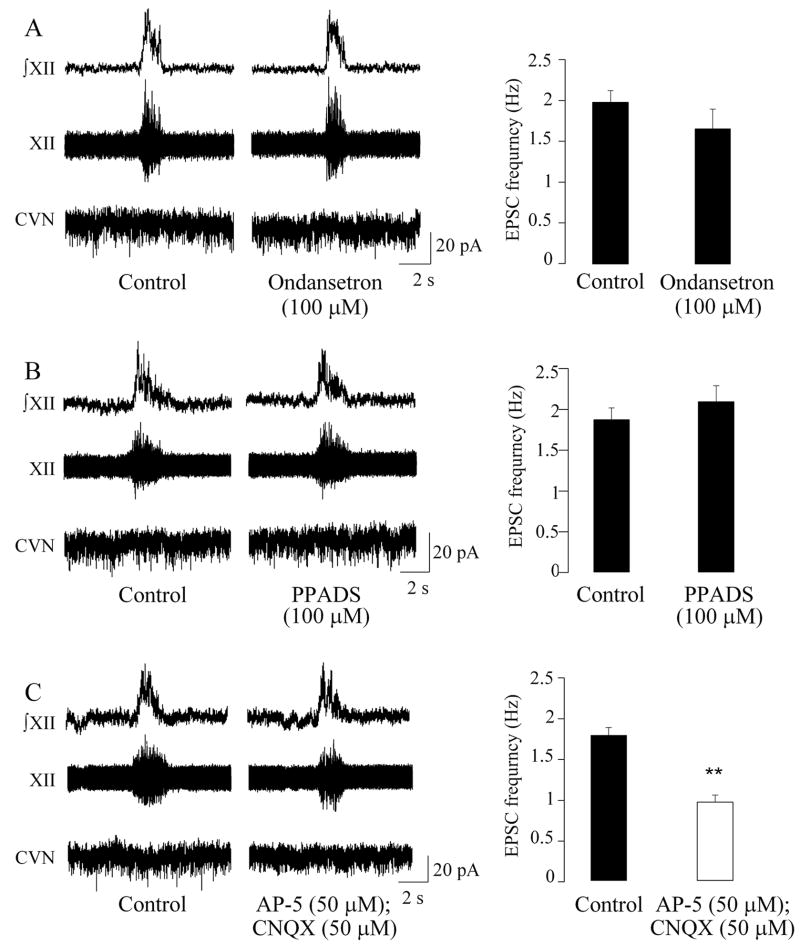
In agreement with previous studies (35), during control conditions neither the 5-HT3 antagonist ondansetron (100μM) nor the P2 receptor blocker PPADS (100μM) significantly altered the frequency of spontaneous EPSCs in CVNs (2.0 ± 0.1 Hz versus 1.7 ± 0.2 Hz, n = 7; P > 0.05, Fig. 2, A, and 1.9 ± 0.1 Hz versus 2.1 ± 0.2 Hz, n = 6; P > 0.05, Fig. 2, B, respectively). However, application of the NMDA and AMPA/kainate glutamatergic antagonists AP-5 (50μM) and CNQX (50μM), respectively, diminished the frequency of spontaneous EPSCs in CVNs from 1.8 ± 0.1 Hz to 1.0 ± 0.1 Hz (n = 6; P < 0.01, Fig 2, C), suggesting that under normal respiratory activity EPSCs in CVNs are primarily mediated by glutamatergic neurotransmission.
Figure 2.
Under normoxic conditions glutamatergic receptors primarily mediate excitation of CVNs. As shown in A, B, and C, excitatory neurotransmission to CVNs was not modulated by respiratory bursts. The frequency of spontaneous EPSCs was not significantly altered with application of either the 5-HT3 antagonist ondansetron (n = 7, A) or the purinergic receptor blocker PPADS (n = 6, B). However, application of the NMDA and non-NMDA glutamatergic antagonists AP-5 and CNQX, respectively, diminished the frequency of spontaneous EPSCs in CVNs (n = 6, C). Typical experiments are shown in the left, whereas the summary data are illustrated in the bar graphs on the right. ** denotes P < 0.01, using Student’s t-test. In this and all subsequent figures, unfilled bars indicate a statistically different response compared to the corresponded period during control conditions.
During hypoxia, in addition to glutamatergic receptors, 5-HT3 receptors participate in excitation of CVNs
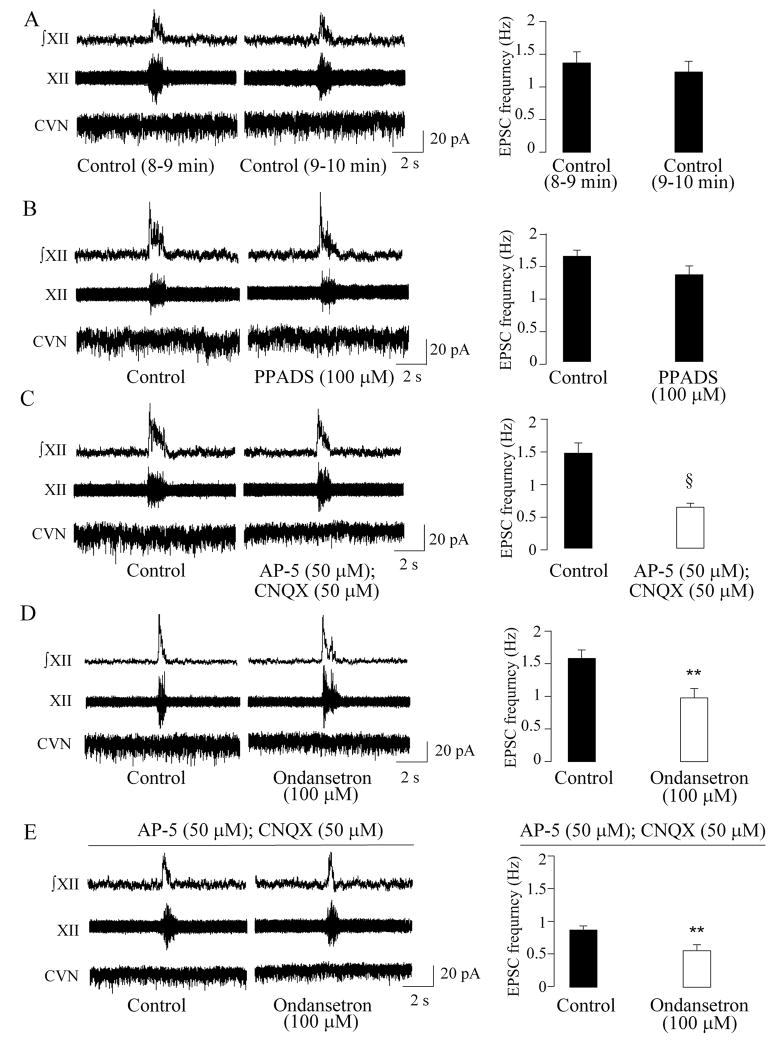
The frequency of EPSCs was not significantly altered within the last 2 minutes of a 10-min period of hypoxia (minutes 8–9: 1.4 ± 0.3 Hz versus minutes 9–10: 1.2 ± 0.2 Hz, n = 8, P > 0.05), Fig. 3, A. Application of PPADS (100μM) did not significantly alter the frequency of spontaneous EPSCs in CVNs (1.6 ± 0.1 Hz versus 1.3 ± 0.1 Hz, n = 11; P > 0.05, Fig. 2, B), suggesting purinergic neurotransmission is not involved in the excitation of CVNs during hypoxia. However, AP-5 (50μM) and CNQX (50μM) evoked a significant decrease in the EPSC frequency from 1.5 ± 0.2 Hz to 0.6 ± 0.1 Hz (n = 10, P < 0.001, Fig 3, C). Similarly, application of the 5-HT3 antagonist ondansetron (100μM) during hypoxia significantly decreased the EPSC frequency from 1.6 ± 0.1 Hz to 1.0 ± 0.1 Hz (n = 9, P < 0.01, Fig 3, D). This significant decrease in the EPSC frequency persisted in the presence of AP-5 (50μM) and CNQX (50μM), from 0.9 ± 0.1 Hz to 0.6 ± 0.1 Hz (n = 9, P < 0.01, Fig. 3, E).
Figure 3.
During hypoxia, both glutamatergic receptors and 5-HT3 receptors mediate excitation of CVNs. As shown in A, B, C, D, and E, excitatory neurotransmission to CVNs was not modulated by respiratory bursts under hypoxic respiration. The frequency of EPSCs was not significantly altered within the last 2 minutes of the 10-min period of hypoxia (n = 8, A). Application of PPADS did not significantly alter the frequency of spontaneous EPSCs (n = 11, B). However, application of AP-5 and CNQX evoked a significant decrease in the EPSC frequency (n =10, C). Similarly, application of the 5-HT3 antagonist ondansetron during hypoxia significantly decreased EPSC frequency (n = 9, D). This significant decrease in the EPSC frequency persisted in the presence of AP-5 and CNQX (n = 9, E). Typical experiments are shown in the left, whereas the summary data are illustrated in the bar graphs on the right. ** denotes P < 0.01, and § denotes P < 0.001 using Student’s t-test.
In recovery from hypoxia both glutamatergic and purinergic receptors mediate neurotransmission to CVN
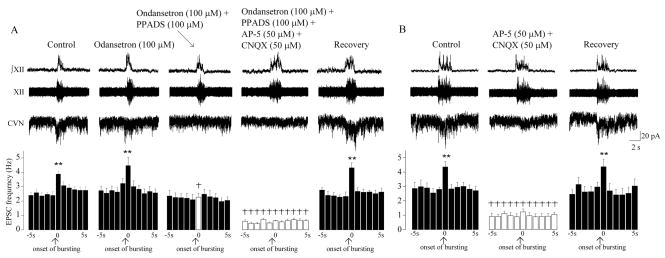
Unlike during hypoxia, in the recovery period application of ondansetron (100μM) did not significantly alter the spontaneous EPSC frequency (n = 7; P > 0.05, Fig. 4, A). Similarly, respiratory related excitatory neurotransmission to CVNs was not significantly changed by application of ondansetron (100 μM, 3.9 ± 0.2 Hz versus 4.5 ± 0.6 Hz, n = 7; P > 0.05, Fig. 4, A). However, in agreement with previous work, sequential addition of PPADS (100μM) blocked the respiratory-related increase in the EPSC frequency (from 3.9 ± 0.2 Hz to 2.3 ± 0.3 Hz, n = 7; P < 0.01, Fig 4, A), while spontaneous EPSCs were unchanged by PPADS (100 μM, n = 7; P > 0.05, Fig. 4, A). Subsequent addition of AP-5 (50μM) and CNQX (50 μM) significantly diminished spontaneous excitatory neurotransmission (n = 7; P < 0.01, Fig. 4, A). The effects of all drugs applied were reversible (see Fig. 4, A). In another set of experiments, AP-5 (50μM) and CNQX (50μM) were applied alone. AP-5 and CNQX reversibly blocked the frequency of both spontaneous and respiratory related EPSCs (n = 7, P < 0.01, Fig 4, B).
Figure 4.
During the recovery from hypoxia both glutamatergic and purinergic receptors mediate neurotransmission to CVN. During posthypoxia CVNs received inspiratory-related modulation of EPSCs (n = 7, ** denotes P < 0.01, using one-way ANOVA with repeated measures). Application of ondansetron did not significantly alter either spontaneous or respiratory-related EPSC frequency (n = 7, A). However, sequential addition of PPADS blocked the respiratory-related increase in the EPSC frequency, while spontaneous neurotransmission remained unchanged by PPADS (n = 7, A). Subsequent addition of AP-5 and CNQX significantly diminished spontaneous excitatory neurotransmission (n = 7, A). The effects of all drugs applied were reversible (n = 7, A). AP-5 and CNQX applied alone reversibly diminished the frequency of both spontaneous and respiratory related EPSCs (n = 7, B). † denotes P < 0.001, using two-ways ANOVA with repeated measures.
DISCUSSION
The main findings of this study are 1) during normal respiratory activity excitation of CVNs is primarily mediated by glutamatergic neurotransmission, while 5-HT3 and purinergic receptors are not involved in control of CVNs under normoxic conditions. 2) Hypoxia recruits a 5-HT pathway to CVNs that maintains spontaneous excitation of CVNs via activation of 5-HT3 receptors in CVNs, in addition to glutamatergic neurotransmission. 3) In recovery from hypoxia, CVNs continue to receive glutamatergic neurotransmission. In addition, purinergic receptor mediated signaling is recruited to excite CVNs during respiratory bursts, while 5-HT3 receptors are not involved in control of CVNs during the posthypoxia period.
The results of this study suggest excitatory glutamatergic signaling is involved in the control of CVNs under all conditions studied: normoxia, hypoxia, and recovery. However, hypoxia evokes a dramatic alteration in 5-HT system function within the brainstem. Hypoxia induces Fos-like immunoreactivity in 5-HT neurons in the nucleus raphe pallidus, the nucleus raphe magnus, and along the ventral medullary surface (36, 37). Within the ventral respiratory group, an area located close to CVNs, 5-HT levels significantly increased and reached their maximum during 9-min of hypoxia and then gradually declined posthypoxia (13). In agreement with these findings, in this study, we demonstrate that during either normal respiration, or in the recovery from hypoxia, excitation of CVNs is not mediated by 5-HT3 receptors. However, during the hypoxia challenge excitatory 5-HT neurotransmission to CVNs in the NA is recruited activating postsynaptic 5-HT3 receptors in CVNs. Other studies demonstrate an important recruitment of 5-HT pathways in response to hypoxia. 5-HT acting on 5-HT1A receptors in the nucleus raphe magnus plays no role under normal conditions but modulates breathing during hypoxia (38). In the anteroventral preoptic region, both 5-HT1A and 5-HT7 receptors are involved in the inhibitory modulation of the hypoxic ventilatory response (39). Activation of central 5-HT2A receptors is required to sustain hypoxic gasping and to restore respiratory activity during posthypoxia (40, 41). Central 5-HT2A receptors are also critical for long-term facilitation in respiratory activity followed by intermittent hypoxia (40, 42, 43).
Unlike during hypoxia, during recovery from hypoxia 5-HT3 receptors do not mediate excitation of CVNs, but rather excitation of CVNs during posthypoxia is mediated by purinergic and glutamatergic receptors. In agreement with this conclusion, within the ventral respiratory group adenosine 5′-triphosphate is released at low levels during hypoxia and adenosine 5′-triphosphate levels peak and remain elevated after termination of hypoxia (13). Thus, purinergic receptor activation is recruited to maintain excitation of CVNs during respiratory bursts posthypoxia. It is likely spontaneous EPSCs are mediated by glutamatergic receptors while purinergic receptors presynaptically facilitate glutamatergic receptor mediated respiratory-related excitation of CVNs posthypoxia.
While some results obtained in this study are similar other results are different from those obtained in previous work that tested the role of excitatory neurotransmission in central cardiorespiratory responses to hypoxia-hypercapnia (31). In both this and the previous study, there are no respiratory-related increases in EPSC frequency during the periods of either hypoxia or hypoxia-hypercapnia. Glutamatergic receptor mediated signaling is the major contributor to spontaneous EPSCs in CVNs during both hypoxia and hypoxia-hypercapnia. During recovery from both hypoxia and hypoxia-hypercapnia, respiratory-related purinergic receptor-mediated excitatory neurotransmission is recruited. However, there are some differences in the responses to hypoxia compared to those from hypoxia-hypercapnia. During hypoxia, 5-HT pathways are recruited to CVNs, but 5-HT pathways are not active posthypoxia. In contrast, 5-HT pathways do not mediate excitatory neurotransmission to CVNs during hypoxia-hypercapnia, but 5-HT3 receptors are involved in control of excitation of CVNs during recovery from hypoxia-hypercapnia.
Previous work (12) has demonstrated hypoxia-induced bradycardia likely also partly results from disinhibition of CVNs due to the decrease in inhibitory GABAergic and glycinergic inputs to CVNs. The results from this study suggest another central neurochemical mechanism may be involved in bradycardia induction during hypoxia. Here we demonstrated that hypoxia recruits excitatory 5-HT3 receptor mediated neurotransmission to maintain an excitation of CVNs during hypoxia. Therefore, a combination of two mechanisms: disinhibition of CVNs via withdrawal of GABAergic and glycinergic neurotransmission, and excitation of CVNs via activation of 5-HT3 receptors is likely important in the bradycardia evoked during hypoxia. The inhibition of GABAergic neurotransmission to CVNs during hypoxia may also be mediated by activation of 5-HT pathways and stimulation of presynaptic 5-HT receptors. In support of this hypothesis previous work has demonstrated 5-HT2B receptors exert an inhibitory action on GABAergic inputs to CVNs (44). In conclusion, this study demonstrated an essential role of 5-HT3 mediated excitation of CVNs during hypoxia. Exaggeration of 5-HT pathways may lead to altered cardiorespiratory responses to hypoxia including an exaggerated 5-HT3 receptor mediated excitatory neurotransmission or/and decreased inhibitory GABAergic neurotransmission to CVNs that may be responsible for the exaggerated bradycardia that occurs in infants that succumb to sudden infant death.
Acknowledgments
Supported by NIH grant HL 59895 and 49965 (D.M.)
Abbreviations
- 5-HT
serotonergic
- AP-5
d-2-amino-5-phosphonovalerate
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CVNs
cardiac vagal neurons
- EPSCs
excitatory postsynaptic currents
- NA
nucleus ambiguous
- NTS
nucleus tractus solitarii
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid
References
- 1.Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics. 1994;93:44–49. [PubMed] [Google Scholar]
- 2.Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res. 1999;45:350–354. doi: 10.1203/00006450-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Kinney HC, Filiano JJ, White WF. Medullary serotonergic network deficiency in the sudden infant death syndrome: review of a 15-year study of a single dataset. J Neuropathol Exp Neurol. 2001;60:228–247. doi: 10.1093/jnen/60.3.228. [DOI] [PubMed] [Google Scholar]
- 4.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 5.Caroff J, Girin E, Alix D, Cann-Moisan C, Sizun J, Barthelemy L. Neurotransmission and sudden infant death. Study of cerebrospinal fluid. C R Acad Sci III. 1992;314:451–454. [PubMed] [Google Scholar]
- 6.Cann-Moisan C, Girin E, Giroux JD, Le Bras P, Caroff J. Changes in cerebrospinal fluid monoamine metabolites, tryptophan, and gamma-aminobutyric acid during the 1st year of life in normal infants. comparison with victims of sudden infant death syndrome. Biol Neonate. 1999;75:152–159. doi: 10.1159/000014091. [DOI] [PubMed] [Google Scholar]
- 7.Guntheroth WG, Kawabori I. Hypoxic apnea and gasping. J Clin Invest. 1975;56:1371–1377. doi: 10.1172/JCI108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gozal D, Gozal E, Reeves SR, Lipton AJ. Gasping and autoresuscitation in the developing rat: effect of antecedent intermittent hypoxia. J Appl Physiol. 2002;92:1141–1144. doi: 10.1152/japplphysiol.00972.2001. [DOI] [PubMed] [Google Scholar]
- 9.Taylor EW, Butler PJ. Nervous control of heart rate: activity in the cardiac vagus of the dogfish. J Appl Physiol. 1982;53:1330–1335. doi: 10.1152/jappl.1982.53.6.1330. [DOI] [PubMed] [Google Scholar]
- 10.Schuen JN, Bamford OS, Carroll JL. The cardiorespiratory response to anoxia: normal development and the effect of nicotine. Respir Physiol. 1997;109:231–239. doi: 10.1016/s0034-5687(97)00052-2. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande P, Khurana A, Hansen P, Wilkins D, Thach BT. Failure of autoresuscitation in weanling mice: significance of cardiac glycogen and heart rate regulation. J Appl Physiol. 1999;87:203–210. doi: 10.1152/jappl.1999.87.1.203. [DOI] [PubMed] [Google Scholar]
- 12.Neff RA, Simmens SJ, Evans C, Mendelowitz D. Prenatal nicotine exposure alters central cardiorespiratory responses to hypoxia in rats: implications for sudden infant death syndrome. J Neurosci. 2004;24:9261–9268. doi: 10.1523/JNEUROSCI.1918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514:567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- 15.Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory response. J Neurosci. 2005;25:1211–1218. doi: 10.1523/JNEUROSCI.3763-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. discussion 266–259. [DOI] [PubMed] [Google Scholar]
- 17.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 18.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi Y, Kojima M, Matsuura T, Sano Y. Serotonergic innervation on the motoneurons in the mammalian brainstem. Light and electron microscopic immunohistochemistry. Anat Embryol (Berl) 1983;167:321–333. doi: 10.1007/BF00315670. [DOI] [PubMed] [Google Scholar]
- 20.Izzo PN, Deuchars J, Spyer KM. Localization of cardiac vagal preganglionic motoneurones in the rat: immunocytochemical evidence of synaptic inputs containing 5-hydroxytryptamine. J Comp Neurol. 1993;327:572–583. doi: 10.1002/cne.903270408. [DOI] [PubMed] [Google Scholar]
- 21.Cohen ML, Bloomquist W, Gidda JS, Lacefield W. Comparison of the 5-HT3 receptor antagonist properties of ICS 205–930, GR38032F and zacopride. J Pharmacol Exp Ther. 1989;248:197–201. [PubMed] [Google Scholar]
- 22.Malinowska B, Gothert M, Godlewski G, Wrobel B, Bonisch H, Buczko W. Inhibitory effect of ethanol on the 5-hydroxytryptamine-induced Bezold-Jarisch reflex--involvement of peripheral 5-HT3 receptors. Eur J Pharmacol. 1995;293:71–76. doi: 10.1016/0926-6917(95)90020-9. [DOI] [PubMed] [Google Scholar]
- 23.Meller ST, Lewis SJ, Brody MJ, Gebhart GF. Vagal afferent-mediated inhibition of a nociceptive reflex by i.v. serotonin in the rat. II. Role of 5-HT receptor subtypes. Brain Res. 1992;585:71–86. doi: 10.1016/0006-8993(92)91192-h. [DOI] [PubMed] [Google Scholar]
- 24.Merahi N, Orer HS, Laporte AM, Gozlan H, Hamon M, Laguzzi R. Baroreceptor reflex inhibition induced by the stimulation of serotonin3 receptors in the nucleus tractus solitarius of the rat. Neuroscience. 1992;46:91–100. doi: 10.1016/0306-4522(92)90011-p. [DOI] [PubMed] [Google Scholar]
- 25.Leal DM, Callera JC, Bonagamba LG, Nosjean A, Laguzzi R, Machado BH. Microinjection of a 5-HT3 receptor agonist into the NTS of awake rats inhibits the bradycardic response to activation of the von Bezold-Jarisch reflex. Brain Res Bull. 2001;54:7–11. doi: 10.1016/s0361-9230(00)00408-1. [DOI] [PubMed] [Google Scholar]
- 26.Sevoz C, Nosjean A, Callera JC, Machado B, Hamon M, Laguzzi R. Stimulation of 5-HT3 receptors in the NTS inhibits the cardiac Bezold-Jarisch reflex response. Am J Physiol. 1996;271:H80–H87. doi: 10.1152/ajpheart.1996.271.1.H80. [DOI] [PubMed] [Google Scholar]
- 27.Sevoz C, Callera JC, Machado BH, Hamon M, Laguzzi R. Role of serotonin3 receptors in the nucleus tractus solitarii on the carotid chemoreflex. Am J Physiol. 1997;272:H1250–H1259. doi: 10.1152/ajpheart.1997.272.3.H1250. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Ramage AG, Jordan D. Mediation by 5-HT3 receptors of an excitatory effect of 5-HT on dorsal vagal preganglionic neurones in anaesthetized rats: an ionophoretic study. Br J Pharmacol. 1996;118:1697–1704. doi: 10.1111/j.1476-5381.1996.tb15594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Ramage AG, Jordan D. Presynaptic 5-HT3 receptors evoke an excitatory response in dorsal vagal preganglionic neurones in anaesthetized rats. J Physiol. 1998;509:683–694. doi: 10.1111/j.1469-7793.1998.683bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu XW, Nurse CA, Wong V, Cutz E. Hypoxia-induced secretion of serotonin from intact pulmonary neuroepithelial bodies in neonatal rabbit. J Physiol. 2002;539:503–510. doi: 10.1113/jphysiol.2001.013071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamendi HW, Cheng Q, Dergacheva O, Frank JG, Gorini C, Jameson HS, Pinol RA, Wang X, Mendelowitz D. Recruitment of excitatory serotonergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus post hypoxia and hypercapnia. J Neurophysiol. 2008;99:1163–1168. doi: 10.1152/jn.01178.2007. [DOI] [PubMed] [Google Scholar]
- 32.Bouairi E, Kamendi H, Wang X, Gorini C, Mendelowitz D. Multiple types of GABAA receptors mediate inhibition in brain stem parasympathetic cardiac neurons in the nucleus ambiguus. J Neurophysiol. 2006;96:3266–3272. doi: 10.1152/jn.00590.2006. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann N Y Acad Sci. 2001;940:237–246. doi: 10.1111/j.1749-6632.2001.tb03680.x. [DOI] [PubMed] [Google Scholar]
- 34.Evans C, Wang J, Neff R, Mendelowitz D. Hypoxia recruits a respiratory-related excitatory pathway to brainstem premotor cardiac vagal neurons in animals exposed to prenatal nicotine. Neuroscience. 2005;133:1073–1079. doi: 10.1016/j.neuroscience.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 35.Dergacheva O, Wang X, Kamendi H, Cheng Q, Pinol RM, Jameson H, Gorini C, Mendelowitz D. 5HT2 receptor activation facilitates P2X receptor mediated excitatory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuropharmacology. 2008;54:1095–1102. doi: 10.1016/j.neuropharm.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- 37.Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Nucci TB, Branco LG, Gargaglioni LH. 5-HT1A, but not 5-HT2 and 5-HT7, receptors in the nucleus raphe magnus modulate hypoxia-induced hyperpnoea. Acta Physiol (Oxf) 2008;193:403–414. doi: 10.1111/j.1748-1716.2008.01853.x. [DOI] [PubMed] [Google Scholar]
- 39.Gargaglioni LH, Bicego KC, Nucci TB, Branco LG. Serotoninergic receptors in the anteroventral preoptic region modulate the hypoxic ventilatory response. Respir Physiol Neurobiol. 2006;153:1–13. doi: 10.1016/j.resp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.St-John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of {alpha}-1 adrenergic receptors and serotonin 5HT2 receptors. J Appl Physiol. 2007;104:665–673. doi: 10.1152/japplphysiol.00599.2007. [DOI] [PubMed] [Google Scholar]
- 42.Fuller DD, Zabka AG, Baker TL, Mitchell GS. Physiological and Genomic Consequences of Intermittent Hypoxia. Selected Contribution: Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- 43.McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R334–R341. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- 44.Dergacheva O, Griffioen KJ, Wang X, Kamendi H, Gorini C, Mendelowitz D. 5-HT(2) receptor subtypes mediate different long-term changes in GABAergic activity to parasympathetic cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2007;149:696–705. doi: 10.1016/j.neuroscience.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]