Abstract
Objective
Human fetal membranes (FM) at term have been shown to contain a weak zone in the region overlaying the cervix which exhibits characteristics of increased collagen remodeling and apoptosis. It has been hypothesized that the FM rupture initiation site is within this weak zone. Although the FM weak zone has been partially characterized, it is unclear what structural differences in the extracellular matrix result in its decreased rupture strength. A screen for differentially expressed proteins in the amnion of the weak zone versus other FM areas demonstrated that fibulin 1 was decreased. We investigated potential regional differences in all fibulin protein family members.
Methods
FM fibulins were localized by immunohistochemistry. Detected fibulins were screened by Western Blot for differences in abundance in the amnion of the weak zone versus non-weak zone FM regions. Amnion epithelial and mesenchymal cells were also screened for fibulin production.
Results
Fibulin 1 and 5 were detected in the cytoplasm of and in a pericellular pattern surrounding all FM cells, and in a dense extracellular pattern in the amniotic compact zone. Fibulin 3 was detected within the cytoplasm of amnion epithelial and chorion trophoblast cells. Fibulins 2 and 4 were not detected. Fibulins 1, 3 and 5 demonstrated decreased abundance of 33%, 63% and 58% (all P<0.01) in amnion of the weak zone relative to other FM regions. Amnion cells produced all three detected fibulins. Furthermore, TNF inhibited amnion cell fibulin production in a dose dependent manner.
Conclusion
Fibulins 1, 3 and 5 were localized coincident with major microfibrillar networks in amnion. Each showed decreased abundance in the amnion component of the FM weak zone. Amnion epithelial and mesenchymal cells produced all three fibulins and their abundance was inhibited by TNF. We speculate that the amnion microfibrillar layer undergoes significant remodeling with the development of the FM weak zone.
Introduction
Untimely rupture of the fetal membranes (FM), the amnion and choriodecidua, is a major cause of preterm birth and results in significant infant mortality and morbidity (1). The physiological mechanisms which normally lead the FM to weaken and fail prior to birth are not known. Conventional thinking that FM rupture is precipitated by the stress of uterine contractions during labor fails to explain the 10% of term deliveries and 40% of preterm deliveries in which FM rupture is the sentinel event, preceding any uterine contractions (2–3). Recent studies from several laboratories indicate that the FM undergo a genetically-programmed, biochemically-mediated, maturation process, near term, which is characterized by collagen remodeling and apoptosis (4–5). In human FM, in contrast to rat membranes, these changes are more limited to the region of the FM overlying the cervix (6). In a series of publications, our group has demonstrated that human FM have a zone of physical weakness (decreased force and energy required to rupture relative to the other areas of the same FM) overlying the cervical opening of the uterus (7, 8). We have further demonstrated that this same weak zone is characterized by specific markers of increased collagen remodeling and apoptosis. These regional characteristics develop prior to the onset of contractions of labor and persist until delivery (7, 8). Furthermore, the rupture tear line of the FM transects this weak zone and thus the rupture process is hypothesized to initiate in this weak zone (5).
A proteomics approach was utilized to investigate how differences between the biomechanical properties of the FM weak zone and that of the remaining stronger FM areas are reflected in their extra-cellular matrix proteins. Amnion alone, rather than full thickness FM was utilized for the proteomics analysis because it is the strongest FM component (9). Further, use of amnion alone eliminated a technical problem: the variable amount of decidua attached to the choriodecidua in different regions of the FM would have distorted the analysis. The initial proteomics 2D-DIGE screening demonstrated differences in fibulin 1 protein abundance between the weak zone of the FM and the remaining areas. Potential regional differences in all fibulin protein family members were investigated.
The fibulins are a family of seven secreted extracellular proteins defined by two structural features: calcium binding epidermal growth factor (EGF) like modules and a unique C-terminal fibulin module. Fibulins are classified into two subgroups. The first subgroup, including fibulin 1 and fibulin 2, consists of larger proteins containing an extra domain with 3 anaphylatoxin modules and additional EGF-like modules. These proteins are often expressed in the basement membrane between epithelium and mesenchyme. They both bind fibronectin, proteoglycans, tropoelastin and other elastic fiber and basement membrane proteins. Fibulins in the second subgroup are smaller and relatively homogeneous (10–11). Fibulins 6 and 7 are very recently described and their functions are relatively unknown (12–14). Fibulins 3, 4, and 5 are all associated with abnormalities of development in knock-out mice, and with disease in humans, including macular degeneration and cutis laxa syndrome (15–18). Fibulin 5 is markedly up-regulated after vascular injury, wound healing, and in the vaginal wall post-partum (19–21). All of the proteins in this family are involved in making bridges in the ECM resulting in supramolecular structures (22, 23), thus this protein family is clearly of potential interest in the remodeling process that weakens FM prior to rupture. There have been no previous publications describing any fibulin family members in amnion. Our objective was to immunohistologically localize fibulin family members in amnion and determine their abundance relative to the para-cervical weak zone.
Methods
Reagents were obtained from Sigma Chemical Company, St. Louis, MO unless stated otherwise.
Tissue procurement
The study protocol was approved by the Institutional Review Board of the MetroHealth Medical Center, Case Western Reserve University, Cleveland, Ohio. FM were collected from patients after normal, unlabored, term (37–38 weeks), elective caesarian section (FM from 16 placentas used for all experiments). Prior to removal of the placenta and FM from the patient, the area overlying the cervix was marked with gentian violet by the Obstetrician (7, 8).
Membrane cutting procedure and the production of a FM map
The placenta and its membranes were placed on a specially designed cutting board (1m × 1m) covered with white paper. After identifying the area overlying the cervix (i.e., the marked area), it was used as a reference point to lay out the FM. Each FM was systematically and completely sectioned into fragments utilizing our previously reported procedure. Those FM fragments from within a five centimeter radius of the marked region were considered part of the para-cervical weak zone; FM fragments outside the weak zone are referred to as other areas or remaining FM (7, 8). Full thickness FM fragments were washed briefly in Hank’s Balanced Salt Solution (HBSS; pH 7.4) and then kept moist throughout strength testing.
Determination of physical properties
FM physical properties were determined by American Society for Testing and Materials standards using modified industrial rupture testing equipment (Com-Ten Industries, St. Petersburg, FL) as previously reported (7–10). Membranes were supported within a 2.5-cm diameter fixture. A motor driven, 1-cm-diameter spherical plunger was forced perpendicular to the membrane surface at a speed of 8.4 cm/min. Displacement of the membrane and the resultant force were collected continuously and analyzed by data reduction software. Rupture forces were determined from the generated force/displacement curves.
Selection of FM fragments for Proteomic study
FM form 4 patient’s placentas were used for the proteomic studies. FM were cut into fragments as above and strength tested. Tissue fragments were divided into three groups according to rupture force (Rupture force < 4.45 N, ≥ 4.45 N and < 8.9 N, and ≥ 8.9 N). FM in the weakest group were all from the para-cervical weak zone as defined above, while those in the strongest group were all from other areas of the FM. Fragments from the intermediate strength group were discarded. The amnion component was manually separated from each of the tested full thickness FM fragments and snap frozen in liquid nitrogen.
Protein extraction and fluorescence dye labeling
Frozen amnion fragments from each strength group were combined and pulverized using a liquid nitrogen cooled stainless-steel mortar and pestle. Amnion powders were homogenized in two volumes 30mM TRIS, 7M urea, 2M thiourea and 4% CHAPS containing 1X Focus-protease inhibitor cocktail (EMD Biosciences, La Jolla, CA) for 30s on ice using a Tekmar Tissuemizer (setting 60). Homogenized samples were allowed to stand for 10m on ice and then frozen at −70°C overnight. Samples were then thawed on ice and the homogenization-freeze/thaw protocol was repeated two additional times. Tissue lysates were clarified by centrifugation at 15000 × g/15min/10°C and the supernatants were frozen at −70°C until proteomics analysis.
Sample preparation for the proteomics analysis was conducted as previously described (24). Briefly, the samples were thawed from −70°C to ice temperature, cleaned, and protein concentrations were determined utilizing the RC/DC protein assay kit (Bio-Rad, Hercules, CA) per the Manufacturer’s instructions. Aliquots, each with 25 μg protein, were collected from the weak (para-cervical weak zone) and strong samples and pooled to prepare internal standard samples. Thus, samples from either weak or strong amnion membrane were labeled with Cy3 or Cy5 cyanine dyes while internal standard sample was labeled with Cy2 dye by the addition of 400 pmol of Cy dye in 1 μL of anhydrous N,N dimethylformamide per 50 μg of protein for 30 min and then quenched with 10 mM lysine and additionally incubated for 10 min. A dye-swapping scheme, as shown in Table 1, was used such that the four samples for any condition were variously labeled with Cy3 or Cy5 to control for any dye-specific labeling artifacts.
Table 1.
Experimental design for 2D-DIGE proteome profiling. Four biological replicate samples for each group (SM; strong membrane, WM; weak membrane) were used and labeled with Cy3 or Cy5. Each gel contained the pooled standard (Pooled Std; equal aliquots of all the samples in the two groups) and two other subject samples. Thus, the eight samples were analyzed in triplicate by running 4 gels. (For detailed descriptions refer to Methods).
| Gel | Cy3 | Cy5 | Cy2 |
|---|---|---|---|
| 1 | SM_1 | WM_2 | Pooled internal standard sample (SM_1,3,5,7 + WM_2,4,6,8) |
| 2 | WM_4 | SM_3 | Pooled internal standard sample (SM_1,3,5,7 + WM_2,4,6,8) |
| 3 | SM_5 | WM_6 | Pooled internal standard sample (SM_1,3,5,7 + WM_2,4,6,8) |
| 4 | WM_7 | SM_8 | Pooled internal standard sample (SM_1,3,5,7 + WM_2,4,6,8) |
Gel Electrophoresis
The quenched Cy3 and Cy5-labeled samples, to be partitioned in the same gel according to the experimental design in Table 1, were then combined and mixed with an aliquot of Cy2-labeled standard and an equal volume of 2X sample buffer (8 M urea, 4% w/v CHAPS, 2% w/v DTT, 2% v/v Pharmalytes 3–10 NL) was added. The mixed samples were then partitioned according to their isoelectric point (pI) and then molecular weight in two dimensional gels as previously described (24).
Image acquisition and spot quantification
Gel images acquisition and spot quantification were carried out as described previously (24). Briefly, a total of 12 gel images consisting of four biological replicate images from the weak zone, four replicates from the strong zone, and four technical replicates from the internal standards were used for spot quantification. The pick gel image was also processed with the rest of the gel images as a pick gel image but not included in the analysis. In order to compare protein spots across the four gels, image analyses were conducted in two steps using DeCyder v6.5 2D Differential Analysis Software (GE Healthcare). In the first step, the set of three images from a single gel was loaded in the DIA (Differential in-gel analysis) algorithm within the DeCyder software. In this module, intra-gel spot detection and quantification were performed. For the subsequent inter-gel differential analysis, the DIA workspaces for all the gels were saved and loaded into the BVA (Biological variation analysis). Once the spots from the common standards were matched across the four gel images and with the pick gel image, the standardized volume ratio for each standard image from the different gels was set to the value 1.0 in order to compare ratios between matched protein spots in the different gels (groups). Thus, the ratios of the log standardized protein spot abundances (differences in expression) between the groups were computed. A total of 28 Spots with 1.2 and above or −1.2 below fold changes with P-value of 0.05 in the weak membrane compared to strong membrane were assigned as proteins of interest. A pick list for these spots along with the post-stained pick gel was transferred to the automated Ettan spot picker and gel plugs were excised and recovered into 96-well plates for the subsequent in-gel digestion and mass spectrometry identification.
In gel digestion and protein identification
The proteins in the gel plugs were digested with trypsin (Promega), extracted from the gel matrix, and concentrated by SpeedVac avoiding complete drying using a standard protocol adapted in our laboratory. Tandem mass spectra for 28 gel plugs were then acquired using an LTQ-FT mass spectrometer (Thermo Electron Corp., Bremen, Germany) equipped with Ultimate 3000 series (Dionex). Full MS spectra (MS survey scan) were recorded in the ICR cell and dependent MS2 spectra for the three most intense ions were subsequently acquired by the LTQ. The tandem mass spectra were annotated and peak list files were generated, commonly referred to as .DTA files, by running SEQUEST extract_msn algorithm in Bioworks version 3.2 (Thermo Electron, Bremen, Germany). The mass range m/z 400–3500 from full scan data, with an absolute threshold of 100, a minimum ion count of 12, and a precursor ion tolerance of 1.4 Da were used to generate the .DTA file. The resulting peak list (.DTA) files were then used to interrogate sequences present in an indexed human subset database (137607 sequences stored locally) by running SEQUEST SEARCH algorithm of Bioworks software version 3.2. The criteria for each protein identification was, a minimum of two peptides with a significant peptide expectation (P<0.001), peptide Xcorr 1.9, 2.7, and 3.5 for the charge states and +1, +2, and +3 respectively, and a minimum Delta CN (Delta correlation) of 0.1. The correlation of theoretical molecular weight and pI with the gel region were also generally considered. In addition all the MS/MS spectra identified by SEQUEST were manually verified for spectral quality and matching y and b ion series.
Immunohistochemistry
For immunohistochemical localization of expressed fibulin species, representative full thickness FM from outside the weak zone of three different vaginally-delivered patient’sFM were examined. FM fragments were fixed in 10% neutral formalin, processed for histology, and 10uM sections were affixed to glass slides. Specimens were deparaffinized in 2 changes of xylene, re-hydrated through changes of 100/95/70/50/0% ethanol:distilled water and blocked in 5% normal isotype specific serum with/without 5uM blocking peptides:PBS (absorption control) or 5% non-immune IgG2b (isotype control) for 1h. After washing in PBS, specimens were then incubated with specific fibulin antibodies (8ug/ml) for 1h at 37°C, then overnight at 10°C. Blocking peptides and antibodies for Fibulins 1(B-5), 2(D-15), 3(mab3–5) and 5(S-20) were obtained from Santa Cruz Biotechnology, Santa Cruz, CA. Fibulin 4 (IgG2b) antibody was graciously provided by Dr. Zsolt Urban, Washington University School of Medicine, St. Louis, MO. Detection was performed using a commercial horseradish peroxidase/diaminobenzidine cell and tissue staining kit following the Manufacturers instructions (R&D Systems, Minneapolis, MN: proprietary concentrations). Briefly, after washing in PBS, sections were incubated in isotype-specific (anti-mouse or anti-goat) biotinylated secondary antibody for 1h then washed in PBS. Washed slides were incubated with streptavidin-horseradish peroxidase conjugate for 30m, rinsed in PBS, and developed with diaminobenzidine chromogen for 20m. Immunostained sections were counterstained for 3m in Harris Hematoxylin, blued in tap water, dehydrated through the aforementioned increasing [ethanol] series, through xylene, and mounted using Permount. Stained specimens were reviewed by one of the authors (RWR) and photographed at 600× magnification.
Amnion cell culture
Reflected amnion was peeled from choriodecidua, washed three times in cold PBS and minced. Amnion epithelial and mesenchymal cells were isolated by sequential digestion with trypsin and collagenase as previously reported (25).
Amnion epithelial cell isolation
Amnion fragments were incubated in 100ml MEM:antibiotic-antimycotic:10mM Hepes, pH 7.2, containing 0.2% trypsin (Sigma #T8128) (TMEM) for 10m at 37°C with intermittent agitation. After straining through 4mm2 stainless steel mesh, the initial eluate was discarded. Fragments were then incubated with 150ml TMEM for 30m at 37°C, strained and the eluate was maintained on ice. Digestion with TMEM was repeated three times. Following digestion, fragments were washed in 500ml cold PBS, strained, and this eluate was pooled along with the previous digests. Epithelial cells were pelleted by centrifugation at 300×g for 8m at 10°C, resuspended in MEM containing 10% FBS, antibiotic-antimycotic, 50ug/ml gentamycin sulfate (EMEMC), and plated in 150mm2 tissue culture dishes containing 30ml EMEMC. Dishes were incubated at 37°C:5%CO2 with 48h medium changes until confluent (5-7d).
Amnion mesenchymal cell isolation
Following trypsinization and washing, de-epithelialized amnion fragments were incubated in 100ml MEM:0.1% collagenase (Sigma #C2139), 25mg DNAase, 10mM Hepes, pH 7.2: for 30m at 37°C, strained through 1mm2 stainless mesh and the eluate was centrifuged 400×g for 10m at 10°C. Pelleted mesenchymal cells were plated in EMEMC on 6 × 150mm2 tissue culture dishes for 6hr then medium was removed and replaced.
Purity and viability of isolated amnion epithelial and Mesenchymal cells were routinely monitored by cytokeratin/vimentin differential immunohistochemistry and trypan-blue exclusion as previously described (20). Purity and viability of isolated cell types were greater than 97% and 99%, respectively.
Amnion cell culture experiments
Upon confluency (5-7d), epithelial and mesenchymal cells were trypsinized (0.25% trypsin/0.02% EDTA:PBS) and plated on 24 chamber, multi-well, plates in EMEMC. At 90–95% confluency (48–72h) medium was replaced with antibiotic-antimycotic free EMEM containing 0.2% lactalbumin hydrosylate (EMEML) for 24h prior to experimentation. Isolated amnion epithelial or mesenchymal cells were treated in 24-well dishes with increasing doses of TNF(0–50ng/ml-triplicate cultures at each dose) in fresh EMEML for 24h. Medium was removed following incubation and monolayers were washed gently with 2ml cold PBS. Cells were disrupted by sonication (2 × 10s on ice at setting 40:ARTEK, Farmington, NY) in 250ul Zymogram Buffer added directly to each well. Well contents were transferred to microtubes and BME was added to 5% v/v. Samples were incubated for 5m in a boiling waterbath and frozen until PAGE analysis. This experiment was repeated three times using amnions obtained from three different patients undergoing repeat cesarian section.
Western Blotting
Fibulin family members were further characterized in amnion stripped from weak and strong FM fragments, and in cultured amnion cell experiments by 1D reducing-PAGE and Western Blot analysis. “Strong” or “Weak” amnion fragments from strength-tested FM of six different vaginally delivering patients were homogenized and frozen/thawed, in 5 volumes cold 2X RIPA Buffer (Santa Cruz Biotechnology, Santa Cruz, CA) containing 1X Focus-protease inhibitor cocktail and clarified by centrifugation. Protein was measured using the RC/DC protein assay kit (Bio-Rad, Hercules, CA). Zymogram Buffer (Bio-Rad) containing 5%β-Mercaptoethanol (BME), was added to tissue lysates (20ug protein) and samples were incubated in a boiling waterbath for 5m.
Denatured tissue or cell lysates were run on 4–15% tris/HCl acrylamide gels (Bio-Rad, Hercules, CA) and resolved proteins were electrophoretically transferred to polyvinylidene fluoride membrane (GE Healthcare, Piscataway, NJ) according to Bio-Rad Protocols. Membranes were blocked in blocking buffer: 5% non-fat dry milk/tris-buffered saline:0.5% Tween-20 (TBST) for 30m, then incubated overnight at 10°C with Fibulin or matrix metalloproteinase (MMP) antibodies (2ug/ml in blocking buffer). Fibulin 1, 2, 3 and 5(as described for Immunohistochemistry above), MMP2(C-19) and MMP9(6-6B) antibodies, as well as isotype-specific HRP-conjugated secondary antibodies, were obtained from Santa Cruz Biotechnology, Santa Cruz, CA. Fibulin 4 (IgG2b) antibody was graciously provided by Dr. Zsolt Urban, Washington University School of Medicine, St. Louis, MO. Membranes were washed three times in TBST, incubated with isotype-specific HRP-conjugated secondary antibody(2ng/ml) in blocking buffer for 30m, then again washed three times in TBST.
Chemiluminescent detection was performed using Lumigen-PS according to the manufacturer’s protocol (Amersham/GE, Piscataway, NJ) and blots were exposed against Super RX film (Fuji, Tokyo, Japan) for equivalent time periods.
Quantitation and statistical analysis
Developed films were scanned using an EPSON Perfection 1200U scanner. Densitometry was performed using ImageJ software (NIH, Bethesda, MD). The value for an equivalent scanned blank region within a “dye-only” lane of each blot was subtracted from the value for each lane containing protein. Data points for cumulative rupture force and western blot analyses represent the mean +/− SD. Data were analyzed by Student’s t-test and/or ANOVA using Statview Software. Data and results are termed significant when P <0.05.
Results
Proteomics
Amnions from five weak and five strong (biomechanically determined) FM fragments of four different placentas (total 20 weak and 20 strong) were used in the analysis. Fibulin 1 was identified as an extracellular matrix protein with decreased (at least 2 fold difference) abundance in weaker amnion regions (P<0.05). As very little has been published about the fibulin family of proteins in human placenta, and nothing has been reported in amnion, we expanded our investigation to include additional fibulin family members.
Immunohistochemistry localization of fibulins in FM
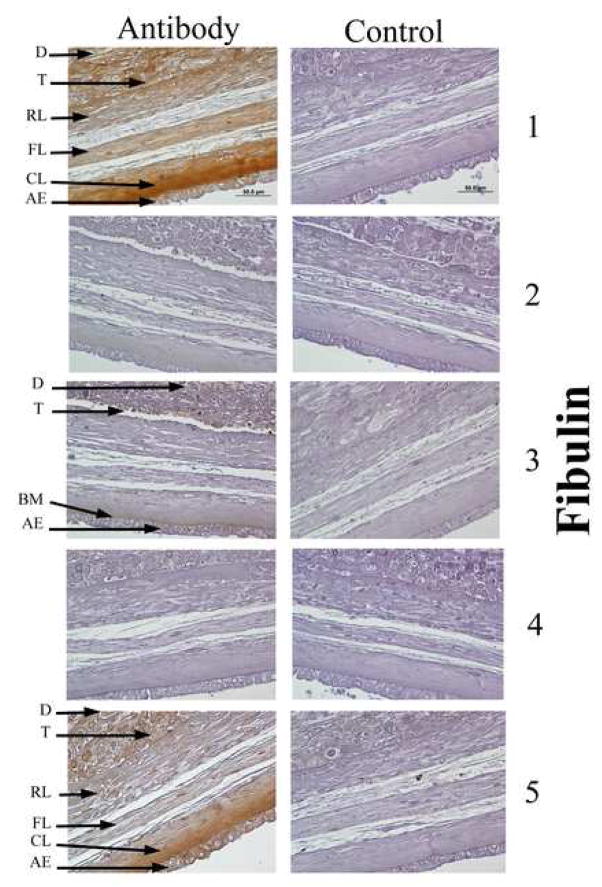
In order to localize the fibulins within the FM, and especially within the amnion, immunohistochemistry was performed. Fibulin 1 and fibulin 5 had similar distributions by immunohistochemistry. Their antibodies stained the cytoplasm of all FM cells and a pericellular region surrounding all FM cells. In addition they densely stained the extracellular matrix of the amnion compact zone (Figure 1). Fibulin 3 was present only in the cytoplasm of the amnion epithelial cells and the chorion laevae trophoblast. Fibulin 2 and fibulin 4 were not detected.
Figure 1.
Immunohistochemical localization of fibulin proteins in intact FM: Localization of immuno-reactive (brown staining) fibulin family members 1–5 are shown in representative cross-sections of fetal membranes (left panel). Pre-treatment of sections with blocking peptides against fibulin antibodies prior to incubation (right panel). Abbreviations: AE amnion epithelial layer, BM basement membrane, CL compact layer, D decidua, FL fibroblast layer, RL reticular layer, T trophoblast (magnification 600X).
Biomechanics
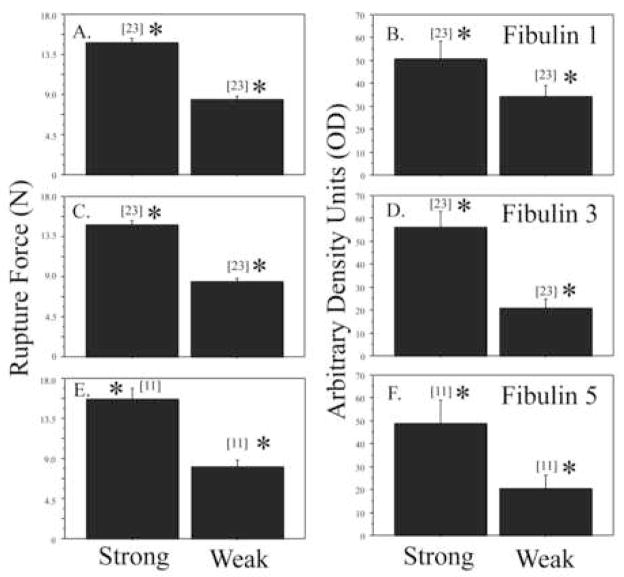
FM from 6 placentas were used to select FM fragments from the weak zone for Western blot studies (below) to confirm the results of the Proteomics. The breaking strength of the fragments from the weak zone was 8.32 ± 1.99 N while that of that of the fragments from outside the weak zone was 14.64 ± 2.41 N (P<0.001), consistent with our previous reports (7, 8).
Western Blots of amnion from para-cervical weak zones and other FM regions
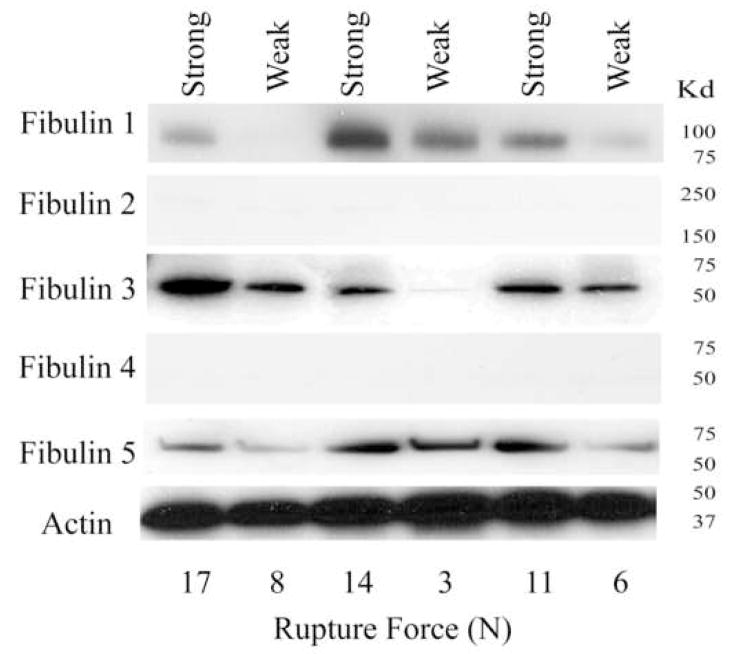
Fibulin 1, fibulin 3, and fibulin 5 all showed decreased abundance in the weak zone compared with other regions. They were decreased 33%, 63% and 58% respectively (all P<0.01; Figure 2 and 3). Fibulins 2 and 4 were not detected by Western Blot.
Figure 2.
Fibulin protein expression in amnion from para-cervical weak zone and other FM areas: Panels indicate Fibulin 1–5 protein expression, as assessed by western blot analysis, of amnion from FM fragments from the para-cervical weak zone and other FM areas from three representative patients. Actin expression is shown for comparison of protein loading. Rupture force in Newtons (N) for each fragment is indicated along the bottom.
Figure 3.
Comparison of rupture force and fibulin protein expression in para-cervical weak zone and other FM areas: Rupture force in Newtons (A, C, E) for intact FM fragments, and fibulin 1, 3 and 5 protein expression (B, D, F) in the amnion component from these intact fragments, as assessed by western blot analysis, are shown. Note that the number of samples in each group appears above each bar [n] and differences between fragments from the para-cervical weak zone and other FM areas for rupture force, as well as fibulin expression, for each group is statistically significant (* P<0.01).
Production of fibulins by amnion cells
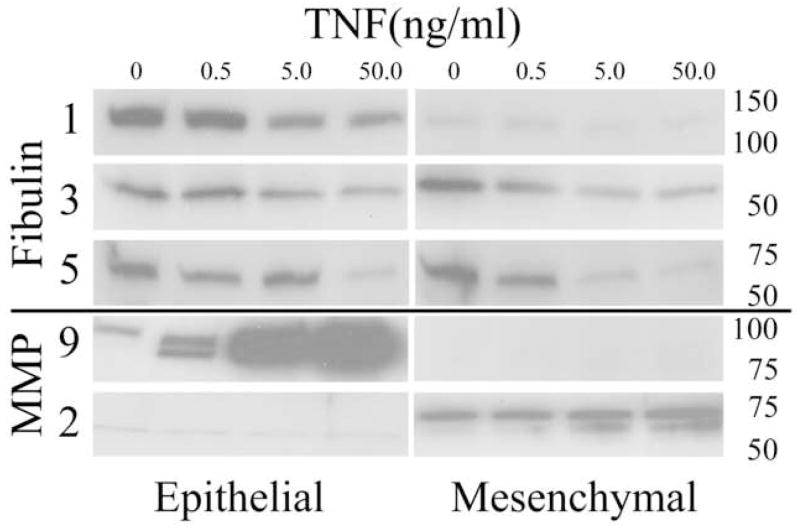
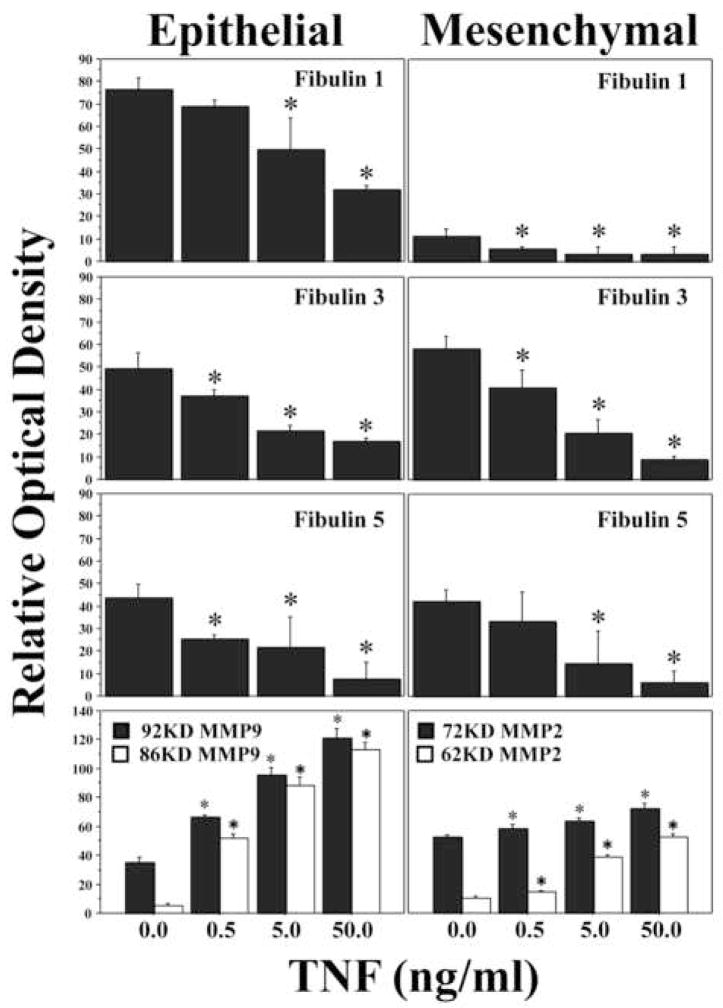
In order to determine the cellular source for the fibulins in amnion, Western blots of amnion epithelial cells and mesenchymal cells were examined (Figure 4). Amnion epithelial cells and amnion mesenchymal cells each produced fibulins 1, 3 and 5. We have demonstrated in previous reports that FM fragments can be weakened in vitro with TNF in a dose dependent manner (26). We thus examined the effect of TNF on amnion cell production of the fibulin family proteins. TNF caused a dose dependent decrease in fibulins 1, 3 and 5 (all P< 0.05; Figure 4). In contrast, TNF-induced dose dependent increases in matrix metalloproteinases 9 and 2 (MMP9 and MMP2) in amnion epithelial cells and amnion mesenchymal cells, respectively, consistent with our previous work (27).
Figure 4.
Fibulin expression is down-regulated by TNF in cultured amnion cells: TNF treatment (0–50ng/ml) of cultured amnion epithelial (left panel) or amnion mesenchymal cells (right panel) for 24h induced a dose dependent decrease in fibulin 1, 3 and 5 expression in both cell types. Dose dependent induction of MMP9 and MMP2 by TNF in amnion epithelial and mesenchymal cells, respectively, is shown for comparison. A representative western blot from one experiment(4a). Molecular weight markers in KD are indicated on the right. Graphical representation of compiled data (relative optical density: Mean+/−SD) obtained from four scanned western blots (as above) for experiments using amnion cells prepared from four separate FM(4b). TNF dose is indicated along the horizontal axis and relative optical density is shown on the vertical axis. Data is considered significant (*) at P<0.05 (N=4).
Discussion
In previous reports we have demonstrated that human FM contain a biomechanically weak zone overlying the cervix which exhibits characteristics of increased collagen remodeling and apoptosis relative to other membrane areas. Specifically we have demonstrated that the para-cervical weak zone has increased MMP9 and decreased TIMP3 (7, 8, 26). In this study we have focused upon the amnion, the strongest component of the FM. In this first report of fibulins in amnion, we localized fibulin 1, fibulin 3 and fibulin 5 by immunohistochemistry. Fibulins 2 and 4 were not detected. Fibulin protein family members showed differential abundance in the weak zone when compared with other FM regions. All three detected fibulins showed decreased abundance in the amnion component of the FM weak zone. All three detected fibulins were produced by both amnion epithelial and mesenchymal cells and all were inhibited by TNF in both cell types.
Fibulin 1 and fibulin 5 were detected in the cytoplasm of all FM cells and in the compact layer of the amnion by immunohistochemistry. Both are particularly abundant immediately deep to the basement membrane underlying the amnion epithelial cell layer. This location has been shown to contain microfibrils of the elastic fiber system, although, whether elastin itself is present in amnion remains controversial (28–30). These microfibrils have been reported to bind amnion epithelial cells, basement membrane structures, and the amniotic compact layer to the underlying fibroblast layer (28). In many tissues Fibulin 1 has been localized in basement membranes, and elastic fibers (10, 11, 22) consistent with this localization in FM. Decreased fibulin 1 in the weak zone, reflecting a possible deterioration of the basement membrane and microfibrillar layer, would thus be consistent with decreased rupture strength. Fibulin 5 also has been shown to be important for the assembly of elastic type microfibrils. Mutation of its gene leads to the cutis laxa syndrome characterized by loose skin, emphysematous lungs and tortuous blood vessels (31–36). Decreased fibulin 5 in the FM weak zone may thus inhibit microfibril assembly, adversely affecting biomechanical properties. Fibulin 3 was seen only in the epithelial cells of the amnion and the trophoblast cells of the chorion. Its mutation leads to macular degeneration but its exact function has not yet been determined (10). It is not clear how its decreased abundance might affect FM strength.
It is unclear how TNF decreases fibulins 1, 3, and 5 production by the amnion cells. TNF generally controls transcription through AP-1 and both canonical and noncanonical NF-kB promoter sites in many genes (37). These promoter elements have not been described in the fibulin genes (38–41). It is thus unlikely that TNF directly effects fibulin transcription. Fibulins are stimulated by TGFb, however, whose action is frequently opposed by TNF in other tissues (42). Alternately, TNF also stimulates numerous proteases as specifically shown in Figure 4 and also in previous studies (43, 44, 26) with MMP9. TNF could decrease fibulin production secondarily through these mechanisms.
In summary, the fibulin family proteins have decreased abundance in the para-cervical weak zone compared with the other areas of the FM. Their location is coincident with an extensive microfibrillar network that is integral to the epithelial cell basement membrane and the amniotic compact layer suggesting that fibulins may be integral proteins of these very important strength maintaining, extracellular matrix structures. Degradation or remodeling of the microcellular network in the amnion may be a critical element of the weakening process leading to FM rupture.
Acknowledgments
Support: NIH HD48476 Grant to John J Moore, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87(6):590–600. doi: 10.1080/00016340802005126. [DOI] [PubMed] [Google Scholar]
- 2.Parry S, Strauss JF. Premature rupture of the fetal membranes. N Engl J Med. 1998;338:663–70. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 3.Lockwood CJ, Kuczynski E. Markers of risk for preterm delivery. J Perinat Med. 1999;27(1):5–20. doi: 10.1515/JPM.1999.001. [DOI] [PubMed] [Google Scholar]
- 4.Menon R, Fortunato SJ. The role of matrix degrading enzymes and apoptosis in rupture of membranes. J Soc Gynecol Investig. 2004;11(7):427–37. doi: 10.1016/j.jsgi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2006;27(11–12):1037–51. doi: 10.1016/j.placenta.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.McLaren J, Malak TM, Bell SC. Structural characteristics of term human fetal membranes prior to labour: identification of an area of altered morphology overlying the cervix. Hum Reprod. 1999;14(1):237–41. doi: 10.1093/humrep/14.1.237. [DOI] [PubMed] [Google Scholar]
- 7.El Khwad M, Stetzer B, Moore RM, Kumar D, Mercer B, Arikat S, Redline RW, Mansour JM, Moore JJ. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod. 2005;72(3):720–6. doi: 10.1095/biolreprod.104.033647. [DOI] [PubMed] [Google Scholar]
- 8.El Khwad M, Pandey V, Stetzer B, Mercer BM, Kumar D, Moore RM, Fox J, Redline RW, Mansour JM, Moore JJ. Fetal membranes from term vaginal deliveries have a zone of weakness exhibiting characteristics of apoptosis and remodeling. J Soc Gynecol Investig. 2006;13(3):191–5. doi: 10.1016/j.jsgi.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Arikat S, Novince RW, Mercer BM, Kumar D, Fox J, Mansour JM, Moore JJ. Separation of amnion from choriodecidua is an integral event to the rupture of normal fetal membranes and constitutes a significant component of the work required. Am J Obstet Gynecol. 2006;194:211–17. doi: 10.1016/j.ajog.2005.06.083. [DOI] [PubMed] [Google Scholar]
- 10.Timpl R, Sasaki T, Kostka G, Chu ML. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol. 2003;4(6):479–89. doi: 10.1038/nrm1130. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi N, Kostka G, Garbe JH, Keene DR, Bächinger HP, Hanisch FG, Markova D, Tsuda T, Timpl R, Chu ML, Sasaki T. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J Biol Chem. 2007 Apr 20;282(16):11805–16. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- 12.Vogel BE, Muriel JM, Dong C, Xu X. Hemicentins: what have we learned from worms? Cell Res. 2006;16(11):872–8. doi: 10.1038/sj.cr.7310100. [DOI] [PubMed] [Google Scholar]
- 13.de Vega S, Iwamoto T, Nakamura T, Hozumi K, McKnight DA, Fisher LW, Fukumoto S, Yamada Y. TM14 is a new member of the fibulin family (fibulin-7) that interacts with extracellular matrix molecules and is active for cell binding. J Biol Chem. 2007;282(42):30878–88. doi: 10.1074/jbc.M705847200. [DOI] [PubMed] [Google Scholar]
- 14.Schultz DW, Weleber RG, Lawrence G, Barral S, Majewski J, Acott TS, Klein ML. Hemicentin-1 (fibulin-6) and the 1q31 AMD locus in the context of complex disease: Review and Perspective. Ophthalmic Genet. 2005;26(2):101–5. doi: 10.1080/13816810590968023. [DOI] [PubMed] [Google Scholar]
- 15.Blackburn J, Tarttelin EE, Gregory-Evans CY, Moosajee M, Gregory-Evans K. Transcriptional regulation and expression of the dominant drusen gene FBLN3 (EFEMP1) in mammalian retina. Invest Ophthalmol Vis Sci. 2003;44(11):4613–21. doi: 10.1167/iovs.03-0112. [DOI] [PubMed] [Google Scholar]
- 16.Loeys B, Van Maldergem L, Mortier G, Coucke P, Gerniers S, Naeyaert JM, De Paepe A. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum Mol Genet. 2002;11(18):2113–8. doi: 10.1093/hmg/11.18.2113. [DOI] [PubMed] [Google Scholar]
- 17.Markova D, Zou Y, Ringpfeil F, Sasaki T, Kostka G, Timpl R, Uitto J, Chu ML. Genetic heterogeneity of cutis laxa: a heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am J Hum Genet. 2003;72(4):998–1004. doi: 10.1086/373940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, Urban Z. Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet. 2006;78(6):1075–80. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowal RC, Richardson JA, Miano JM, Olson EN. EVEC, a novel epidermal growth factor-like repeat-containing protein upregulated in embryonic and diseased adult vasculature. Circ Res. 1999;84(10):1166–76. doi: 10.1161/01.res.84.10.1166. [DOI] [PubMed] [Google Scholar]
- 20.Lee MJ, Roy NK, Mogford JE, Schiemann WP, Mustoe TA. Fibulin-5 promotes wound healing in vivo. J Am Col Surg. 2004;199(3):403–10. doi: 10.1016/j.jamcollsurg.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Rahn DD, Acevedo JF, Word RA. Effect of vaginal distention on elastic fiber synthesis and matrix degradation in the vaginal wall: potential role in the pathogenesis of pelvic organ prolapsed. Am J Physiol Regul Integr Comp Physiol. 2008;295(4):R1351–R8. doi: 10.1152/ajpregu.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4(12):1127–31. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cirulis JT, Bellingham CM, Davis EC, Hubmacher D, Reinhardt DP, Mecham RP, Keeley FW. Fibrillins, Fibulins, and Matrix-Associated Glycoprotein Modulate the Kinetics and Morphology of in Vitro Self-Assembly of a Recombinant Elastin-like Polypeptide. Biochemistry. 2008 Oct 31; doi: 10.1021/bi8005384. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yohannes E, Chang J, Christ GJ, Davies KP, Chance MR. Proteomics analysis identifies molecular targets related to diabetes mellitus associated bladder dysfunction. Mol Cell Proteomics. 2008;7(7):1270–85. doi: 10.1074/mcp.M700563-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar D, Moore RM, Elkhwad M, Silver RJ, Moore JJ. Vitamin C exacerbates hydrogen peroxide induced apoptosis and concomitant PGE2 release in amnion epithelial and mesenchymal cells, and in intact amnion. Placenta. 2004;25(6):573–9. doi: 10.1016/j.placenta.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Kumar D, Fung W, Moore RM, Pandey V, Fox J, Stetzer B, Mansour JM, Mercer BM, Redline RW, Moore JJ. Inflammatory mediators found in amniotic fluid induce collagen remodeling, apoptosis, and biophysical weakening of cultured fetal membranes. Biol Reprod. 2006;74:29–34. doi: 10.1095/biolreprod.105.045328. [DOI] [PubMed] [Google Scholar]
- 27.Moore RM, Novak JB, Kumar D, Mansour JM, Mercer BM, Moore JJ. Alpha-lipoic acid inhibits Tumor Necrosis Factor-induced remodeling and weakening of human fetal membranes. Biol Reprod. 2008 doi: 10.1095/biolreprod.108.073205. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachmaier N, Graf R. The anchoring zone in the human placental amnion: bunches of oxytalan and collagen connect mesoderm and epithelium. Anat Embryol (Berl) 1999;200(1):81–90. doi: 10.1007/s004290050262. [DOI] [PubMed] [Google Scholar]
- 29.King BF, Blankenship TN. Immunohistochemical localization of fibrillin in developing macaque and term human placentas and fetal membranes. Microsc Res Tech. 1997;38(1–2):42–51. doi: 10.1002/(SICI)1097-0029(19970701/15)38:1/2<42::AID-JEMT6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 30.Hieber AD, Corcino D, Motosue J, Sandberg LB, Roos PJ, Yu SY, Csiszar K, Kagan HM, Boyd CD, Bryant-Greenwood GD. The detection of elastin in the human fetal membranes: proposed molecular basis for elasticity. Placenta. 1997;18(4):301–12. doi: 10.1016/s0143-4004(97)80065-3. [DOI] [PubMed] [Google Scholar]
- 31.Katsuta Y, Ogura Y, Iriyama S, Goetinck PF, Klement JF, Uitto J, Amano S. Fibulin-5 accelerates elastic fibre assembly in human skin fibroblasts. Exp Derm. 2008;17(10):837–42. doi: 10.1111/j.1600-0625.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 32.Freeman LJ, Lomas A, Hodson N, Sherratt MJ, Mellody KT, Weiss AS, Shuttleworth A, Kielty CM. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem J. 2005;388:1–5. doi: 10.1042/BJ20050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiemann WP, Blobe GC, Kalume DE, Pandey A, Lodish HF. Context-specific effects of fibulin-5 (DANCE/EVEC) on cell proliferation, motility, and invasion. J Biol Chem. 2002;277(30):27367–277. doi: 10.1074/jbc.M200148200. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kubuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo R, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415(6868):171–5. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen AD, Itoh S, Jeney V, Yanagisawa H, Fujimoto M, Ushio-Fukai M, Fukai T. Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circ Res. 2004;95(11):1067–74. doi: 10.1161/01.RES.0000149568.85071.FB. [DOI] [PubMed] [Google Scholar]
- 36.Spencer JA, Hacker SL, Davis EC, Mecham RP, Knutsen RH, Li DY, Gerard RD, Richardson JA, Olson EN, Yanagisawa H. Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc Natl Acad Sci U S A. 2005;102(8):2946–51. doi: 10.1073/pnas.0500058102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 2006;20(10):1589–98. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 38.Bardin A, Moll F, Margueron R, Delfour C, Chu ML, Maudelonde T, Cavailles V, Pujol P. Transcription and posttranscriptional regulation of fibulin-1 by estrogens leads to differential induction of messenger ribonucleic acid variants in ovarian and breast cancer. Endocrinology. 2005;146(2):760–8. doi: 10.1210/en.2004-1239. [DOI] [PubMed] [Google Scholar]
- 39.Castoldi M, Chu ML. Structural and functional characterization of the human and mouse fibulin-1 gene promoters: role of Sp1 and Sp3. Biochem J. 2002;362:41–50. doi: 10.1042/0264-6021:3620041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuruga E, Yajima T, Irie K. Induction of fibulin-5 gene is regulated by tropoelastin gene and correlated with tropoelastin accumulation in vitro. Int J Biochem Cell Biol. 2004;36(3):395–400. doi: 10.1016/s1357-2725(03)00238-3. [DOI] [PubMed] [Google Scholar]
- 41.Singh U, Sun T, Larsson T, Elliott RW, Kostka G, Fundele RH. Expression and functional analysis of fibulin-1 (Fbln1) during normal and abnormal placental development of the mouse. Placenta. 2006;27(9–10):1014–21. doi: 10.1016/j.placenta.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Kuang PP, Joyce-Brady M, Zhang XH, Jean JC, Goldstein RH. Fibulin-5 gene expression in human lung fibroblasts is regulated by TGF-β and phosphatidylinositol 3-kinase activity. Am J Physiol Cell Physiol. 2006;291(6):C1412–21. doi: 10.1152/ajpcell.00087.2006. [DOI] [PubMed] [Google Scholar]
- 43.Fortunato SJ, Menon R, Lombardi SJ. Role of tumor necrosis factor – alpha in the premature rupture of membranes and preterm labor pathways. Am J Obstet Gynecol. 2002;187(5):1159–62. doi: 10.1067/mob.2002.127457. [DOI] [PubMed] [Google Scholar]
- 44.Arechavaleta-Velasco F, Ogando D, Parry S, Vadillo-Ortega F. Production of matrix metalloproteinase-9 in lipopolysaccharide-stimulated human amnion occurs through an autocrine and paracrine proinflammatory cytokine-dependent system. Biol Reprod. 2002;67(6):1952–8. doi: 10.1095/biolreprod.102.004721. [DOI] [PubMed] [Google Scholar]